Abstract
In this work, we present a new approach for development of metallomesogen (MOM) eutectic mixtures for potential applications in liquid crystal display devices. Through molecular engineering and physical mixing techniques, we aimed to resolve the major drawbacks of MOMs including their inaccessable transition temperature and low solubility in liquid crystal hosts. Accordingly, we studied the phase diagrams of blends of model structures of rod-like MOMs based on a mono-ligand Pd alkyl/alkoxy-azobenzene complex and bi-ligand Cu, Ni and Pd salicylal-diaminate complexes. These phase diagrams indicate mesogenic miscibility, distinct eutectic points and wide mesogenic range. In addition, the phase diagrams of a eutectic MOM mixture with commercial TN10427, TNO623 and E43 liquid crystals exhibit complete mesogenic miscibility, which geneally qualifies MOMs as potential guest materials to improve the performance of commercial liquid crystal materials in display devices.
Keywords
Metallomesogens, Eutectic mixture, Miscibility, Ligand, Commercial nematics, Guest-host
Introduction
The metal-containing liquid crystals, known as “metallomesogens” (MOMs) incorporating metal centres into selected organic structures have been studied for decades as potential and effective materials for technological applications. It has been demonstrated that the presence of metal complexation in liquid crystal chemical structures could add many physical and optical features not present in organic mesogenic systems. Such features have been the main driving forces for potential applications of MOMs in a wide range of electrooptical applications including additional selective absorptions; large electrical polarizability, refractive indices and birefringences; high order parameters, mesogenic stability and dichroic ratios, which by a combination of the supramolecular mesogenic ordering of organic with additional presence of metal complexation provide new opportunity for utilization of MOMs in liquid crystal display devices.
In spite of many previous studies on the chemistry of MOMs, there have been only few practical attempts on their potential applications [1-8]. In recent years a great variety of MOM materials with photoluminescence and water-free proton conduction, electroluminescence, magnetic and electric properties have been synthesised. Some scientific and patent literature are also reported on the potential applications of calamitic and discotic MOMs as dichroic dyes, non-linear optics, thermal recording, thermochromism, passive optical filters, photo-sensing, laser addressing, optical and thermal recording, polarizing flms, radiation absorbing films, ferroelectricity, ferromagneticity, electroconductivity, reaction catalysts, LC intermediates, ink jet and security printing, medicinal and agricultural components [9-27].
In spite of these developments in synthesis and characterization of MOMs, however these scientific works have not yet been able to provide proper materials for commercial applications, even in the simplest guest-host systems. Some of the major problems in development of MOMs have been due to their inaccessible and high transition temperatures, risk of decompositions at high temperatures, small mesophase range and low chemical stability. Therefore, the key to application of MOMs is not only through their molecular engineering, synthesis and chemical structure, but rather through blending and miscibility by physical and chemical mixing approach to overcome their above mentioned drawbacks. In order to develop MOMs for application, one requires to provide appropriate materials and systematic characterization to qualify for specific device applications. The real challenge to qualify MOMs for application is not necessary in finding the properties found in organic mesogens or coordination chemistry, but also to discover new features that are not found in either material. In the present study, we studied the physical mixing of few rod-like MOM model structures based on mono-ligands alkyl/alkoxy-azobenzene Pd metal-complex and bi-ligands based on Cu, Ni and Pd complex salicylal-diaminates chemistries. Accordingly, we studied the binary phase diagrams, mesogenic miscibility and eutectic behaviour in these model MOM materials. In addition, we utilized a eutectic mono-ligand MOM-ligand mixture and studied its phase diagrams with two MOMs and three commercial nematic mixtures TN10427, TNO623 and E43, in order to demonstrade their potential MOMs in commercial liquid crystal materials. The results of these studies are mentioned in the following sections.
