Abstract
The origin of life can be explained by the intermolecular interactions among molecules via a spiral structure of water. DNA is composed of D-type saccharides, and proteins are composed of L-type amino acids in most organisms on Earth, production of biomolecules is controlled by two types of chirality. The homochirality of biomolecules is evidence of the existence of a spiral structure of water. The chirality of molecules affects the selective chaining of L-type amino acid in protein. The main components of the cell membranes of living organisms today are C 16 H 34 (melting point 18°C, boiling point 287°C) and C18H38 (melting point 28~30°C, boiling point 317°C). There was a time when the H+ of the solar wind collided with CO2, the main component of the primordial Earth’s atmosphere, at 500 km/sec to produce hydrocarbon molecules, and the long-chain molecules of C16H34 and C18H38 floated as a membrane on the surface of the waters. When a spiral structure is formed on the surface of the water, homochiral molecules are selectively incorporated into vacant channels of the helix. With the oil film as the surface, the spiral structure group are aligned in water, and rearranged by thermal motion. Lipid molecules invade the vacant channel of the helix and are arranged perpendicular to the membrane surface by hydrophobic interactions, thus forming a cell membrane. The three-dimensional structure of amino acid molecules and water molecules interact to form a structure adapted to amino acids. DNA was created for storing amino acids sequences of proteins in a double spiral structure with intermolecular bonds in the middle part of the protein replication region. By converting long-lived DNA into short-lived RNA, RNA modified by amino acids becomes m-RNA that stores amino acid sequences and t-RNA that selectively carries molecules of other RNA amino acids. After a protein detached by formation of a ribbon, the same amino acids can be attached to the remained RNA. The genetic code (codon) corresponds to one amino acid in three sets of four bases, and all organisms on Earth have codons in common. The genetic code (codon) is nothing but a label of molecular structure that is a key-lock relationship between m-RNA and t-RNA. Thus, origin of life depended upon the movement of water molecules and spiral structures.
Keywords
Intermolecular bond, Spiral structure, Homochirality, Protein, DNA, RNA, Codon
Introduction
It is highly likely that life in the universe exists only on Earth [1] 2014. Theories of how life first originated from nonliving matter are classified into two categories: replicator first and metabolism first [2] 2007. Wherever something is produced, the producer and the resulting product must exist at the same time. Nucleosides, which are the building blocks of DNA, and amino acids which are the basic blocks of proteins, must exist at the same time to establish a relationship between them. Therefore, replicator and metabolism are necessary concurrently. Hunding et al. discussed that life began as a prebiotic ecology of co-evolving populations of macro-molecular aggregates [3] 2006. However, they ignored the origin of homochirality of biomolecules and the origin of the genetic code. F. Crick and J. Watson described the double helix structure of DNA which is composed solely of D-type sugars [4] 1953. This paper puts forward the hypothesis that water molecules form spiral structures. When liquid oil is dropped into liquid water, the hydrophobic hydrocarbon molecules gather on the surface of the water to form an oil film. Water molecules become organized in a “spiral structure (α-quartz lattice structure [5] 1974.) under the influence of the oil film. Although hydrocarbon molecules pass through vacant channel, lipid molecules insert into the vacant channels of the helix of water, and the hydrophobic molecules align vertically to the surface, and the membrane of a cell has formed. After a while, carbohydrates are produced on the surface of hydrocarbons floating on the surface of the water. The left-handed L-type amino acid and the left-handed D-type glycans that make up nucleic acids are concurrently generated in the living creatures of the Earth. The three-dimensional structure of water molecules around both regions interact to form an adapted structure where the three-dimensional structure of molecules characterized by individual amino acids is formed in contact with amino acids and t-RNA, but these two structures do not coalesce due to different chirality. Evolution is achieved by partial improvement during activities of the life to prolong its life. Each living creature was formed by individual organization of itself. Leveraging formed tissues is not a random process. Approximately 3.5~4 billion years ago, the intermolecular interaction of water molecules caused the structures of molecules to influence each other, and the DNA gene system was established.
Spiral Structure of Water Molecules
Evidence that Indicates a Spiral Structure of Water Molecules in Carbonated Water
Liquid water contributes to the building and repairing of cellular membranes, as well as relaying mechanical and chemical messages. Eisenberg and Kauzmann describe the structure and properties of water in detail [6] 1969. However, the dynamic properties of water such as organized movements of atoms in liquid water for metabolism remain unclear. The microscopic structure of liquid water changes every 10-12 s. Such instantaneous change of state is difficult to observe using modern technologies.
