Abstract
We investigated the spike protein-specific IgG antibody levels against COVID-19 in a 64-year-old male medical staff periodically after two doses of COVID-19 vaccination and a third booster shot during a one-year period. The antibody levels increased after the two doses vaccination; however, it rapidly decreased in the first 3 months. The antibody levels increased again after the third booster vaccination. The antibody levels were remarkably higher than that after the two doses of vaccination and remained high for several months. We demonstrated that the third booster shot was significant in maintaining a high level of immunity against COVID-19.
Keywords
COVID-19, Vaccine, Antibody, Spike protein, Booster
Introduction
The COVID-19 pandemic [1], which began in early 2020, affected the examination and treatment of patients in hospitals, healthcare, and nursing care facilities in various ways. Vaccination for COVID-19 [2-4] initially started in the United Kingdom in December 2020 and subsequently started in Japan in February 2021, which has been effective in preventing the onset and reducing the severity of the disease [5-7] owing to its high immune induction potency [8]. However, some cases of breakthrough infections have been reported [9-11], and it has been pointed out that one of the causes is a decrease in the levels of antibodies against the disease over time [12]. However, booster vaccination has been shown to be effective in preventing the onset of the disease and reducing the risk of severe disease [13-15]. Therefore, we consider it meaningful to assess COVID-19 antibody levels periodically after vaccination and booster shots to prevent such infections. We measured the antibody levels of our medical staff against COVID-19 monthly after COVID-19 vaccination and examined changes in antibody levels after administration of additional vaccination during a one-year period.
Materials and Methods
Subjects
The subject was a 64-year-old male medical staff of our corporation who received the COVID-19 vaccine by Pfizer-BioNTech twice and received an additional vaccine dose by Moderna eight months after the second vaccine dose and was administered COVID-19 antibody testing monthly for a year.
Ethical Principles
The present study was conducted in accordance with the Declaration of Helsinki, and the Seikokai Group Ethics Committee approved the study protocol. Informed consent was obtained from the staff.
Methods
The staff member received two doses of the COVID-19 vaccine by Pfizer-BioNTech between 10 June and 1 July 2021 and was subsequently administered COVID-19 antibody testing over time. Following that, he received an additional booster COVID-19 vaccine by Moderna on 21 February 2022 and was subsequently administered COVID-19 antibody testing over time. Antibody levels were measured monthly: one month after the completion of the second vaccination [16], two months after the second vaccination, and three months after the second vaccination. Subsequently, the fourth, fifth, and sixth measurements were performed every one month. An additional vaccine dose was administered eight months after the second vaccination. The seventh antibody level was measured one month after the additional vaccine dose, and the eighth, ninth, and tenth measurements were performed every one month, respectively. In total, the fluctuation of antibody levels was monitored for a year. Antibody levels were measured by quantification of spike protein-specific IgG antibodies, which have neutralizing activity against the receptor-binding domain of the virus. Abbott SARS-CoV-2 IgG (Abbott Japan, Minato-ku, Tokyo, Japan; cutoff value: 50 AU/mL) was used to perform this measurement.
Results
The time course of the antibody levels after two doses of the vaccine for the first 6 months is shown in Figure 1. The antibody level at one month after the second vaccine dose was 3,337 AU/mL, but that at the next month was 1,510 AU/mL, which is a decrease of 55%. The antibody level at three months after the vaccination was 749 AU/mL, a 78% decrease from that at the first measurement. Antibody levels at 4, 5, and 6 months after treatment were 455, 287, and 245 AU/mL, respectively. The decline in antibody levels was relatively slow compared with that in the first three months (Figure 1: arrows). The time course of the antibody levels from six months to 12 months after two doses of the vaccine is shown in Figure 2. After the administration of booster vaccine dose, the antibody level was remarkably increased to 22,900 AU/mL (at 9 months) and declined to 17,100 AU/mL (at 10 months), 12,900 AU/mL (at 11 months), and 7,990 AU/ml (at 12 months). However, the antibody level after the additional vaccine dose was relatively higher than that after the second dose. The total course of vaccination and antibody levels for a year period are shown in Figure 3.
