Abstract
Background: Type 2 Diabetes Mellitus (T2DM) and Metabolic Syndrome (MS) continuously rise among South-Asian women. However, few studies explored the association between these chronic conditions and modifiable risk factors among South-Asian women. Therefore, this study evaluated the incidence of T2DM and MS and its association with modifiable risk factors among women with and without a history of Gestational Diabetes Mellitus (GDM).
Methods: We conducted the study using a retrospective and prospective follow-up component (an ambidirectional cohort study). We retrospectively identified women with GDM from 1999 to 2005 from the medical record system of the Aga Khan University (AKU) Hospital Karachi, Pakistan. We prospectively enrolled 226 women with GDM and 423 without GDM (1:2) between 2008 and 2010. The outcomes were the development of T2DM or impaired glucose tolerance (IGT) and MS among women with vs. without GDM and their association with modifiable risk factors, such as body mass index (BMI), body fat%, diet, and physical activity. Using multivariable logistic regression, we created two models to understand the association between modifiable risk factors and the development of T2DM and MS. In the first model, we used BMI as a measure of obesity, while in the second; we replaced it with body fat percentage. Other variables (diet and physical activity) were present in both models.
Results: We observed 10.91 times (CI 4.57-26.04) higher odds of developing T2DM/IGT among women with vs. without GDM. In addition, BMI (kg/m2) (OR 1.09, CI 1.02-1.15) and diet scores (high in fat, sugar, and bakery items) (OR 1.22, CI 1.01-1.49) were found significant. In the second model of T2DM, women with GDM (OR 11.02, CI 4.63-26.22) and body fat% (OR 1.10, CI 1.04-1.17) were found significant. For MS, we observed 2.78 times (CI 1.04-7.41) higher odds of developing MS among women with vs. without GDM. In addition, BMI (kg/m2) (OR 1.32, CI 1.22-1.42) and body fat% (OR 1.35, CI 1.23-1.48) were found significant in the first and second models, respectively.
Conclusions: BMI or body fat%, and, possibly, diet are potential modifiable risk factors for T2DM/IGT and MS among women with GDM.
Keywords
Gestational diabetes mellitus, Modifiable risk factors, Type 2 diabetes mellitus, Metabolic syndrome, South-Asian women, Low-and middle-income countries
Background
Diabetes is a major growing public health concern affecting about half a billion people worldwide [1]. The recent global estimates on diabetes prevalence report that 537 million adults aged 20 to 79 (one out of ten people) are affected by diabetes [2]. One in every four adults in Pakistan has diabetes mellitus (26.7%), reaching the highest national prevalence globally [2].
Type 2 Diabetes Mellitus (T2DM) is a progressive disease resulting from insufficient insulin secretion or resistance [3]. Several environmental risk factors, such as unhealthy diet, physical inactivity, and obesity, are further implicated in the pathogenesis of T2DM [4,5]. One of the metabolic risk factors for the development of T2DM is Gestational Diabetes Mellitus (GDM) [4]. It has been estimated that about 30% to 70% of women with GDM develop T2DM within approximately 15 years of the index pregnancy [6,7]. Among South Asian women, about 17% to 33% develop T2DM within 5 to 10 years after the index pregnancy [8].
GDM is defined as high blood glucose levels first recognized during pregnancy or subsequent pregnancies [9]. GDM is a highly prevalent metabolic issue during pregnancy, affecting about 1.8% to 31.5% of all pregnancies worldwide, depending on the screening methods, diagnostic criteria, and population characteristics [10]. The prevalence of GDM among low-and middle-income countries (LMICs) varies between 9.2% to 12.7% [11]. There has been a rise in the prevalence of GDM in Pakistan over the last few decades, with an increase from 6.3% in 2003 to 19% in 2018 [12].
GDM, on the one hand, increases the risk of developing many short-term complications, including preeclampsia, caesarean section, preterm births, macrosomia, and prenatal and perinatal mortality [13]. On the other hand, GDM tends to increase the risk of developing adverse health in the long term, such as T2DM, Cardiovascular Diseases (CVDs), and Metabolic Syndrome (MS) [7]. MS is defined as the presence of biological indicators such as abnormal waist circumference, high systolic and diastolic blood pressure, increased triglyceride levels, low high-density lipoprotein (HDL) levels, and impaired blood glucose levels [14].
Since these long-term issues can be preventable, lifestyle factors, i.e., diet, physical activity, and weight reduction, play a pivotal role in preventing and delaying the onset of T2DM and MS [15]. However, few studies have explored the association of lifestyle factors with T2DM and MS in women with GDM [16,17]. In addition, the existing evidence mainly based on the West, like Yang et al., demonstrated a lower risk of developing T2DM among women with a history of GDM who effectively managed modifiable risk factors [18]. There is a dearth of evidence, particularly from LMICs, on the role of modifiable risk factors in the development of T2DM and MS among women with a history of GDM. Therefore, this study evaluated the incidence of T2DM and MS and its association with modifiable risk factors among women with and without a history of GDM attending a tertiary healthcare facility in Karachi, Pakistan.