Figure 1 indicates the activation energy of water. calculated from temperature characteristics of water vapor pressure on ice (Kaye and Laby), and water: by Wagner and Pruss) [7] p.421. The melting heat of ice is 1.4 kcal/mol which is one 3.7th of the hydrogen bond formation energy. The small energy difference is caused by clusters of hydrogen bonds in the liquid water. When carbonated water is slowly frozen, CO2 forms spikes of bubbles within the ice which are similar in appearance to the spikes of sea urchins (Figure 2). This is the evidence that CO2 forms cocoon like molecule of surrounded by water molecules. Figure 3 shows the vacant channel of a helix, in which pairs of hydrogen atoms of a water molecule shown in yellow are lined up toward the vacant channel. A linear molecule of CO2, in which the electron cloud of the carbon atom is covered by the electron clouds of the oxygen atoms, fits in the vacant channel.
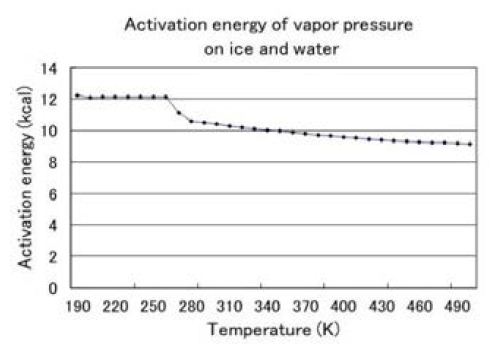
Figure 1: The small energy difference between ice and water

Figure 2: Elongated bundle of CO2 bubbles in the ice of carbonated water

Figure 3: Structure of through-holes in liquid helix structure Blue allows indicate electric dipole moment Oxygen atom: blue, Hydrogen atom: yellow.
The spiral structure of α-quartz has the smallest size and lowest energy among various SiO2 crystal structures. The spiral structure of liquid water would be similar to that of α-quartz. At β- quartz to α-quartz phase transition, a systematic movement takes place as shown in Figure 5.There are vacant channels along the rotational axes of the spiral structure (Figures 4 and 5).
Figure 5 shows a model in which tetrahedral of H2O molecules in a helix make systematic thermal motions. Alternate vibration of rotation θ on each electric axis (± δ θ) involves extension and contraction, enable the entire molecular structure to generate movements that are reminiscent of respiration. If the vibration changes to one-way rotation, the systematic thermal movement of the spiral structure of liquid water molecules makes neighboring two way of streams. These streams can support the replication of protein. One stream is for protein and the other is for a m-RNA.
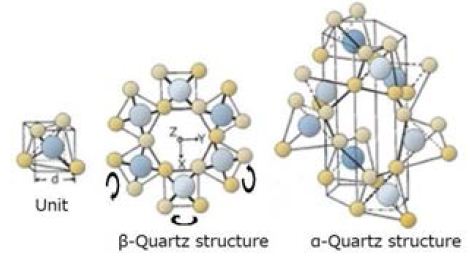
Figure 4: Formation of α-quartz spiral structure (This illustration was reproduced from Ref. [5])
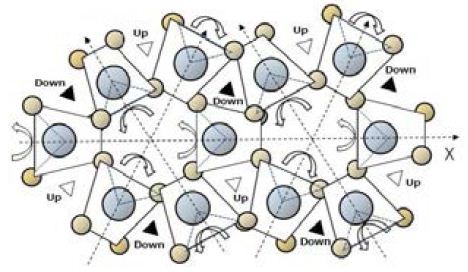
Figure 5: Movement of water molecules in spiral white triangle indicates upward movement, and black triangle indicates downward movement.
Thermal Motion of H2O in a Spiral Structure Formed in the Vicinity of the Membrane of CO2 Bubbles
The downsize of helix and the energy decreases at the phase transition from β-quartz structure to α-quartz structure as shown Figure 4, the coupling angle of the connection points of the tetrahedron intermittently.
The downsize ratio depends on the rotational angle (θ). Contraction ratio for the optical axis (γ) is expressed by Eq. (1)
γz= (cos θ). (1)
The contraction ratio for the electrical axis is expressed by Eq. (2)
γxy={(1+31/2cos θ)/(1+31/2)}. (2)
The rotation of θ results a large temperature-dependence of α-quartz typed phase as shown in Figure 6.