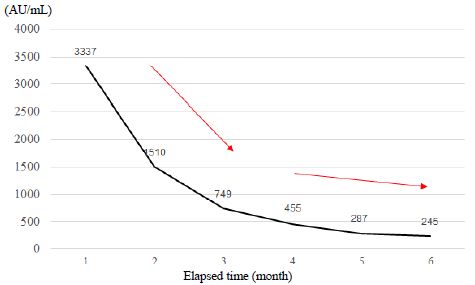
Figure 1: Time course of the antibody levels for six months after administration of a second vaccine dose
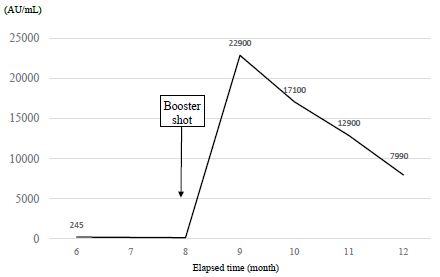
Figure 2: Changes in the antibody levels after administered an additional vaccine dose
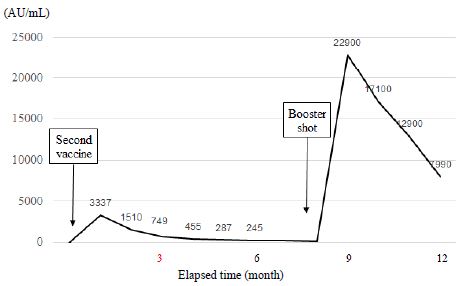
Figure 3: Total time course of vaccine doses and antibody tests for a-year period
Discussion
How antibody levels change after COVID-19 vaccine dose is now a matter of concern not only for healthcare professionals but also for the general population. In this study, we evaluated the antibody levels of our medical staff following vaccination and booster shot for COVID-19 every month during a one-year period. After two doses of the vaccine, the antibody level at three months decreased by 78% from baseline and at six months, decreased by 93% from baseline. This result indicates that the degree of decrease in antibody levels in the first three months was higher than that in the second three months, and the current results support our previous study [17]. We also demonstrated that after the administration of the booster shot, the antibody levels were remarkably increased and significantly higher than that after the second vaccine dose (Figure 3). Notably, the antibody levels remained high even after several months of the booster shot. Our current data also showed similar results to those of the previous studies [18-21]. It has been thought that the vaccinations increased at the same time as the outbreak of the delta strain that began in May last year from India [22-24], contributing to the convergence [25] of COVID-19 worldwide; however, the subsequent decrease in antibody levels may have contributed to the new omicron strain outbreak. However, the administration of additional doses of the vaccine is considered a significant countermeasure against COVID-19 because it was shown that the antibody levels against the disease increased again after the additional doses and that the severity and mortality rate from COVID-19 were reduced by the additional vaccine doses [26,27]. This remarkable increase in antibody levels after the additional vaccine dose may also contribute to the convergence of the omicron strain. We believe that the current data may help infection measures against COVID-19 for doctor, nurse, and other medical staff.
Study Limitations
The serum sample was obtained from one medical staff, and a large-sample investigation is needed to confirm our current study.
Conclusion
We measured the antibody levels of our medical staff over time after COVID-19 vaccination and examined changes in antibody levels after the administration of the booster vaccination for a year. Although the antibody levels declined with time after vaccination, we showed that the antibody levels significantly increased again after booster vaccination and remained high for several months.
Acknowledgment
The authors deeply indebted Saeki Ishiwata for providing serum samples and technical assistance for this research. The authors also thank to Bio Medical Laboratories Incorporated (BML, Inc.) for valuable support.
The preliminary data of this study was presented at a 4th European Congress on Infectious Diseases via live on 11 November 2022 at Paris, France, and on demand web streaming.
Funding
The authors declare that they have nothing to disclose regarding funding.
Conflict of Interest
The authors state that they have no conflict of interest to declare.
Author Contributions
Ikuma Kasuga conceived the work and designed the study protocol. Yuko Ishii contributed to the data curation and laboratory analysis. Yoshimi Yokoe and Osamu Ohtsubo supervised the project. Ikuma Kasuga contributed to the writing of the original draft, and all authors contributed to the revision of the manuscript and approved the final manuscript version.