Methods
This study involved retrospective and prospective follow-up components (an ambidirectional cohort study). We retrospectively identified women from the medical record system who identified as having GDM based on International Classification of Diseases (ICD) 10 code and received prenatal care during 1999-2005 at the Aga Khan University Hospital (AKUH). All women identified through the Medical Record (MR) system with singleton birth in the index pregnancy, spoke Urdu, and residents of Karachi were included in the study. Those living outside Pakistan, with incomplete medical records, who used drugs influencing blood glucose concentration, such as glucocorticoids, antipsychotic drugs, or metformin, were excluded. We also excluded women diagnosed with T2DM before the initiation of the study as that was the key outcome, and they might have modified their lifestyle after a diagnosis of T2DM which may bias the association between the risk factors and development of T2DM.
All potential GDM women were contacted through mail as well as via phone calls as part of the recruitment process. Both mailing addresses and phone numbers were obtained from the MR system. All GDM women were first issued an invitation letter outlining the aims and methods of the study, a consent form, and a pre-paid, self-addressed mail-back envelope. The enclosed consent form was to be filled out by the women, and then mailed back. All GDM women were contacted by phone in addition to being mailed out. The phone calls were made a week after the mailings were completed. The letters were sent out, and the phone calls were made by the trained research staff. The detailed recruitment plan and participant response rate were published elsewhere [19]. During the phone calls, initial consent was obtained from the women to review their MRs for further basic medical information.
Women without GDM (matched for age at pregnancy and gestational age) were also identified from the MR system and contacted via phone to recruit them for the study. Women with GDM were classified as exposed, and those who did not develop GDM were considered non-exposed. We enrolled women as exposed and non-exposed in the 1:2 ratio. Those who consented and were eligible to participate were invited to AKUH to provide written informed consent and for further study assessments during 2008-2010. Structured questionnaire was used to assess sociodemographic, dietary intake, and physical activity through a face-to-face interview. In addition, anthropometric assessments and blood tests for evaluating T2DM or Impaired Glucose Tolerance (IGT) and MS status were also done.
Retrospective Data Collection
Data collected from MRs included maternal age, parity, known hypertension, mode of delivery, gestational age, use of insulin/diet for treating GDM, and GDM during a subsequent pregnancy.
Prospective Data Collection
Blood Work
All participants underwent an oral glucose tolerance test (OGTT) which involved a fasting blood sample followed by a 2-hour post-glucose sample (75-gram glucose liquid). At the same time, a 12-hour fasting lipid profile test was also performed to assess the status of dyslipidemia and MS. The details of the blood work and their cut-off values are described in additional file (see Additional file 1).
Anthropometry
Stature
Height and weight were measured at the time of the interview by the research officer. Height was measured using a wall-mounted measuring scale to the nearest cm, while weight was measured using a Tanita Body composition analyzer (Tanita Corp. CA. USA).
Waist Circumference and Waist-to-Hip Ratio
Waist and hip circumferences were measured using non-stretchable tape. These measurements were used to estimate waist circumference and waist-to-hip ratio among participants. The research officer was trained in measuring the circumferences at the correct point, i.e., waist circumference was measured between the uppermost part of the hip bone and the lowest rib margin (tenth rib), and the hip circumference was measured at the widest point over the buttocks.
Body Composition
Body composition was measured by a body fat analyzer. Total body fat was measured by a non-invasive Tanita body composition analyzer BC 310 (Tanita Corp. CA. USA), which measured fat mass, body fat percentage, abdominal fat mass, and fat-free mass.
Questionnaires and Interview
Two research officers were involved in data collection using an Urdu-translated interviewer-administered questionnaire for the following components:
Sociodemographic Questionnaire
A sociodemographic questionnaire collected information on participants’ educational level, occupation, household income, and socioeconomic status.
Food frequency Questionnaire
Diet was assessed using a Food Frequency Questionnaire (FFQ) that was developed and validated among Pakistani women [20]. The questionnaire has food items with their frequencies and portion sizes. The frequency of all food items consumed was multiplied by the portion sizes and then converted into the daily intake.
Using principal component analysis (PCA), we reduced dietary intake data to generate one variable to assess the diet-disease association. The new diet variable loadings were high in fat, sugar, and other bakery items (see Additional file 2).
Physical activity Questionnaire
To assess participants’ physical activity, a Monica Optional Study of Physical Activity (MOSPA) questionnaire was used. The questionnaire has been adapted from the World Health Organizations’ Monitoring Trends and Determinants of Cardiovascular Disease study [21] and has been validated among Pakistani women [22]. The MOSPA questionnaire assessed physical activity in four broader categories, including leisure, occupational, transportation, and household chores. Detailed information on calculating Energy Expenditure (EE) was provided in additional file (see Additional file 3).
