Abstract
Purpose: Limited research has been conducted on the long-term use of metformin in patients with and without diabetes. Metformin is considered one of the most effective and affordable drugs used in patients with diabetes. However, only a few studies have examined the effects of metformin on prostate cancer mortality. This study aimed to understand the impact of metformin use on prostate cancer mortality among men, adjusted for diabetes.
Methods: SEER-Medicare data between 2007 and 2013 were used to extract demographic information from patients and cancer characteristics, including age, race, marital status, comorbidities (i.e., diabetes), and metformin use. Descriptive statistical analysis and logistic regression were performed to investigate the association between mortality rates (12, 60, and 120 months) and metformin use in men with prostate cancer with and without diabetes.
Results: We identified 222,289 prostate cancer patients enrolled in Medicare Part D. Among them, 17% received metformin, and 35% were diagnosed with diabetes. Among metformin users, a significantly higher proportion of cancer patients had diabetes (34.75%). Prostate cancer patients who took metformin had 0.54(AOR: 95% CI: 0.48-0.61), 0.58 (AOR: 95% CI: 0.55-0.62), and 0.56 (AOR: 95% CI: 0.54-0.59) lower odds of dying at 12 months, 60 months, and 120 months since the first diagnosis of prostate cancer than those without using metformin.
Conclusion: Metformin is one of the most commonly used diabetes treating agents. However, few studies have examined its role in treating prostate cancer. Our study showed that patients with prostate cancer who received metformin had a higher mortality rate than those who did not. Further studies are needed to determine whether metformin can positively affect patients with prostate cancer.
Keywords
Diabetes mellitus, Elderly, Metformin, Mortality, Prostate cancer, SEER-Medicare
Introduction
According to the Centers for Disease Control and Prevention (CDC), prostate cancer in the United States is the second leading cause of death, surpassed by heart disease, and one of the top four common cancers [1]. Furthermore, it is also one of the leading causes of cancer death among men of all races and populations of Hispanic origin populations [2,3]. Research from the Prostate Cancer Foundation revealed information about the probability that African American men are about 1.6 times more likely than all other men to get prostate cancer and twice more likely to die from it [4]. Specifically, approximately more than 200,000 new cases of prostate cancer were reported among men in 2017, and more than 30,000 men died from this cancer [5]. The Mayo Clinic estimates that approximately 60% of older adults (50 years or older) are at an increased risk of developing prostate cancer [6].
Diabetes mellitus is one of the most common comorbidities in patients with prostate cancer patients [7-9]. Diabetes is associated with an increased incidence of prostate cancer and mortality due to prostate cancer-specific mortality and/or all-cause mortality [10]. However, the biological link between diabetes and prostate cancer is poorly understood [11].
According to the American Diabetes Association, metformin is an inexpensive generic drug used to treat diabetes [12]. Recently, physicians have widely prescribed metformin because of its specific effects in treating and curing diabetes [13,14] as well as to prevent the development of diabetes [15].
Recent studies have shown that metformin can be used to reduce cancer incidence and mortality [16-19]. However, little is known regarding metformin in patients with prostate cancer. The results of studies evaluating the role of metformin in prostate cancer are contradictory. There are different perspectives on whether metformin use improves or deteriorates health conditions. One study showed that patients with diabetes mellitus taking metformin had a lower risk of prostate cancer [20]. However, a study conducted in Toronto found that metformin use is associated with an increased risk of being diagnosed with prostate cancer [21]. Another study suggested that metformin did not have proven benefits in cancer cells [22]. In 2020, the U.S. Food and Drug Administration (FDA) conducted recalls for different types of metformin. In other words, the FDA has not approved the use of certain types of metformin for patients with diabetes, as it can cause lactic acidosis [23]. Given these conflicting findings and the generally limited research, this study aimed to examine the association between metformin use and prostate cancer mortality, adjusted for diabetes status in patients. This study aimed to obtain data from Medicare beneficiaries (Medicare) and Surveillance, Epidemiology, and End Results (SEER).