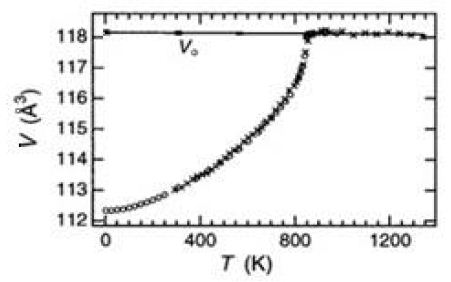
Figure 6: Temperature-dependence on the size of α-quartz1 (from Carpenter et.al. [8] 1998)
Structure of Spirally Arranged Water Molecules
Observations of Bubbles in Carbonated Water and Systematic Movements of Molecules
Karasawa reported how the acidity of carbonated water varied with the addition of iron powder [9] 2014. Metabolic reaction takes place in the material of intermolecular bond. As an example, material of intermolecular bonds was observed by mixing iron powder with carbonated water. Bubbles made of carbonated water and iron atom are always formed by intermolecular interactions at the boundary conditions between liquids and gases. So, even if the bubbles begin to crack, the defects are quickly repaired through the bubble formation mechanism. Adding changes to the bubbles from the outside helps with metabolism and thus prolongs the life of the membrane (https://youtu.be/GCL6Sv3WvOI). The intermolecular reaction is expressed by Fe(OH)2 + 2(CO2) ⇆ Fe(HCO3)2. This material generates the membrane of bubble that exhibits a metabolism.(https://youtu.be/M6n9kURDNg8). But the bubbles are disappeared, and only Fe2O3 remained.
The systematic movement of liquid water can be observed by the behavior of bubbles generated when thawing ice of carbonated water as shown in (https://youtu.be/BbaMVCgKrA8). We observed occasional phenomena such as CO2 bubbles that moved suddenly, coalescence of moving bubbles, and a moving bubble taken into the intermediate region of paired bubbles. This is evidence of the connection of liquid water by small size and low energy. When sudden movements of bubbles were observed, these occurred when the flow of oxidized carbon through the vacant channel of the spiral structure underwent a continuous swirling motion. Observed activities are difficult to explain without by spiral structure of molecules of low energy downsize structure formed from the plane of the membrane. When carbonated water is frozen, CO2 bubbles form and appears like spikes on sea urchins. This is evidence that CO2 molecules form linear molecules surrounded by water molecules as shown in Figure 7. The systematic movement of water molecules can be observed from the behavior of CO2 bubbles generated when thawing ice with carbonated water as shown in previous section. In the final state of thawing the ice of carbonated water, clusters of water like grapes are observed as is the coalescence of bubble of CO2 and the movement of bubbles in pair. If a stream of CO2 takes place in the helical structure, the alternate thermal vibration changes to rotation. White triangle indicates upward movement, and black triangle indicates downward movement. The movements of neighboring opposite stream of up-word and down-word are necessary for replication of protein as shown in Figure 5.
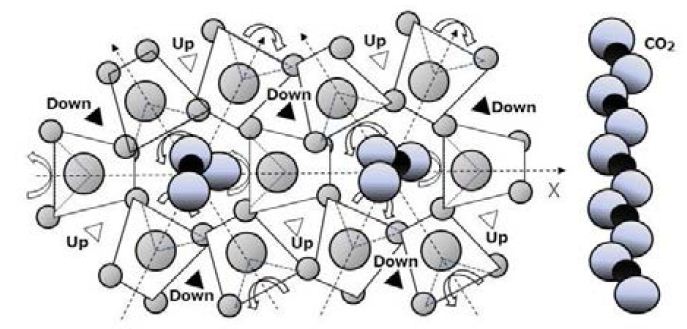
Figure 7: An illustration of thermal motion of molecules in helix structure of a carbonated water
Liquid water becomes the lowest-energy structure (α-quartz type lattice) in which tetrahedral molecules are arranged in a spiral shape, and phase transition takes place. The spiral structure of water has channels perpendicular to the X-Y plane, and the molecules inner wall exposes hydrogen atom pairs to be rotated three times symmetry, and the molecules in the channels move up and down by the thermal rotational vibration of the hydrogen pairs. The systematic thermal motion of the spirally arranged water molecules synthesized the homochiral molecule. Most of biochemical reactions are carried out by the network of reactions in which adjacent atoms and molecules are exchanged by the thermal motion, so the amount of consumption on energy and nutrients are small.