Materials and Methods
Materials
The general chemical formula of ligands and mono-ligand MOMs based on a common class of Palladium (Pd) metal complex and alkyl/alkoxy-azobenzenes are presented in Figure 1. The structural variations are obtained by changing the structure of the ligand’s terminal groups R and R’, which may be also different in the same molecule, as well as variations of coordinated metal complex. The details of the synthetic procedures of this class of ligands and MOMs have been mentioned elsewhere [28-30]. The utilized commercial liquid crystal mixtures TN10427, TNO623 where procured from Hofmann LaRoche and E43 was purchased from Merck. All materials were used as such. According to Figure 1, the chemical structures of MOMs are obtained by three different ligands incorporated in three Pd-alkyl/alkoxy-azobenzene complex chemical structures. With reference to the general formula of Figure 1, the nomenclature and chemical structures of utilized parent ligand and MOMs in this study are as follows:
- HL2: R:C6H13, R’: O(CH2)2CH=CH2
- Pd-L2: R:C6H13; R’: O(CH2)2CH=CH2
- Pd-L5: R: OC7H15; R’: O(CH2)3CH2CH=C(CH3)2
- Pd-L6: R: OC7H15;R’: O(CH2)2CH(CH3)-(CH2) 2CH=C(CH3)2
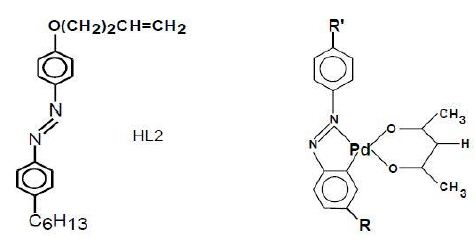
Figure 1: The general formula of ligand and MOM
In Table 1, we tabulate the crystal-mesogenic and mesogenic-isotropic transition temperatures on heating (TCM and TMI) and cooling (TMC and TIM) modes, as well as the mesogenic phases of the ligand, MOMs and utilized commercial nematic materials. All three commercial liquid crystals TN10427, TNO623 and E43 exhibit enantiotropic nematic phase. The synthesized bi-ligand MOM compounds pertain to a common class of salicylaldiminates metal complexes which corresponds to the general chemical formula presented in Figure 2. The structural variations of the general formula in Figure 2 are obtained by changing the structure of the ligand’s terminal groups R and R’, which are different or the same having the same or different metal complex ion M. The details of general synthetic procedures of this class of MOM’s chemistry, as part of an extensive industrial research and development projects, have been reported elsewhere [31-35]. With reference to the general formula mentioned in Figure 1, the nomenclature and chemical structures of synthesized MOMs based on Ni and Pd complexes and their ligands (R and R’) are as follows:
- A11O-6ON-Ni: R=-(CH2)3-O-CH2-CH3; R’=-O-(CH2)11-OOC-CH=CH2);
- A11O-6ON-Pd: R=-(CH2)3-O-CH2-CH3; R’=-O-(CH2)11-OOC-CH=CH2.
Table 1: The transition temperatures of ligand, MOMs and commercial liquid crystals on cooling mode
|
Compound |
Transition Temperature (°C) |
Mesophase |
|
|
TIN |
TNC |
||
| H-L2 |
48.1 |
14.8 |
Monotropic Nematic |
| Pd-L2 |
43.1 |
– 12 |
Monotropic Nematic |
| Pd-L5 |
39.1 |
23.8 |
Monotropic Nematic |
| Pd-L6 |
63.6 |
33.8 |
Enantiotropic Chiral Nematic |
| TN10427 |
114.5 |
– 40 |
Enatiotropic Nematic |
| TNO623 |
101.9 |
– 35 |
Enantiotropic Nematic |
| E43 |
77.8 |
– 4.5 |
Enantiotropic Nematic |
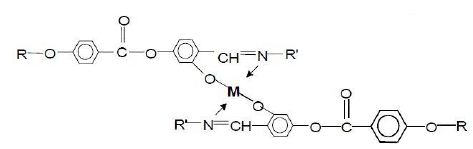
Figure 2: The general formula of the salicylaldiminates MOMs
In Table 2, we tabulate the nematic-crystal (TNC) and isotropic-nematic (TIN) transition temperatures of the studied MOM components obtained by DSC at 5°/min on cooling mode. All four synthesized bi-ligand MOM materials exhibit enantriotropic nematic phase with overall mesogenic stability within 80-150°C range.