Outcomes and Their Assessments
The outcomes were defined as the development of T2DM or IGT and MS among women with and without a history of GDM and their association with modifiable risk factors such as Body Mass Index (BMI), body fat%, diet, and physical activity. Women with fasting blood sugar ≥126 mg/dL and 2-hour post glucose ≥200 mg/dL were considered T2DM, whereas those with one value abnormal, either fasting blood sugar ≥126 mg/dl or 2-hour post glucose ≥200 mg/dl considered having IGT [3]. Metabolic Syndrome (MS) was defined as women with waist circumference > 80 cm with any one of the following conditions (HDL <50 mg/dl and Triglyceride >150 mg/dl) OR (HDL <50 mg/dl and FBS >100 mg/dl) OR (Triglyceride >150 mg/dl and FBS >100 mg/dl) [14].
Modifiable Risk Factors and Their Association with T2DM/IGT and MS
We evaluated the independent effect of BMI, body fat%, diet, and total reported physical activity (energy expenditure (kcal)/day) as modifiable risk factors on the development of T2DM/IGT and MS while adjusted for education, wealth index, and family history of diabetes.
Sample Size
Based on the findings of Feig et al. [23] and Cianni et al. [24] for the risk of developing T2DM and MS among women with GDM vs. without GDM, respectively, a sample size of 62 in exposed and 124 in non-exposed (1:2) for T2DM, whereas a sample size of 140 in exposed and 280 in non-exposed (1:2) for MS was required assuming 80% power and 5% level of significance.
However, we prospectively enrolled 226 women with a history of GDM who were eligible and consented to participate in the study as exposed and their comparators 423 women without a history of GDM as non-exposed (1:2) matched on age at the time of pregnancy and gestational age.
Statistical Analysis
Sociodemographic characteristics of women with and without a history of GDM were presented as mean ± SD for age at the time of pregnancy and age at the time of follow-up and median and range for the number of children. The frequencies and percentages were reported for education, occupation, wealth index, parity, mode of delivery, family history of diabetes, hypertension, treatment of GDM during pregnancy, and GDM during a subsequent pregnancy. The anthropometric and body composition distribution among the two groups were presented as mean ± SD or frequencies and percentages as appropriate. A chi-square test for all categorical variables, whereas an independent t-test for all continuous variables was computed to compare any differences between the two groups.
Binary logistic regression analysis was performed using the development of T2DM/IGT and MS as the dependent variable and women with a history of GDM vs. Non-GDM as an exposure variable, whereas four modifiable risk factors as an independent variable (BMI, body fat%, diet, and physical activity). A multivariable logistic regression analysis was performed to assess the association between modifiable risk factors and the risk of developing T2DM/IGT and MS among women with and without a history of gestational diabetes. Univariate analysis was performed to compute crude regression coefficients with 95% CIs. A stepwise approach was used during multivariable analysis while adjusting for confounders such as education, wealth quintiles, and family history of diabetes. A p-value of <0.05 was considered significant. Data were analyzed using Stata (V.17, Statacorp).
Results
Sociodemographic Characteristics of the Study Population
The sociodemographic characteristics of women with vs. without a history of GDM were compared in Table 1. Both groups were comparable in terms of almost all variables, except for number of children (p=0.020), mode of delivery (p=0.001), family history of diabetes (p=0.001), and presence of hypertension (p=0.009).
Table 1: Sociodemographic characteristics of the study population
|
GDM Mean ± SD or n (%) |
Non-GDM n=423Mean ± SD or n (%) |
P-value |
|
| Age at the time of pregnancy (years) |
31.18 ± 4.87 |
31.06 ± 4.75 | 0.77 |
| Age at the time of follow-up (years) | 37.15 ± 5.16 | 37.38 ± 5.05 |
0.58 |
| Education | 0.58 | ||
|
Primary to secondary (Class 6-10) |
23 (10.2) | 42 (9.9) | |
| Intermediate (Class 12) |
44 (19.5) |
69 (16.3) | |
|
Graduation (Class 14 and above) |
159 (70.4) | 312 (73.8) | |
| Occupation | 0.14 | ||
| Housewife |
196 (86.7) |
369 (87.6) |
|
|
Employed |
30 (13.3) |
46 (10.9) |
|
| Others |
0 |
6 (1.4) | |
|
Wealth Index (quintiles) |
0.89 |
||
| Lowest |
91 (40.3) |
171 (40.4) | |
|
Middle |
58 (25.7) |
101 (23.9) |
|
| Highest |
77 (34.0) |
151 (35.7) | |
|
Parity |
0.12 |
||
| Primiparous |
48 (21.6) |
69 (16.6) | |
|
Multiparous |
174 (78.4) |
346 (83.4) |
|
| Numbers of children; Median (range) |
1 (0-7) |
1 (0-6) | 0.020* |
|
Mode of delivery |
0.001* |
||
| Spontaneous vaginal delivery |
114 (50.7) |
267 (65.3) | |
|
Assisted (Forceps/vacuum) |
110 (48.9) |
140 (34.2) |
|
| Caesarean section |
1 (0.4) |
2 (0.5) | |
|
Family history of diabetes |
175 (77.8) | 273 (65.0) |
<0.001* |
| Hypertension | 0.009* | ||
|
Essential hypertension |
19 (8.4) |
20 (4.7) |
|
| Pregnancy-induced hypertension |
24 (10.6) |
24 (5.7) |
|
| Treatment of GDM during pregnancy | |||
| Insulin |
34 (15.5) |
– | |
|
Diet |
186 (84.5) | – | |
| GDM during subsequent pregnancy |
41 (18.7) |
– |
GDM: Gestational diabetes
*P-Value <0.05
Incidence of T2DM and MS among the Study Population
With a median follow-up of 6 years, we found 34 (15%) incident cases of T2DM/IGT among women with a history of GDM, whereas 7 cases (1.7%) among women without a history of GDM (p <0.001). We also evaluated the status of MS in our study population and found that one out of every 15 women was diagnosed with MS in the GDM group. In contrast, among the non-GDM group, the diagnosis was less (one out of every 36 women) (6.7% vs. 3.1%, p <0.033). Moreover, women in the GDM group were found with a higher level of fasting blood glucose (41.3% vs. 14.7%), total cholesterol (25.2% vs. 16.4%), triglycerides (30.2% vs. 14%), and LDL (72.1% vs. 61%) and a lower level of HDL (70.3% vs. 64.6%), all with a p-value of <0.05 except for the HDL (Figure 1).