Methods
Study Design and Data Source
Our study is a secondary analysis of merged data from the SEER and Medicare datasets, Medicare beneficiaries (Medicare), and Surveillance, Epidemiology, and End Results (SEER). The National Cancer Institute sponsors the SEER program [24]. Additionally, the dataset provided information on different stages of cancer and was designed to track cancer incidence and mortality rates in the United States [25]. Staging information is based on classification schemata that vary according to cancer site and year of diagnosis [26]. The SEER dataset also covers diverse populations with detailed cancer-related diagnoses and social demographics and covers more than 26% of the U.S. population in different demographics [26,27].
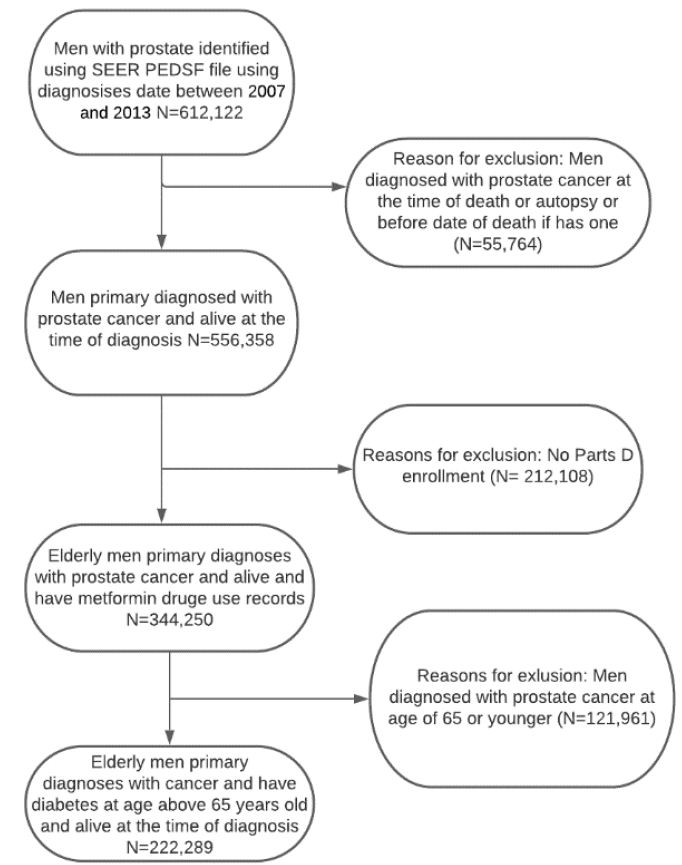
Figure 1: Flow chart for the inclusion and exclusion of studies among Medicare beneficiaries diagnosed with diabetes and incident prostate cancer
The Medicare dataset consists of claim data and billing information, including inpatient, outpatient, and home health information [28]. Medicare is the primary health insurer for over 90% of the U.S. population aged 65 years and older in the U.S. [29]. All Medicare data were de-identified to protect patient information so that no protected health information could be traced to individual patients.
Combining the two datasets represents over 20% of the U.S. population from 1991 to 2013 (What Is SEER?, n.d.) [27]. The merging of the two datasets, SEER and Medicare claims, allows for a longitudinal analysis of cancer patients’ healthcare utilization and health outcomes.
Sample Selection
Our study included 222,289 patients, and all male patients aged 66 years and older who had prostate cancer diagnoses between January 1, 2007, and December 31, 2013, were selected from SEER-Medicare. Patients were excluded if their cancer diagnosis date was missing, death occurred before cancer diagnosis, or there was no follow-up medical care. We also excluded patients identified as having cancer through a death certificate. Furthermore, patients were excluded if they did not enroll in Medicare Part A, B, or D during the study follow-up. Some people may want to enroll in Medicare later could be that they have to pay a premium for Part A. It is also possible that a person who contributes to a health savings account (HSA) may consider delaying Medicare Part A [30]. Exclusion criteria were used to ensure that the long-term prostate cancer survivors/medication use data were complete for all patients. There were no missing values for age, metformin use, or diabetes based on the inclusion criteria. However, 27,188 (12.23%) individuals were classified as unknown for marital status, and 1,314 (0.59%) did not disclose their race/ethnicity.