Evidence that Surface Water Molecules form a Planar Structure
Reflected light has polarization that oscillates almost parallel to the reflective surface. When observing the surface of water with a slight wind blowing, the surface of the water appears to sparkle because the light polarized horizontally from the surface of the water enters the human eye. This is evidence that the water molecules on the surface of the water form a planar structure. Liquid water is a large molecular structure that frequently changes its three-dimensional structure by changing partner of hydrogen bonds. In the cell, all molecules cooperate and easily move by thermal vibration. Since three-dimensional structure of water molecules contains a spiral structure, the biomolecules that make up the organisms that inhabit the entire Earth are homochiral. Sunlight irradiates the primordial Earth, but there is no material that changes chirality in outer space along the way. The homochirality of biomolecules is evidence of the existence of a spiral structure of water. The generated homochiral biomolecules induce the formation of spiral structures in water to support biochemical reactions. That is, chiral molecules selectively combine to form large chiral molecules due to the helical structure of water. While hydrothermal vents as the origin of life are local areas and cannot be diffused to other regions.
The Oldest Organic Molecules in the Primitive Earth
There was a time when CO2 was the main component in the atmosphere of the primitive Earth. Researchers at UCLA and the University of Wisconsin–Madison have confirmed that microscopic fossils discovered in a nearly 3.5-billion-year-old piece of rock in Western Australia are the oldest fossils ever found and the earliest direct evidence of life on Earth [10] 2017. Tice, M. M., and Low were discovered in South Africa in large quantities of carbonaceous mud produced 3.4 billion years ago. [11] 1999. The cell membranes of current organisms have a structure in which C16 H34 or C18H38 are aligned vertically on the water surface. These molecules were synthesized in the atmosphere. That is, H+ at a high speed of 500 km/sec of the solar wind synthesized organic molecules in the upper atmosphere due to the collision of the H+ of the solar wind [12] 2022. The small hydrocarbon molecules produced remain in the atmosphere and undergo repeated synthesis reactions. C16H34 and C18H38 are hydrophobic long-chain molecules with a melting point of 20°C and a boiling point of 300°C, which float as an oil film on the surface of water [13] 2022. There are systematic thermal movements in which spiral structures line up in water based on the oil film surface. Under the hypothesis of spiral structured liquid water, formation of biological cell membrane will understand as follows. There are vacant channels surrounded by a tetrahedron of water molecules as shown in Figure 5 facilitates uptake of a long-chain alkane CnH2n+2, because such molecules have an elongated shape and are covered with hydrogen atoms [14] p.52, 2017. The hexadecane (C16H34) and octadecane (C18H38) are involved in transport of hydrocarbons through cell membranes.
How Cell Membranes are Constructed?
The cell membrane is one of the most important for the creature. Dyson came up with the world theory of “garbage bags”, in which many “garbage bags” containing miscellaneous molecules were formed in the primordial ocean [15] 1999. However, he did not describe the process of how cell membrane had formed. Figure 8 shows an illustration of a cell membrane.
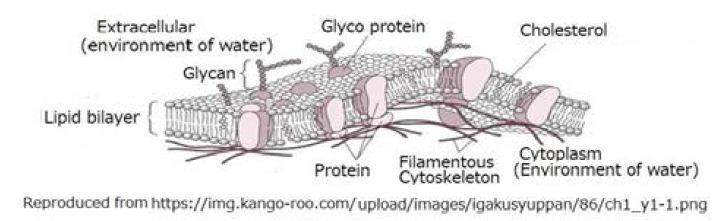
Figure 8: An illustration of a cell membrane
Origin of the Mechanism of Protein Production
Biomolecules are Homochiral because They are Produced by the Information Stored in DNA
The control system consists of two factors that are activated at the same time. The controller makes action to the object one directionally. The replication of protein is carried out where L-type of amino acids and D-type of sugars exist. The different chirality of DNA makes possible to control the production of proteins. Here, L-type of amino acid chain and D-type of sugar chains precede in the opposite direction similarly the positive screw and the reverse screw precede in the opposite direction with the same rotation. If working tools of D-type of sugar chains at first, the products of L-type of amino acid chain are controlled at a selected portion by difference of the chirality. In DNA, the region connecting the rows of two main chains is an intermolecular bond and can be separated. Since the base pair in the region of DNA where the amino acid is released has a key-lock relationship between amino acids, the same amino acids as the separated amino acids can be bonded. Under the helical movement of water molecules, the cell membrane syntheses proteins by systematic thermal motions of spiral structure. Proteins produced by peptide-bonding between L-type amino acids are released like ribbons. The protein linked with water molecules to form various three-dimensional structures.