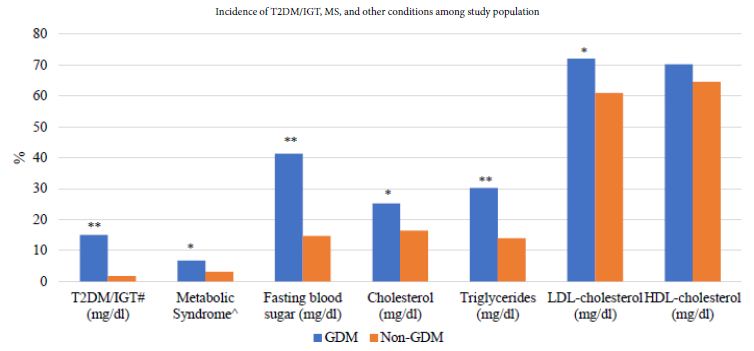
Figure 1: Incidence of T2DM/IGT, MS, and other conditions among study population.
T2DM: Type 2 Diabetes Mellitus, IGT: Impaired Glucose Tolerance, MS: Metabolic Syndrome, GDM: Gestational Diabetes, LDL: Low-density lipoprotein, HDL: High-density lipoprotein
#also included women with one value abnormal, i.e., IGT
^MS defined as women with waist circumference > 80 cm with any one of the following conditions (HDL <50 mg/dl & Triglyceride >150 mg/dl) OR (HDL <50 mg/dl & FBS >100 mg/dl) OR (Triglyceride >150 mg/dl & FBS >100 mg/dl)
*P-value <0.05
**P-value <0.001.
Modifiable Risk Factors among the Study Population
While comparing the anthropometric and body composition of the study population, there were significant differences between the two groups in terms of BMI (kg/m2) (28.4 vs. 27.4, p 0.017), waist circumference (cm) (66.7 vs. 64.9, p 0.036), body fat (%) (36.1 vs. 34.5, p 0.007), and visceral fat (%) (6.6 vs. 5.9, p 0.025) in women with vs. without GDM, respectively.
There were differences in physical activity between groups in occupation and household chores-related activity; however, none of these differences were found to be statistically significant (Table 2).
Table 2: Description of modifiable risk factors among the study population
|
|
GDM Mean ± SD or n (%) |
Non-GDM N=423Mean ± SD or n (%) |
|
P-value |
|
| Anthropometric | |||||
| Weight (kg) |
68.31 ± 12.63 |
66.60 ± 13.16 | 0.11 | ||
|
BMI (kg/m2) |
28.42 ± 5.29 | 27.37 ± 5.33 |
0.017* |
||
| Normal weight (<23) |
28 (12.4) |
77 (18.3) | 0.014* | ||
|
Overweight (23-26.9) |
67 (29.8) | 150 (35.6) | |||
| Obese (≥ 27) |
130 (57.8) |
194 (46.1) | |||
|
Waist circumference (cm) |
66.69 ± 9.80 | 64.85 ± 11.06 |
0.036* |
||
| High (≥ 80) |
19 (8.4) |
32 (7.6) | 0.70 | ||
|
Waist-to-hip ratio |
0.82 ± 0.08 | 0.81 ± 0.10 |
0.059 |
||
| High (≥ 0.8) |
133 (58.8) |
218 (51.5) | 0.075 | ||
|
Body Composition |
|||||
| Body fat (%) |
36.12 ± 6.18 |
34.51 ± 7.63 | 0.007* | ||
|
Visceral fat (%) |
6.56 ± 2.73 | 5.95 ± 2.70 |
0.025* |
||
| Muscle mass (kg) |
40.47 ± 35.16 |
42.96 ± 34.24 | 0.38 | ||
|
Bone mass (kg) |
2.20 ± 0.23 | 2.39 ± 1.97 |
0.24 |
||
| Total body water (kg) |
31.57 (2.90) |
31.18 (3.22) | 0.15 | ||
|
Physical Activity (energy expenditure; kcal/day) |
|||||
| Total reported physical activity |
655.47 ± 26.48 |
688.67 ± 21.29 | 0.35 | ||
|
Occupation |
620.96 ± 33.64 | 521.14 ± 33.92 |
0.06 |
||
| Transportation |
135.25 ± 22.29 |
172.69 ± 19.11 | 0.24 | ||
|
Household chores |
463.17 ± 19.18 | 514.24 ± 16.81 |
0.06 |
||
| Leisure time |
154.89 ± 18.87 |
158.97 ± 15.53 |
0.87 |
||
GDM: Gestational diabetes, BMI: Body mass index
*P-Value <0.05
Modifiable Risk Factors and the Risk of Developing T2DM/IGT
We created two models to understand the association between modifiable risk factors and the development of T2DM and MS in women with vs. without GDM. In the first model, we used BMI as a measure of obesity, while in the second, we replaced it with body fat percentage. Other variables, such as diet and physical activity were present in both the models. We observed that women with a history of GDM had 10.91 times (CI 4.57-26.04) higher odds of developing T2DM/IGT than women without a history of GDM. Furthermore, among women with a history of GDM, with every one unit increase in the BMI (kg/m2) and diet scores (high in fat, sugar, and other bakery items), the odds of developing T2DM/IGT increased by 1.09 (CI 1.02-1.15) and 1.22 (CI 1.01-1.49), respectively. In the second model, the odds of developing T2DM/IGT among women with GDM (OR 11.02, CI 4.63-26.22) and body fat% (OR 1.10, CI 1.04-1.17) were significant. Both models were adjusted for education, wealth index, and family history of diabetes (Table 3).