Measures
Long-term prostate cancer survivors were defined as those who survived for >12 months after being diagnosed with prostate cancer [31,32]. Mortality at 12, 60, and 120 months was the key dependent variable in this study. Our study design also included the analysis of patients who survived more than 60 and 120 months after being diagnosed with prostate cancer. The initial selection of independent variables was based on both current data and existing literature [14,22]. Metformin use was a key independent variable. Most independent variables were categorized as binary variables. For example, age at diagnosis was divided into two different age groups, 66-74 and 75 years or older. Race/ethnicity was classified into four types: white non-Hispanic, black, Hispanic, and others. The five contrasting marital statuses were single, married, separated/divorced, widowed, and unknown. The presence of diabetes was identified using at least one hospital visit or more than one physician visit, with a primary or secondary diagnosis code for diabetes. The physician used several ICD-codes to identify diabetes, such as ICD-9-CM codes: 250.x0 or 250.x2. Diagnosis codes were extracted from Medicare Provider Analysis and Review (MEDPAR), carrier claims (NCH), and outpatients (OUTPAT). Metformin use was extracted from Part D Event (PDE).
Statistical Analysis
Descriptive statistics analysis using the chi-square test was used to investigate the association between mortality at 12, 60, and 120 months and each independent variable. Specifically, we examined the risk factors for patient mortality (12, 60, and 120 months) after being first diagnosed with prostate cancer and metformin use among men with prostate cancer. Additionally, we evaluated the mortality rates among those who used metformin and were diagnosed with incident prostate cancer with and without diabetes. Bivariate analyses examined the differences between basic demographic variables, metformin use, prostate cancer, diabetes, and mortality. Logistic regression models were built to estimate the direction and size of the association between live length and metformin use. Logistic regression was used to analyze the relationship between diabetes and mortality among metformin users. Several covariates, including age, race/ethnicity, and marital status, were used to adjust these models to estimate adjusted odds ratios and 95% confidence intervals for mortality of prostate cancer patients with or without diabetes using metformin.
Results
Baseline Characteristics
Table 1 shows the characteristics of the sample and bivariate associations between metformin use status and each independent variable and diabetes. The study sample included 222,289 patients with prostate cancer. Most of the patients were in the age group 66-74 116,344 (52.34%), and non-metformin users 96,654 (83.08%). In our study, there were more white non-Hispanic 170,598 (76.7%), and among the total population, there were more married 147,081 (66.17%) compared to other races and marital status. A significantly higher proportion of metformin users (32,076 patients) were diagnosed with diabetes, while there was still a high proportion of patients; 60,219 non-metformin users also had diabetes. Significant associations were also observed between diabetes, race/ethnicity, and white non-Hispanic individuals who were more likely to use metformin.
Table 1: Characteristics of the patient in a cohort of older adults with prostate cancer: SEER-Medicare database, 2007-2013.
|
Characteristics |
Overall sample
n (%) |
Metformin users
n (%) |
Non-metformin
users n (%) |
P-value |
| Unweighted Observations |
n=222,289 (100%) |
n=37,500 (16.87%) | n=184,789 (83.13%) | |
| Age |
<0.476 |
|||
| 66-74 |
116,344 (100%) |
19,690 (16.92%) |
96,654 (83.08%) |
|
| 75 or older |
105,945 (100%) |
17,810 (16.81%) |
88,135 (83.19%) |
|
| Diabetes |
<0.001 |
|||
| Yes |
92,295 (100%) |
32,076 (34.75%) |
60,219 (65.25%) |
|
| No |
129,994 (100%) |
5,424 (4.17%) |
124,570 (95.83%) |
|
| Race/Ethnicity |
<0.001 |
|||
| White non-Hispanic |
170,598 (100%) |
26,181 (15.35%) |
144,417 (84.65%) |
|
| Black non-Hispanic |
29,344 (100%) |
6,047 (20.61%) |
23,297 (79.39%) |
|
| Hispanic |
6,098 (100%) |
1,666 (27.32%) |
4,432 (72.68%) |
|
| Other |
16,249 (100%) |
3,606 (22.19%) |
12,643 (77.81%) |
|
| Marital Status |
<0.237 |
|||
| Married |
147,081 (100%) |
24,851 (16.90%) |
122,230 (83.10%) |
|
| Unmarried |
15,675 (100%) |
2,676 (17.07%) |
12,999 (82.93%) |
|
| Divorced /Separated |
32,345 (100%) |
5,338 (16.50%) |
27,007 (83.50%) |
|
| Unknown |
27,188 (100%) |
4,635 (17.05%) |
22,553 (82.95%) |
Table 2 presents descriptive statistics of the sample with the three different outcomes, mortality at 12 months, 60 months, and 120 months among patients with prostate cancer patients. Age, race/ethnicity, marital status, metformin use, and diabetes were statistically significant differences (p-value < 0.05) for the three outcomes: 12 months mortality, 60 months mortality, and 120 months mortality after being diagnosed with prostate cancer. A total of 219,551 (98.77%) patients survived more than 12 months, and 2,738 (1.23%) died. There were 204,579 (93.18%) patients who survived more than 60 months, and 14,972 (6.82%) did not survive more than 60 months. There were 184,433 (90.15%) patients who survived more than 120 months, and 20,146 (9.85%) did not survive more than 120 months after being first diagnosed with prostate cancer.