Table 2: The phase transitions of bi-ligand MOM components on cooling mode
|
MOM Compound |
Transition Temperature (°C) |
Mesophase |
|
|
TIN |
TNC |
||
| 12-8N-Cu |
129 |
103.5 |
Enatiotropic Nematic |
| A6O-N8-Cu |
116 |
81.6 |
Enatiotropic Nematic |
| A11O-6ON-Ni |
124 |
107 |
Enatiotropic Nematic |
| A11O-6ON-VO |
144 |
119 |
Enatiotropic Nematic |
Methods
The phase transition temperatures of the MOM mixtures, including the nematic-crystal (TNC) and isotropic-nematic (TIN) transition temperatures were determined by a Perkin Elmer DSC7 Differential Scanning Calorimeter (DSC) and Nikon Eclipse-50i polarizing optical microscope (POM) equipped with a temperature-controlled Mettler FP5 microscopic hot stage. The binary MOM mixtures were prepared by physical mixing method of binary MOM components. The phase diagrams of the mixtures were carried out by direct weigting of the components in the DSC pan through repeated heating (at 10°C/min) and cooling (at 5°C/min) scanning rates until there was no change in their thermograms and mixings were completed.
Results and Discussion
Momo-Ligand MOMs Mixtures
According to Table 1, the studied ligand (HL2) and MOMs (Pd-L2, Pd-L5 and Pd-L6) exhibit low temperatures transitions. With respect to mesogenic type, the ligand HL2 exhibits an entantiotrpic nematic phase, the MOM components Pd-HL2 and Pd-HL5 exhibit monotropic nematic phase, whille Pd-HL6 shows an enantiotropic chiral nematic phase. The phase diagrams of binary and ternary of mono-ligand MOMs were carried out on the following mixtures:
- Binary MOM and parent liqand: Pd-L2+HL2
- Ternary eutectic Pd-L2+HL2 and MOMs: Pd-L2/HL2/Pd-L5, Pd-L2/HL2/Pd-L6
- Ternary eutectic Pd-L2+HL2 and commercial liquid crystals: TN10427, TNO623 and
In Figure 3 we present the phase diagram of binary Pd-L2 MOM and H-L2 ligand mixtures at cooling modes. Both H-L2 and Pd-L2 exhibit similar isotropic-nematic (TIN) transitions, whereas the presence of metal complex in PD-L2 shows a wider nematic range (52°C) and lower than that of H-L2 ligand (33°C), which is due to supper cooling of Pd-L2 and lowering of nematic-crystal (TNC) to -12°C. Also according to Figure 3, the Pd-L2/H-L2 phase diagram shows a complete linear trend of TIN transitions within the whole composition range, which is due to total nematic miscibility of MOM and ligand. In addition, this mixture also exhibits a distinct eutectic point at the composition of around Pd-L 2=62.5%wt. At the eutectic point the nematic range is expanded to 80°C with TNC=-35°C and TIN=45.9°C, which makes this eutectic mixture itself as a potential candidate material for application in commercial liquid crystals.
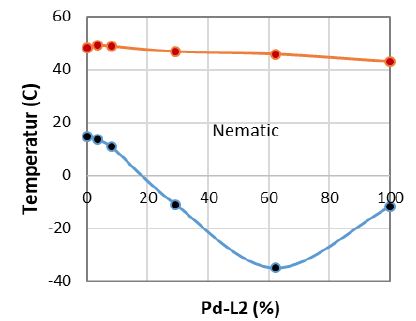
Figure 3: The phase diagram of Pd-L2 / HL2 mixtures
In Figure 4, we provide the phase diagrams of ternary mixtures consisting of eutectic composition Pd-L2/L2 (62.5/37.5) with Pd-L5 and Pd-L6 MOMs at the cooling modes. According to Figure 4, the Pd-L2/HL2-Pd-L5 mixtures show nematic miscibility of the components due to predominantly linear trends of their TIN transions and a small eutectic point at around Pd-L5=50%wt with TNC=-38°C. The nematic stability range at this eutectic composition is around 73°C. On the other hand, the phase diagram of Pd-L2/L2-Pd-L6 exhibits a chiral nematic (cholesteric) phase within the total composition range. The chiral nematic miscibility is the result of a predominantly linear trend of their TIN* transition. This tertiary mixture also exhibits a small eutectic point at the composition of around Pd-L6=10%wt and a mesomorphic stability range of around 79°C. Although the addition of Pd-L5 and Pd-L6 to the eutectic Pd-L2/HL2 do not substantially improve the mesogenic stability in the present model mixtures, but it provides the possibilty of developing alternative MOM mixtures with appropriate ligand and metal complex, as well as stable chemical structures to provide the benefits of in potential MOMs material for wide range of applications.