Table 3: Association of T2DM/IGT and modifiable risk factors among women with vs. without a history of GDM
|
Variables |
T2DM/IGT | ||
| Model 1 |
Model 2a |
||
|
Crude OR (95% CI) |
ORadj (95% CI) |
ORadj (95% CI) |
|
| Group | |||
| Non-GDM |
Ref |
Ref |
Ref |
| GDM |
10.52 (4.58-24.17)* |
10.91 (4.57-26.04)* | 11.02 (4.63-26.22)* |
|
BMI |
1.09 (1.03-1.15)* | 1.09 (1.02-1.15)* |
NA |
| Body fat% |
1.10 (1.05-1.16)* |
NA | 1.10 (1.04-1.17)* |
|
Diet |
1.08 (0.91-1.28) | 1.22 (1.01-1.49)* |
1.21 (0.99-1.48)^ |
| PA expenditure (kcal/day) |
1.00 (0.99-1.01) |
1.00 (0.99-1.01) |
1.00 (0.99-1.01) |
T2DM: Type 2 diabetes mellitus, IGT: Impaired glucose tolerance, GDM: Gestational diabetes, BMI: Body mass index, CI: Confidence interval, NA: Not applicable, OR: Odds ratio, ORadj: Adjusted odds ratio, PA: Physical activity,
PCA included diet high in fat, sugar, and other bakery items
Women were matched on age and time of delivery
Models 1 and 2 also adjusted for education, wealth index, and family history of diabetes
aIn model 2, BMI was replaced by fat%
OR for BMI and PCA are for one unit increase, and for fat% and physical activity are for a 10% and 10 minutes increase, respectively
*P-value <0.05
^P-value=0.05
Modifiable Risk Factors and the Risk of Developing MS
Similarly, in the first model, we observed that women with a history of GDM had 2.78 times (CI 1.04-7.41) higher odds of developing MS than women without a history of GDM. Furthermore, among women with a history of GDM, with every one-unit increase in the BMI (kg/m2), the odds of developing MS increased by 1.32 (CI 1.22-1.42). When we replaced BMI with body fat% in the second model, we found that the odds of developing MS among women with a history of GDM (OR 2.80, CI 1.12-7.03) and body fat percentage (OR 1.35, CI 1.23-1.48) were significant (Table 4).
Table 4: Association of MS and modifiable risk factors among women with vs. without a history of GDM
|
Variables |
MS | ||
| Model 1 |
Model 2a |
||
|
Crude OR (95% CI) |
ORadj (95% CI) |
ORadj (95% CI) |
|
| Group | |||
| Non-GDM |
Ref |
Ref | Ref |
|
GDM |
2.25 (1.05-4.81)* | 2.78 (1.04-7.41)* |
2.80 (1.12-7.03)* |
| BMI |
1.30 (1.21-1.40)* |
1.32 (1.22-1.42)* | NA |
|
Body fat (%) |
1.33 (1.22-1.45)* | NA |
1.35 (1.23-1.48)* |
| Diet |
1.03 (0.83-1.27) |
1.20 (0.92-1.55) | 1.17 (0.91-1.51) |
|
PA expenditure (kcal/day) |
1.00 (0.99-1.01) | 1.00 (0.99-1.01) |
1.00 (0.99-1.01) |
MS: Metabolic Syndrome, GDM: Gestational diabetes, CI: Confidence interval, NA: Not applicable, OR: Odds ratio, ORadj: Adjusted odds ratio, PA: Physical activity
PCA included diet high in fat, sugar, and other bakery items
Women were matched on age and time of delivery
Models 1 and 2 also adjusted for education, wealth index, and family history of diabetes
aIn model 2, BMI was replaced by fat%
OR for BMI and PCA are for one unit increase, and for fat% and physical activity are for a 10% and 10 minutes increase, respectively
*P-value <0.05
Discussion
This study evaluated the incidence of T2DM and MS and its association with modifiable risk factors among women with and without a history of GDM attending a tertiary healthcare facility in Karachi, Pakistan. We found a higher incidence of T2DM (15%) and MS (6.7%) in women with GDM compared to those without GDM, 1.7%, and 3.1%, respectively. In addition, the two modifiable risk factors, such as BMI and diet, predicted T2DM. Replacing BMI with body fat% in a similar model led to comparable estimates for the two indicators of obesity. However, in our second model, the history of GDM and BMI or body fat percentage were associated with MS.