Table 2: Descriptive statistics for mortality at 12 months, 60 months, and 120 months
|
Variables |
One-Year Mortality (12 months) | P-value | Five-Year Mortality (60 months) | P-value | Ten-Year Mortality (120 months) |
P-value |
|||
|
Yes n (%) |
No
n (%) |
Yes
n (%)1 |
No
n (%)0 |
Yes
n (%) |
No
n (%) |
|
|||
| Age |
<0.001 |
<0.001 |
<0.001 |
||||||
| 66-74 |
1,524 (1.31%) |
114,820 (98.69%) | 8,008 (6.97%) | 106,812 (93.03%) | 10,073 (9.43%) |
96,739 (90.57%) |
|||
| 75 or older |
1,214 (1.15%) |
104,731 (98.85%) | 6,964 (6.65%) | 97,767 (93.35%) | 10,073 (10.30%) |
87,694 (89.70%) |
|||
| Race |
0.034 |
<0.001 |
<0.001 |
||||||
| White-non-Hispanic |
2,089 (1.22%) |
168,509 (98.78%) | 11,294 (6.70%) | 157,215 (93.30%) | 15,271 (9.71%) |
141,944 (90.29%) |
|||
| Black-non-Hispanic |
383 (1.31%) |
28,961 (98.69%) | 2,176 (7.51%) | 26,785 (92.49%) | 2,912 (10.87%) |
23,873 (89.13%) |
|||
| Hispanic |
92 (1.51%) |
6,006 (98.49%) | 495 (8.24%) | 5,511 (91.76%) | 637 (11.56%) |
4,874 (88.44%) |
|||
| Other |
174 (1.07%) |
16,075 (98.93%) | 1,007 (6.26%) | 15,068 (93.74%) | 1,326 (8.80%) |
13,742 (91.20%) |
|||
| Marital Status |
<0.001 |
<0.001 |
0.002 |
||||||
| Married |
1,733 (1.18%) |
145,348 (98.82%) | 9,617 (6.62%) | 135,731 (93.38%) | 13,380 (9.86%) |
122,351 (90.14%) |
|||
| Unmarried |
185 (1.18%) |
15,490 (98.82%) | 1,124 (7.26%) | 14,366 (92.74%) | 1,419 (9.88%) |
12,947 (90.12%) |
|||
| Separated/ Divorced |
373 (1.15%) |
31,972 (98.85%) | 2,136 (6.68%) | 29,836 (93.31%) | 3,061 (10.26%) |
26,775 (89.74%) |
|||
| Unknown |
447 (1.64%) |
26,741 (98.36%) | 2,095 (7.83%) | 24,646 (92.17%) | 2,286 (9.28%) |
22,360 (90.72%) |
|||
| Metformin |
<0.001 |
<0.001 |
<0.001 |
||||||
| No |
2,408 (1.30%) |
182,381 (98.70%) | 12,919 (7.08%) | 169,462 (92.92%) | 17,352 (10.24%) |
152,110 (89.76%) |
|||
| Yes |
330 (0.88%) |
37,170 (99.12%) | 2,053 (5.52%) | 35,117 (94.48%) | 2,794 (7.96%) |
32,323 (92.04%) |
|||
| Diabetes |
<0.001 |
<0.001 |
<0.001 |
||||||
| No |
1,433 (1.10%) |
128,561 (98.90%) | 7,469 (5.81%) | 121,092 (94.19%) | 10,106 (8.35%) |
110,986 (91.65%) |
|||
| Yes |
1,305 (1.41%) |
90,990 (98.59%) | 7,503 (8.25%) | 83,487 (91.75%) | 10,040 (12.03%) |
73,447 (87.97%) |
|||
Most of the patients aged 66 to 74 survived for over one year with a p-value = 0.001). With five-year mortality, most patients survived more than five years after being diagnosed with prostate cancer (p = 0.001). The third result showed that most of the patients stayed alive for more than 120 months, with a p-value of 0.001.