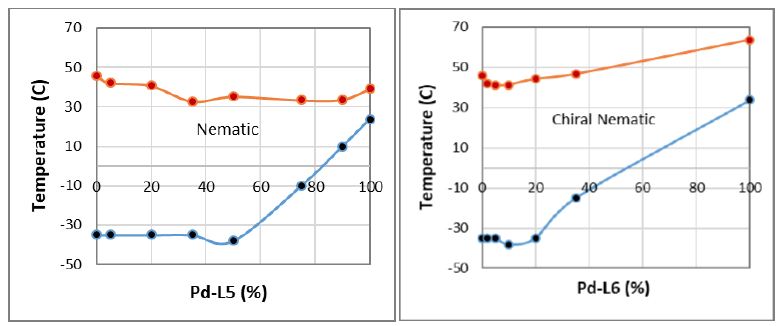
Figure 4: The ternary phase diagrams of eutectic Pd-L2/HL2 with Pd-L5 and Pd-L6 materials
Bi-Ligand MOMs Mixtures
In Figure 5, we present the transition temperatures and phase diagrams of 12-8N-Cu/A6O-8N-Cu and A11O-6ON-Ni/A11O-6ON-Pd binary mixtures, respectively. It should be noticed that, the former mixture consists of the same Cu complex with different ligands (M-L1/M-L2), whereas the latter mixture contains different Ni and Pd complexes (M1-L/M2-L) with the same ligand. According to Figure 5, the linear trends of isotropic-nematic (TIN) transitions within the whole composition range of both phase diagrams is the clear indication of complete mesogenic miscibility of the MOM components. It is also noticed that, both MOM mixtures in Figure 5 exhibit strong eutectic behavior with the lowest nematic-crystal (TNC) transitions and largest nematic stability range. The eutectic composition of 12-8N-Cu/A6O-8N-Cu mixrure appears at around 12-8N-Cu=25%wt, resulting to an expansion of nematic phase to around 46.5°C. The eutectic point of A11O-6ON-Ni/A11O-6ON-Pd mixture occurs at around A6O-6ON-Ni=40%wt with similar nematic phase extension of around 46.0°C.
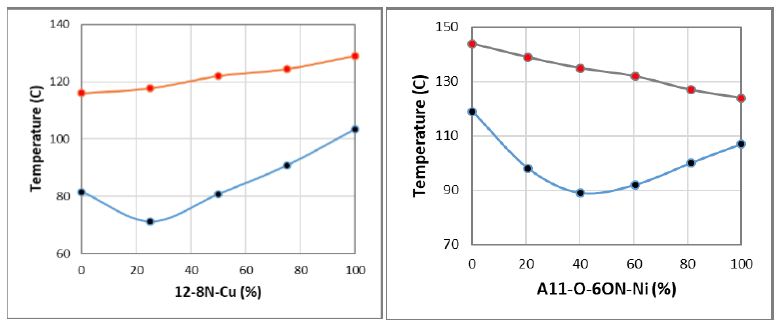
Figure 5: Phase diagrams of 12-8N-Cu/A6O-8N-Cu and A11O-6ON-Ni/A11O-6ON-Pd mixtures
At the eutectic points, the phase diagrams in both MOM mixtures exhibit widest nematic range and lowest TCN transition temperatures than those of single MOM components. In addition, a comparison between the eutectic compositions of the two MOM mixtures indicate that, although both systems exhibit the same range of nematic expansion, the occurrence of eutectic composition in A11O-6ON-Ni/A11O-6ON-Pd mixture at 40% demonstrates the more crystalline structure similarities of its components with respect to 12-8N-Cu/A6O-8N-Cu mixture. This difference indicates that, the same ligand of A11O-6ON-Ni/A11O-6ON-Pd mixture contributes more to similarity of their crystalline structure than the same metal of 12-8N-Cu/A6O-8N-Cu mixture.