Gestational diabetes is an independent risk factor for developing T2DM and other metabolic issues [25,26]. Our findings are similar to a meta-analysis that reported almost ten times higher risk (pooled relative risk) of developing T2DM among women with a history of GDM as compared to their counterparts [27]. Similarly, for MS, a study from Italy reported about 9% of women with a previous history of GDM identified with MS (15 out of 166 women) compared to controls (1%, 1 out of 90) [24]. We also observed similar results where women with a history of GDM had higher odds of developing T2DM and MS than women without a history of GDM.
Previous studies have identified the predictive relationship between the modifiable risk factors and the risk of diabetes and MS [28-30]. However, most of these studies have been conducted among women without GDM. Few studies from the West have explored the association of modifiable risk factors with T2DM in women with GDM [18,31]. For example, a longitudinal cohort study among women with a history of GDM found a 90% relative reduction in the risk of T2DM among those with adequate control of five modifiable risk factors, such as BMI, diet, physical activity, smoking, and alcohol use [18]. We also assessed the association of four modifiable risk factors, such as BMI, body fat%, diet, and physical activity, on the development of T2DM and MS among women with vs. without a history of GDM and found an apparent effect of BMI and body fat% on the risk of T2DM and MS.
In general, Asians are more susceptible to fat deposition and metabolic derangement even at a lower BMI and are at a greater risk of developing T2DM and MS [32]. Additionally, BMI is a crude indicator of adiposity compared to other, more direct measures of body composition, i.e., body fat percentage [33], which can be assessed by DEXA scans and the Bio-Electrical Impedance Analysis (BIA) method [34]. A direct assessment of body fat percentage can be more accurate in predicting the association between adiposity and chronic disease outcomes such as T2DM and MS in women with GDM [33]. Therefore, we also developed a regression model with body fat percentage by replacing BMI for T2DM and MS. Our study provides evidence that the two parameters (BMI and body fat%) had similar strength of association in predicting T2DM and MS among South Asian women in our study.
Other modifiable risk factors, such as unhealthy diet and physical inactivity, play a central role in the development of T2DM and other metabolic issues [35]. For example, there is strong evidence of the cause-and-effect association between unhealthy dietary patterns and the risk of T2DM and MS [36,37]. Similarly, the dose and response relationship between physical activity and the risk of T2DM has been established [38]. However, the association of diet and physical activity with the development of T2DM and MS has not been fully explored among women with a history of GDM in South Asia. Hence, we evaluated the effect of diet and physical activity on the risk of T2DM and MS in our study population and found that increased dietary scores (high in fat, sugar, and other bakery products) in the presence of BMI predicted T2DM among women with previous GDM. However, the study findings were insignificant when we replaced BMI with body fat% in the second model. Unfortunately, we did not find any association between physical activity and the risk of T2DM and MS. The reason may be that the physical activity scores were very low in our study population. In addition, as our study sample was based on AKU hospital, which mainly serves the affluent people of the city; therefore, we might not have been able to accurately estimate the impact of some lifestyle factors, such as diet and physical activity, on the outcome due to lack of variability in the data. However, one cannot underestimate the value of physical activity in preventing the development of T2DM and MS.
Our study has several strengths. To the best of our knowledge, this is the first study that assessed the association between modifiable risk factors and the risk of developing T2DM and MS among South Asian women with and without a history of GDM. The prospective nature of the study allowed us to collect information on the modifiable risk factors prior to the occurrence of the outcomes, minimizing recall and interviewer bias during the data collection. We identified women with a history of GDM from the medical record system of AKUH using the International Classification of Diseases (ICD) code 10 for GDM. We objectively assessed the diagnosis of T2DM and MS using the World Health Organization (WHO) [3] and International Diabetes Federation (IDF) [14] criteria, respectively. We included the comparator group (women without GDM) matched on age at the time of pregnancy and gestational age. In addition, we also adjusted our regression models for other known confounders, such as education, wealth quintiles, and family history of diabetes, and hence provide more accurate estimates of the risk of T2DM and MS and their association with modifiable risk factors among women with and without GDM. Our study does have some limitations. We evaluated the modifiable risk factors at a single time point and have not followed them up to gather information on long-term lifestyle habits, which would have enabled us to estimate a more accurate effect of these risk factors on the outcomes. Additionally, we enrolled our study sample from AKU hospital, which mainly serves the affluent population of the city; therefore, we might not have been able to accurately estimate the impact of some lifestyle factors, such as diet and physical activity, on the outcome due to lack of variability in the data. Furthermore, the hospital-based sample might limit the generalizability of our study findings. However, given the nature of the study, there were no community-based registries of pregnant women present at the time this study was being carried out to answer the research question.