Different Chirality of D-type of Sugar Controls Production of Protein
It is possible that carbohydrates (Cx(H2O)y) are produced on the surface of hydrocarbons floating on the surface of the water. So, there exist long-chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. A region of D-type sugar is added to surround the invading amino acids to form a tissue of helical sugar molecules modified with amino acids. The rotation meshed by the rotation of the gear is combined with the L-type amino acid sequence and D-type helix glycan. That is, direction of progress of L-type amino acid sequence is opposed to that of D-type helix glycan sequence. D-type helical sugar becomes the more stable molecular region through dehydration bonding, and the DNA is formed (Figure 9) [16].
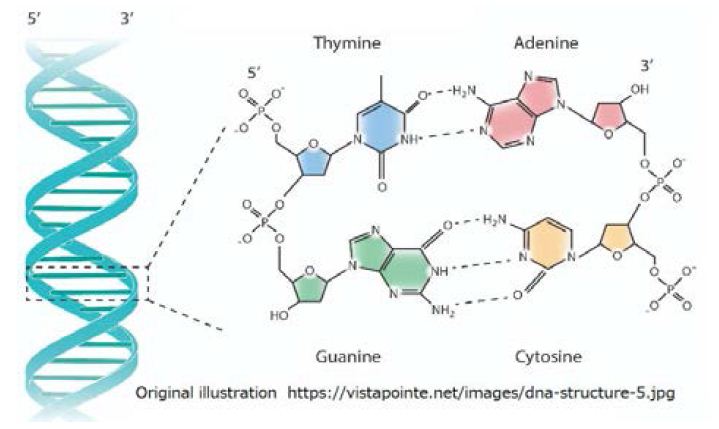
Figure 9: The symmetry of sense chain against antisense chain in the double layered structured DNA are as follows, L-type adenine for D-type thymine, L-cytosine for D-guanine, L-guanine for D-cytosine, and L-thymine for D-adenine.
Processes of Protein Replication by DNA
All living things on Earth have a DNA code with a common design is used. Until the period when living organisms evolved to the level where they had common DNA, the evolutionary process was carried out by intermolecular interactions in water.
The process to establish DNA gene system can be explained as follows:
- When a L-type amino acid invades a cell membrane, and the next L-type of amino acid makes a peptide bond form a ribbon shaped L-type of If D-type sugar is added to surround amino acid, the shift of stem on D-type helix sugar sequence is opposed to that of the protein.
- A D-type helix sugar region for one amino acid forms a region of t-RNA which is used for transporting of an amino acid to m-RNA.
- Progression of L-type amino acid sequence will form a series of D-type of t-RNA. The series of D-type of t-RNA will be surrounded by L-type of sugars, and one L-type of m-RNA will be formed. So, each step of L-helix and D-helix proceed in opposite directions as amino acid unit step.
- L-type of m-RNA is changed to a stable double helix of DNA, and it is kept for the replication of protein.
Codon as the Label that Identifies the Type of Amino Acid
Amino acids and DNA that are active in the vicinity at the same time of day influence each other and incorporate the features of the three-dimensional structure of amino acids into the structure of the region of the molecular row of the double helix of DNA. However, the information of the intermolecular sequence that is intermolecular bound is not changed from the outside due to facing the inside of the molecular row of DNA. Therefore, even after the double strands are separated individually, the same pair can be made by the relationship between the pair of locks and keys in the base sequence. Since it is easier to manipulate DNA in the case of proteins with a small number of amino acids, DNA was made with fewer amino acids in the early stages.