MOMs and Commercial Nematics
In Figure 6, we provide examples of phase diagrams of ternary mixtures consisting of eutectic Pd-L2/L2 (62.5/37.5) MOM and three commercial nematic TN10427, TNO623 and E43 mixtures. According to Figure 6, the phase diagrams of these ternary mixtures indicates that, due to linear trends of their TIN transitions within the whole composition range of phase diagrams and the eutectic Pd-L2/H-L2 is completely miscible in all three commercial nematic hosts. It is also noticed that, the TNC transitions of this mixtures exhibit the linear trends with no ulterior eutectic behavior. The nematic stability of these model mixtures is relatively constant and dependent on the TIN transitions of the nature of the host commercial nematic material.
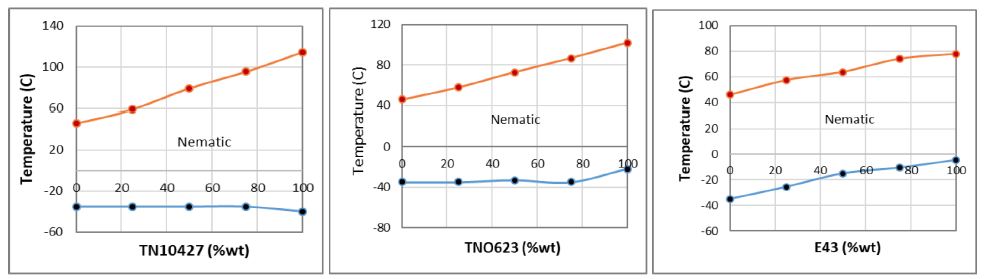
Figure 6: Phase diagrams of eutectic Pd-L2/H-L2 and commercial liquid crystals
Conclusion
In the present study, we utilized few nematic MOMs as model structures and through physical mixing method their binary and ternary phase diagrams, which exhibited eutectic behavior. In addition, we utilized a eutectic MOM/ligand mixture and mixed it with other nematic MOMs, as well as with few commercial nemtic liquid crystals and provided new mixtures with the following criteria:
- Binary MOM/ligand mixtures showed a complete nematic miscibility having a distinct eutectic behavior with mesogenic range of around 80°C.
- Ternary eutectic MOM/ligand-MOM mixtures also exhibited nemtic miscibility, eutectic behavior and mesogenic sability within 73-79°C
- Ternary eutectic MOM/ligand and three commercial nematic liquid crystals also exhibited complete nematic The lack of a distinct eutectic behavior in these mixtures was due to nemtic-crystal transitions, which are not optimized by the present MOMs chemical structures.
Accordingly, we presented the potential introduction of MOM mixtures as guest in the commercial liquid crystal materials, not only to expand the transition temperatures and mesophase range of host liquid crystals but also to exploit the other unique properties of MOMs, such as additional selective absorptions, large electrical polarizability, refractive indices and birefringences; high order parameters and dichroic ratios, for improving the electrooptical properties of commercial liquid crystal materials. Ultimately, if the mesogenic range of eutectic MOM mixtures would be larger or even comparable to those of commercial nematic materials, the eutectic MOMs mixtures could partially or totally substitute the commercial liquid crystals as alternative materials for vast electro-optical applications.