Race/ethnicity was statistically significant among the three outcomes, 12 months mortality (p-value=0.034), 60 months mortality (p-value < 0.001), 120 months mortality (p < 0.001) after the first diagnosis of prostate cancer. Among the same race/ethnicity, most patients, whether white-no Hispanic, black-no Hispanic, Hispanic, or other non-Hispanic, have survived more than half of the population for 12 months after being first diagnosed with prostate cancer.
Metformin use and mortality outcomes were statistically significant (p < 0.001). For the second outcome, the age group between 66 and 74 years had more than half of the population, and 106,812 (93.03%) survived more than 60 months after the first diagnosis of prostate cancer. Among the same race/ethnicity, most patients survived more than 60 months after the first diagnosis of prostate cancer, with a p-value of 0.001. Marital status was statistically significant for all three outcome variables, with a p-value of less than 0.005. The diabetes variables were also statistically significant for the three outcomes, with a p-value less than 0.001. Based on the three different outcomes of logistic regression, most patients survived for > 12, 60, and 120 months.
Table 3 describes the AOR for mortality at 12, 60, and 120 months in patients with prostate cancer treated with metformin. For this subgroup, metformin use between 66 and 74 years was 0.54 (95% CI: 0.48-0.61), 0.58 (95% CI: 0.55-0.62), 0.56 (95% CI:0.53-0.59) times less likely to die after prostate cancer was diagnosed within 12 months, 60 months, 120 months than those who were older than 74 years, respectively. Patients with diabetes were 1.49 (95% CI: 1.38-1.62), 1.64 (95% CI: 1.59-1.70), 1.66 (95% CI:1.60-1.72), 1.74 (95% CI: 1.69-1.79) times more likely to die after being diagnosed with prostate cancer within 12 months and 60 months, and 120 months compared to those without diabetes. Patients taking metformin were 0.54 (95% CI 0.48-0.61), 0.57 (95% CI 0.55-0.62), 0.56 (95% CI: 0.54-0.59) times less likely to die after prostate cancer compared to those who did not take metformin, respectively. Furthermore, black non-Hispanics had an AOR of 1.06 (95% CI: 1.01-1.11), 1.06 (95% CI: 1.02-1.11) times more likely to die within five years and ten years after the first diagnosis of prostate cancer. Hispanics are 1.19 (95% CI: 1.09-1.31) and 1.16 (95% CI: 1.06-1.26) times more likely to die within five and ten years after being diagnosed with prostate cancer than other races/ethnicities. The other marital status groups are 1.10 (95% CI: 1.03-1.17) times less likely to die after being diagnosed with prostate cancer. Otherwise, other marital statuses were not statistically significant.
Table 3: Adjusted odds ratios for mortality at 12 months, 60 months, and 120 months among prostate cancer patients.