References
- Gao Z, Xu Y, Sun C, Wang X, Guo Y, et al. (2021) A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect 54: 12-16. [crossref]
- Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, et al. (2020) Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 586: 589-593. [crossref]
- Kim JH, Marks F, Clemens JD. Looking beyond COVID-19 vaccine phase 3 trials. (2021) Nat Med 27: 205-211.
- Lazarus JV, Ratzan SC, Palayew A, Gostin LO, Larson HJ, et al. (2021) A global survey of potential acceptance of a COVID-19 vaccine. Nat Med 27: 225-228. [crossref]
- Keech C, Albert G, Cho I, Robertson A, Reed P, et al. (2020) Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med 383: 2320-2332.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, et al. (2020) Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 383: 2603-2615. [crossref]
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, et al. (2021) Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384: 403-416.
- Wang Z, Schmidt F, Weisblum Y, Muecksch F, Barnes CO, et al. (2021) mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature 592: 616-622.
- Callaway E (2021) Could new COVID variants undermine vaccines? Labs scramble to find out. Nature 589: 177-178.
- Gupta RK, Topol EJ (2021) COVID-19 vaccine breakthrough infections. Science 374: 1561-1562.
- Hacisuleyman E, Hale C, Saito Y, Blachere NE, Bergh M, et al. (2021) Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med 384: 2212-2218. [crossref]
- Khoury J, Najjar-Debbiny R, Hanna A, Jabbour A, Ahmad YA, et al. (2021) COVID-19 vaccine – Long term immune decline and breakthrough infections. Vaccine 39: 6984-6989. [crossref]
- Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, et al. (2021) Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med 385: 1393-1400.
- Accorsi EK, Britton A, Fleming-Dutra KE, Smith ZR, Shang N, et al. (2022) Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 Omicron and Delta variants. JAMA 327: 639-651. [crossref]
- Garcia-Beltran WF, St Denis KJ, Hoelzemer A, Lam EC, Nitido AD, et al. (2022) mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 185: 457-466. [crossref]
- Zhou W, Xu X, Chang Z, Wang H, Zhong X, et al. (2021) The dynamic changes of serum IgM and IgG against SARS-CoV-2 in patients with COVID-19. J Med Virol 93: 924-933. [crossref]
- Kasuga I, Gamo S, Yokoe Y, Sugiyama T, Tokura M, et al. (2022) Antibody levels over time against novel coronavirus and incidence of adverse reaction after vaccination. Health Evaluation and Promotion 49: 462-469.
- Belik M, Jalkanen P, Lundberg R, Reinholm A, Laine L, et al. (2022) Comparative analysis of COVID-19 vaccine responses and third booster dose-induced neutralizing antibodies against Delta and Omicron variants. Nat Commun 13.
- Zeng G, Wu Q, Pan H, Li M, Yang J, et al. (2022) Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: interim results from two single-centre, double-blind, randomized, placebo-controlled phase 2 clinical trials. Lancet Infect Dis 22: 483-495. [crossref]
- Petrelli F, Luciani A, Borgonovo K, Ghilardi M, Parati MC, et al. (2022) Third dose of SARS-CoV-2 vaccine: A systemic review of 30 published studies. J Med Virol 94: 2837-2844. [crossref]
- Muik A, Lui BG, Wallisch A-K, Bacher M, Mühl J, et al. (2022) Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine-elicited human sera. Science 375: 678-680. [crossref]
- Vaidyanathan G (2021) Coronavirus variants are spreading in India – what scientists know so far. Nature 593: 321-322. [crossref]
- Callaway E (2021) Delta coronavirus variant: scientists brace for impact. Nature 595: 17-18. [crossref]
- Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, et al. (2021) Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 596: 276-280.
- Bernal JL, Andrews N, Gower C, Gallagher E, Simmons R, et al. (2021) Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med 385: 585-594. [crossref]
- Barda N, Dagan N, Cohen C, Hernán MA, Lipsitch M, et al. (2021) Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet 398: 2093-2100.
- Arbel R, Hammerman A, Sergienko R, Friger M, Peretz A, et al. (2021) BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med 385: 2413-2420. [crossref]