Acknowledgment
The authors would like to acknowledge the Electro-Optical Film Group of Snia Riceche, Snia BPD (Fiat Group), Via Pomarico, Pisticci Scalo (MT), Italy, who sponsored and financed the research and development projects on Metallomesogens under collaborations with Prof. M. Ghedini at Universita di Calabria and professors A. Sirigu and A. Roviello at Universita di Napoli, during 1993-1996 period.
References
- Serrano JL (1996) Metallomesogens: Synthesis, Properties and Wiley.
- Donnio B, Bruce DW (1999) Liquid Crystals II Mingos .Springer
- Donnio B, Guillon D, Deschenaux R, Bruce DW (2003) Metallomesogens, Comprehensive Coordination Chemistry II, Oxford: Elsevier.
- Date RW, Iglesias EF, Rowe KE, Elliott JM, Duncan WB (2003) Dalton
- Bruce D W, Deschenaux R, Donnio B, Guillon D (2006) In Comprehensive Organometallic Chemistry III, Oxford.
- Porta B, Khamsi J, Noveron JC (2008) Curr Org Chem. V12, 1298.
- Wang Y, Shi J, Chen J, Zhu W, Baranoff E (2015) J Mater Chem C 3: 7993.
- Wang Y, Fan J, Shi J, Qi H, Baranoff E et al. (2016) Dyes Pigments 133: 238.
- Krikorian M, Liu S, Swager TM (2014) J Am Chem Soc 136: 2952.
- Geng H, Luo K, Cheng H, Zhang S, Ni H. (2017) RSC Adv 7: 11389.
- Materials Cristián Cuerva de Alaíz, PhD thesis, Deprtment of Inorganic Chemistry, University of Madrid.
- Liu SHS, Lin MS, Chen LY, Hong YH, Tsai CH, et al. (2011) Org Electron 12: 15.
- Seredyuk M, Muñoz MC, Ksenofontov V, Gütlich P, Galyametdinov Y, et al. (2014) Inorg Chem 53: 8442.
- Fitzpatrick AJ, Martinho PN, Gildea BJ, Holbrey JD, Morgan GG (2016) Eur J Inorg Chem2025.
- Ionescu A, Godbert N, Crispini A, Termine R, Golemme A et al. (2012) J Mater Chem 22: 23617.
- Su PYS, Tseng JCW, Lee KM, Wang JC, Lin IJB (2014) Inorg Chem 53: 5902.
- Su PYS, Hsu SJ, Tseng JCW, Hsu HF, Wang WJ et al. (2016) Chem Eur J 22: 323.
- Ginord-Godquin M, Maitlis PM (1991) Angew Chem Int Engl 30: 375-402.
- Bruce DW (1992) “Inorganic Materials” W. Bruce & D. O’Hare Eds.
- Blanca Ros. “Metallomesogens – Synthesis, Properties & Applications”, Ed. J. L. Serrano.
- Hakemi, M. Caporusso, M. Santangelo, EP 0747 461 A1.
- Roviello Centore, B, Panunzi, H. Hakemi, IT1394422.
- Hakemi, A. Lofer & E. Peso; US62/065,805; PCT47459 WO2016/06327.
- Hakemi, A. Lofer, E. Peso & D. Gal-Fuss; US62/076,002.
- Binnemans K (2010) Inorganic Materials Series, 61-133.
- Gimenez R, Lydon Serrano JL (2022) Current Opinion in Solid State & Materials Science, 6: 527.
- Ghedini M, Pucci D, Neve F (1996) Chem Commun137.
- Ghedini M, Morrone S, Neve F, Pucci D (1996) Gazz Chim 126: 511.
- Hakemi H (2022) Journal of Materials & Polymer Science 2: 1-6.
- Caruso U, Roviello A, Sirigu A (1990) Liquid Crystals 7: 421.
- Caruso U, Roviello A, Sirigu A (1991) Liquid Crystals 10: 85.
- Caruso U, Roviello A, Sirigu A (1991) Macromoleculess 24: 2606.
- Caruso U, Centore R, Roviello A, Sirigu A (1992) Macromoleculess, 25: 2290.
- Caruso U, Necca, Roviello A, Sirigu A (1995) New Polymeric Mater 4: 309.