Molecules of water move via hydrogen bonds, and those molecules in contact with organic molecules also move via replacing neighboring molecules due to the thermal motion of molecules. Therefore, proteins are replicated in amino acid units by RNA equipped with a backbone region modified with amino acids. RNA and amino acids are connected by intermolecular bonds and have weak bonding force, and L-type amino acid selectively bond with L-type amino acid. Since it is peptide bonded, the protein becomes like a ribbon and is separated from the RNA. RNA that has released amino acids can bind the same amino acid back to the trace where the amino acid was taken away by intermolecular bonds. Genetic code (codon) is used as the label that identifies the type of amino acid. There are 64 species of codons and 20 types of amino acids. If amino acids are directly related to messenger RNA (which indicates the sequence of amino acids), amino acids bind selfishly to messenger RNA and accurate replication is not possible. Therefore, the relationship between t-RNA, (which carries amino acid) and m-RNA is checked by the genetic code that is not related to amino acids, and the amino acid sequence is determined through t-RNA only when the matching conditions are satisfied.
Production of Protein by DNA
The pairs of complementary base sequences of DNA are bounded by weak intermolecular bond. Since the regions of protein and that of DNA must be interacted at each amino acid, modification of amino acid units is carried out in t-RNA. But the pair of complementary bases are placed inward so that it does not change due to external influences. Since one of DNA is disappeared by generation of L-type of m-RNA and D-type of t-RNA, the double helix of DNA is unwound to form two lines of single-stranded DNA. Each single strand of DNA is used as the template to incorporate and replicate complement.
The process on a protein replication is described as follows and it is shown in Figure 9:
- One of DNA is used to produce D-type t-RNA and L-type m-RNA.
- The D-type t-RNA adhered with L-type of amino acid move to at the position assigned L-type m-RNA.
- Progression of L-type amino acid sequence by peptide bonds will form L-type protein (Figure 10).
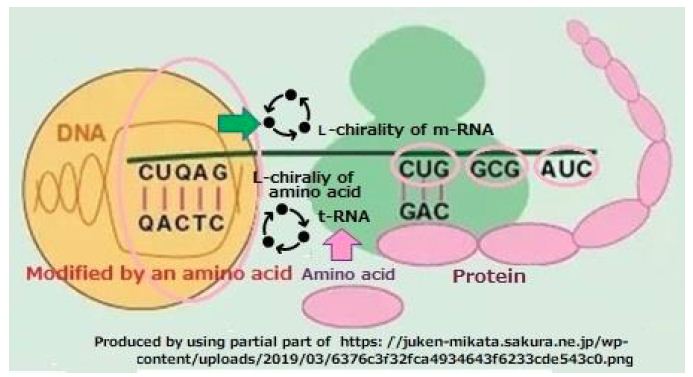
Figure 10: Role of chirality in protein replication. L-type of amino acid joins D-type of t-RNA, and the D-type of t-RNA bond with L-type of m-RNA. The proceed of right screw and left screw are reversed in case of the same rotation. So, L-type m-RNA proceeds in opposite directions to D-type of t-RNA as the unit of amino acid [17] “Molecular Biology of the Gene”, Fig. 8.11, 2004.
The base sequence is used as a label to distinguish the type of amino acid and the genetic code (codon). There are 64 types of codons by combining 3 elements with 4 elements. Any of them corresponds to 20 kinds of amino acids used in living organisms. If amino acid molecules are directly related to m-RNA, amino acids bind as one pleases to m-RNA. Then, the accurate replication is not possible. The genetic code of m-RNA must not physical relationship with amino acids.
The Mechanism of Cell-division is Incorporated into the Cell Cycle
The double spiral structure separates the original and copied DNA in different directions at the replication. There are many separations of double spiral structures. Those separations can be performed simultaneously at the copying DNA. When paired DNA double helix structures are separated in different directions, the central region of the cell that divides in two becomes concave, then the central cell membrane contracts and closes, eventually dividing into two cells. Cell division is built into the cycle of organisms that continue to react without rest [17,18] “Molecular Biology of the Cell”, Chap.13, 1989. Due to the rotation of the Earth, the environment changes in a one-day cycle. With these changes, a cycle of biochemical reactions of cells occurs naturally. Each DNA corresponding to various proteins is replicated. As the DNA that controls biochemical reactions in cells evolves, there will be longer ones. The mechanism of chaining cell activities one after another and circulating those activities has evolved into life activities (Figure 11).