Conclusion
Our study findings reveal that potentially modifiable risk factors, such as BMI or body fat% and possibly diet are associated with the development of T2DM/IGT and MS in South Asian women with a history of GDM. These findings hold significant implications, particularly for countries like Pakistan, where T2DM/IGT and MS are rising. Therefore, establishing prevention programs targeted towards women with GDM during the postpartum period is crucial. These programs should prioritize the promotion of healthy lifestyle behaviors, such as healthy eating habits and increasing physical activity, to reduce fat deposition in individuals with high body fat percentages.
Declarations
Ethical Approval and Consent to Participate
The study was approved by the Ethical Review Committee of Aga Khan University (Ref: 1094-CHS/ERC-08). Informed consent was obtained from study participants and before participation.
Funding
This research was funded by The Aga Khan University Research Council (URC); however, the funding is not available to support publication of the research.
Author Contributions
RI conceptualized the study, wrote the study protocol, and secured funding. RI supervised the study. SN and GB were involved in the execution of the study and data acquisition. RQ facilitated data collection from the medical records as well as approaching women. SN, GB, and UK were involved in the analysis, interpretation, and writing the manuscript. RI and RQ reviewed and provided scientific revisions to the manuscript. All authors agreed prior to submission to take responsibility and be accountable for the contents of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
We would like to thank our study participants who took part in this study. We are also thankful to the research team who contributed to this study.
List of Abbreviations:
T2DM: Type 2 Diabetes
GDM: Gestational Diabetes
LMICs: Low-and Middle-Income Countries
CVDs: Cardiovascular Diseases
MS: Metabolic Syndrome
ICD: International Classification of Diseases
AKU: Aga Khan University
IGT: Impaired Glucose Tolerance
OGTT: Oral Glucose Tolerance Test
FFQ: Food Frequency Questionnaire
PCA: Principal Component Analysis
MOSPA: Monica Optional Study of Physical Activity
LDL: Low-Density Lipoprotein
HDL: High-Density Lipoprotein
BMI: Body Mass Index
WHO: World Health Organization
IDF: International Diabetes Federation
References
- Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, et al. (2018) IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 138: 271-281. [crossref]
- International Diabetes Federation Brussels, Belgium2021 [10th: [Available from: https://diabetesatlas.org/.
- Association AD (2018) Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 42: S13-S28.
- Fletcher B, Gulanick M, Lamendola C (2002) Risk Factors for Type 2 Diabetes Mellitus. Journal of Cardiovascular Nursing 16: 17-23.
- Zheng Y, Ley SH, Hu FB (2018) Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 14: 88-98. [crossref]
- Lee AJ, Hiscock RJ, Wein P, Walker SP, Permezel M (2007) Gestational diabetes mellitus: clinical predictors and long-term risk of developing type 2 diabetes: a retrospective cohort study using survival analysis. Diabetes Care 30: 878-883. [crossref]
- Lauenborg J, Hansen T, Jensen DM, Vestergaard H, Mølsted-Pedersen L, et al. (2004) Increasing incidence of diabetes after gestational diabetes: a long-term follow-up in a Danish population. Diabetes Care 27: 1194-1199. [crossref]
- Gadve SS, Chavanda S, Mukherjee AD, Aziz S, Joshi A, et al. (2021) Risk of Developing Type 2 Diabetes Mellitus in South Asian Women with History of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Indian J Endocrinol Metab 25: 176-181. [crossref]
- Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 43: S14-s31.
- Zhu Y, Zhang C (2016) Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: a Global Perspective. Curr Diab Rep 16: 7. [crossref]
- Wang H, Li N, Chivese T, Werfalli M, Sun H, et al. (2022) IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s Criteria. Diabetes Research and Clinical Practice 183: 109050. [crossref]
- Sheikh A, Sheikh L (2020) Changing prevalence of Gestational Diabetes Mellitus during pregnancy over more than a decade. J pak Med Assoc 70: 1477-1478. [crossref]
- Group HSCR (2009) Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations with neonatal anthropometrics. Diabetes 58: 453-459. [crossref]
- Zimmet P, KG MMA, Serrano Ríos M (2005) [A new international diabetes federation worldwide definition of the metabolic syndrome: the rationale and the results]. Rev Esp Cardiol 58: 1371-1376. [crossref]
- Goveia P, Cañon-Montañez W, Santos DP, Lopes GW, Ma RCW, et al. (2018) Lifestyle Intervention for the Prevention of Diabetes in Women With Previous Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne) 9: 583. [crossref]
- Ratner RE, Christophi CA, Metzger BE, Dabelea D, Bennett PH, et al. (2008) Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab 93: 4774-4779. [crossref]
- Pérez-Ferre N, Del Valle L, Torrejón MJ, Barca I, Calvo MI, et al. (2015) Diabetes mellitus and abnormal glucose tolerance development after gestational diabetes: A three-year, prospective, randomized, clinical-based, Mediterranean lifestyle interventional study with parallel groups. Clin Nutr 34: 579-585. [crossref]
- Yang J, Qian F, Chavarro JE, Ley SH, Tobias DK, et al. (2022) Modifiable risk factors and long term risk of type 2 diabetes among individuals with a history of gestational diabetes mellitus: prospective cohort study. Bmj 378: e070312. [crossref]
- Iqbal R, Haroon A, Jabbar A, Babar N, Qureshi R (2012) What method of contact works best for recruiting participants in a study: lessons for health care researchers? J Pak Med Assoc 62: 1293-1297. [crossref]
- Iqbal R, Rafique G, Badruddin S, Qureshi R, Cue R, et al. (2007) Increased body fat percentage and physical inactivity are independent predictors of gestational diabetes mellitus in South Asian women. Eur J Clin Nutr 61: 736-742. [crossref]
- Pereira MA, FitzerGerald SJ, Gregg EW, Joswiak ML, Ryan WJ, et al. (1997) A collection of Physical Activity Questionnaires for health-related research. Med Sci Sports Exerc 29: 205. [crossref]
- Iqbal R, Rafique G, Badruddin S, Qureshi R, Gray-Donald K (2006) Validating MOSPA questionnaire for measuring physical activity in Pakistani women. Nutr J 5: 18. [crossref]
- Feig DS, Zinman B, Wang X, Hux JE (2008) Risk of development of diabetes mellitus after diagnosis of gestational diabetes. Cmaj 179: 229-234. [crossref]
- Di Cianni G, Lencioni C, Volpe L, Ghio A, Cuccuru I, et al. (2007) C-reactive protein and metabolic syndrome in women with previous gestational diabetes. Diabetes Metab Res Rev 23: 135-140. [crossref]
- Kaiser K, Nielsen MF, Kallfa E, Dubietyte G, Lauszus FF (2021) Metabolic syndrome in women with previous gestational diabetes. Scientific Reports 11: 11558. [crossref]
- Diaz-Santana MV, O’Brien KM, Park Y-MM, Sandler DP, Weinberg CR (2022) Persistence of Risk for Type 2 Diabetes After Gestational Diabetes Mellitus. Diabetes Care 45: 864-870. [crossref]
- Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, et al. (2020) Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. Bmj 369: m1361. [crossref]
- Aravinda J (2019) Risk factors in patients with type 2 diabetes in Bengaluru: A retrospective study. World J Diabetes 10: 241-248. [crossref]
- Ismail L, Materwala H, Al Kaabi J (2021) Association of risk factors with type 2 diabetes: A systematic review. Computational and Structural Biotechnology Journal 19: 1759-1785. [crossref]
- Al Shehri HA, Al Asmari AK, Khan HA, Al Omani S, Kadasah SG, et al. (2022) Association between preventable risk factors and metabolic syndrome. Open Med (Wars) 17: 341-352. [crossref]
- Mishra S, Shetty A, Rao CR, Nayak S, Kamath A (2020) Risk factors for gestational diabetes mellitus: A prospective case-control study from coastal Karnataka. Clinical Epidemiology and Global Health 8: 1082-1088.
- Deurenberg P, Deurenberg-Yap M, Guricci S (2002) Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev 3: 141-146. [crossref]
- Bhowmik B, Munir SB, Diep LM, Siddiquee T, Habib SH, Samad MA, et al. (2013) Anthropometric indicators of obesity for identifying cardiometabolic risk factors in a rural Bangladeshi population. J Diabetes Investig 4: 361-368. [crossref]
- Yi Y, Baek JY, Lee E, Jung H-W, Jang I-Y (2022) A Comparative Study of High-Frequency Bioelectrical Impedance Analysis and Dual-Energy X-ray Absorptiometry for Estimating Body Composition. Life 12: 994. [crossref]
- Raman PG (2016) Environmental Factors in Causation of Diabetes Mellitus. In: Marcelo LL, Sonia S, editors. Environmental Health Risk. Rijeka: IntechOpen 9.
- Czekajło A, Różańska D, Zatońska K, Szuba A, Regulska-Ilow B (2018) Association between dietary patterns and metabolic syndrome in the selected population of Polish adults-results of the PURE Poland Study. European Journal of Public Health 29: 335-340. [crossref]
- Pestoni G, Riedl A, Breuninger TA, Wawro N, Krieger J-P, et al. (2021) Association between dietary patterns and prediabetes, undetected diabetes or clinically diagnosed diabetes: results from the KORA FF4 study. European Journal of Nutrition 60: 2331-2341. [crossref]
- Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, et al. (2012) Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 380: 219-229. [crossref]