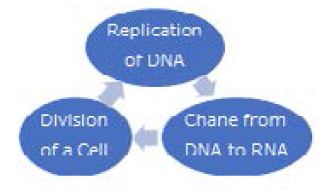
Figure 11: Cycle of intracellular biochemical reactions
Conclusions
This study elucidated how the spiral structure of water led to the evolution of biomolecules, DNA, and, ultimately, life on Earth. It is known that the life of the structure of liquid water is about the minus 12 power of 10 seconds, and it cannot be directly observed. However, there are many phenomena those indicate that the molecules of liquid water have a systematic thermal motion. The fact that most biomolecules in living organisms are homochiral is the evidence that water molecules work together to construct spiral structures. Adjacent molecules in liquid water are exchanged in an intermolecular bonded system. If a spiral-type thermal motion of water molecules works together to create organized motion, we can explain formation of cell membranes, protein production, and protein replication by DNA, which are crucial to the origin of life. The sense and antisense strands in a DNA are connected by inter-molecular bonds and t-RNA is modified by the molecular structure of the region around amino acids at the time of generation. The complementary symmetry of sense chain against antisense chain is formed via mechanism of left-hand type spiral and that of right-hand type spiral. Therefore, even after the double strands are separated individually, the same pair can be made again by locks-and-keys relationship in the base sequence. The structure of DNA indicates how to synthesize protein that carries out activity of life. The primitive biochemical reactions are carried out with small energy consumption. After years of trial and error, the evolution of creature reached the level of DNA. By using mechanisms of DNA acquired, If the species’ evolution fits the environment, they thrive. If it is not suitable to the environment, they become extinct. The evolution of specie is adapted to the environment.
Acknowledgment
I thank Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
References
- Cady Lawrence P, André Brack, Jorge EBP, Charles Cockell, Gerda Horneck, et al. (2014) Aastrobiology editorial board opinions “Where do we go from here?” Astrobiology Editorial Office, Portland, Oregon, USA.
- Shapiro R (2007) A simpler origin for l Scientific American 296: 46-53.
- Hunding A, Kepes F, Lancet D, Minsky A, Norris V, et al. (2006) Compositional complementarity and prebiotic ecology in the origin of life. Bio Essays 28: 399-412, Wiley Periodicals, Inc. [crossref]
- Watson JD, Crick FHC (1953) A Structure for Deoxyribose Nucleic Acid. Nature 171: 737-738.
- Karasawa S (1974) Origin of Piezoelectricity in an α-Quartz. Japanese Journal of Applied Physics 13: 799-803.
- Eisenberg E, Kauzmann W (1969) The structure and properties of water. Pg: 73. Oxford Univ Press.
- Chronological Scientific Tables (2020) pg 421, pg: 872, (On ice: by Kaye and Laby), (on water: by Wagner and Pruss) p.421. Maruzen-Pub. Co. Jpn. 2019.
- Carpenter MA, Salje EKH, Graeme-Barber A, Wruck B, Dove MT, et al. (1998) Calibration of excess thermodynamic properties and elastic constant variations associated with the α↔β phase transition in quartz. American Mineralogist 83: 2-22.
- Karasawa S (2014) Prebiotic reactions in the bubble that was formed in carbonated water by iron atoms. Viva Origino 42: 12-17.
- Schopf JW, Valley JW, “https://news.wisc.edu/oldest-fossils-found-show-life-began-before-3-5-billion-years-ago/” Proceeding of National Academy of Science, 18, 2017.
- Tice MM, Lowe DR (2004) Photosynthesis microbial mats in the 3.416-Myr-old ocean. Nature 431: 549-552.
- Karasawa S (2022) Effects of Solar Wind on Earth’s Climate. Geology, Earth & Marine Sciences 4: 1-5.
- Karasawa S (2022) Earliest BIF and life produced via submarine volcanism in carbonated seawater. Geology, Earth & Marine Sciences 4: 1-5.
- Nelson D L, Cox MM (2017) Lehninger Principles of biology. 7th p.52, W. H. Freeman and Company.
- Dyson F (1999) Origins of Life. 2nd Ed. Cambridge University Press, Cambridge.
- McKee T, McKee JR, (2016) Biochemistry – The molecular bases of life. 6th Fig.1.13, Oxford Univ. Press.
- Watson JD, Baker TA, Bell SP, Gann A, Levine M, et al. (2004) Molecular Biology of the Gene. 5th Ed, Replication fork, Fig.8.11, Benjamin Cummings.
- Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD (1989) Molecular Biology of the Cell. 2th Ed, Chap: 13, Garland Publishing.