|
Variables |
One-Year Mortality (12 months) | Five-Year Mortality (60 months) | Ten-Year Mortality (120 months) | |||
| OR | 95% CI | OR | 95% CI | OR |
95% CI |
|
| Age | ||||||
| 66-74 |
0.85 |
0.79-0.92* | 0.94 | 0.91-0.97* | 1.10 |
1.07-1.13* |
| 75 or older |
(ref.) |
(ref.) |
(ref.) |
|||
| Race/Ethnicity | ||||||
| White-non-Hispanic |
(ref.) |
(ref.) |
(ref.) |
|||
| Black-non-Hispanic |
1.02 |
0.91-1.14 | 1.06 | 1.01-1.11* | 1.06 |
1.02-1.11* |
| Hispanic |
1.21 |
0.98-1.50 | 1.19 | 1.09-1.31* | 1.16 |
1.06-1.26* |
| Other |
0.87 |
0.74-1.02 | 0.91 | 0.85-0.97* | 0.87 |
0.82-0.93* |
| Marital Status | ||||||
| Married |
(ref.) |
(ref.) |
(ref.) |
|||
| Unmarried |
0.99 |
0.85-1.16 | 1.10 | 1.03-1.17* | 1.00 |
0.94-1.06 |
| Separated/ Divorced |
0.99 |
0.89-1.11 | 1.01 | 0.96-1.06 | 1.03 |
0.98-1.07 |
| Metformin | ||||||
| No |
(ref.) |
(ref.) |
(ref.) |
|||
| Yes |
0.54 |
0.48-0.61* | 0.58 | 0.55-0.62* | 0.56 |
0.54-0.59* |
| Diabetes | ||||||
| No |
(ref.) |
(ref.) |
(ref.) |
|||
| Yes |
1.49 |
1.38-1.62* | 1.66 | 1.60-1.72* | 1.74 |
1.69-1.79* |
Discussion
The age at diagnosis is an important predictor of prostate cancer mortality. Race/ethnicity can include various major risk factors such as genetic factors, medical practices such as screening, and complete reporting. Furthermore, there is conflicting evidence regarding the relationship between metformin use and mortality in patients with prostate cancer patients (He et al., 2019)33. The present study provides additional evidence that metformin use reduces the likelihood of mortality in patients with prostate cancer, regardless of diabetes. This finding is consistent with the existing evidence in the literature reviews that we found [33-36].
Furthermore, the results of this study showed that unmarried men were more likely to die within 60 months than married men. The findings showed that family support plays an important role in cancer outcomes; however, more research is needed to study the difference between different years of mortality versus unmarried status. Among metformin users, patients with diabetes have higher odds of mortality than those without diabetes. This may be due to the fact that metformin is the first-line medicine for diabetes. These findings support the notion that metformin is beneficial. These results indicate that metformin users with diabetes and prostate cancer are less likely to die. This finding may have been biased because we considered diabetes the only comorbidity in our analysis. Other comorbidities and diabetes may play a complex role in cancer-related mortality.
This observational study had several limitations. First, our study population included Medicare beneficiaries aged over 65 years. However, our findings may not be generalizable to younger age groups. Second, diabetes and cancer are two common diseases that are complex and have several subtypes. This study did not consider the different types or severity of diabetes, and other comorbidities were not included in the analysis. Therefore, this study may have excluded important risk factors related to patient outcomes. Furthermore, this study did not consider several important factors such as possible individual exposure to different types of treatment with an optimal potential dose, schedule, and duration of metformin treatment, which can contribute to different clinical outcomes.
Despite the study’s limitations, our study used a large sample size to identify the association between mortality due to metformin use among prostate cancer patients with or without diabetes. In general, metformin decreased the mortality of some patients with prostate cancer at 12, 60, and 120 months.
Conclusion
This study provides evidence that metformin use can reduce the mortality risk among patients with prostate cancer, including those with diabetes. The results showed that age, metformin use, and comorbidities influence prostate cancer. Socioeconomic disparities and prolonged use of metformin may explain the differences in mortality. Even the fact that metformin is an inexpensive drug with few side effects and anticancer effects in many types of cancer. Low socioeconomic status may explain why some patients have difficulty accessing treatment. Previous results concluded that our research identified population segments that may benefit from targeted interventions.
More studies, such as clinical trials, are needed to provide evidence of the effectiveness of metformin in patients with prostate cancer. Furthermore, we found a correlation between metformin and mortality in prostate cancer by adding additional information on comorbidities and various disparities. With more variables included in future research, more value can be added to identify better treatment options for older adults.
References
- Cancer Statistics—National Cancer Institute (nciglobal, ncienterprise) (2015) https://www.cancer.gov/about-cancer/understanding/statistics
- CDCBreastCancer (2021) Prostate Cancer Statistics. Centers for Disease Control and Prevention https://www.cdc.gov/cancer/prostate/statistics/index.htm
- Xiao H, Tan F, Goovaerts P, Adunlin G, Ali AA, et al. (2016) Impact of Comorbidities on Prostate Cancer Stage at Diagnosis in Florida. American Journal of Men’s Health 10: 285-295. [crossref]
- Prostate cancer in Black men: Risk factors, symptoms, and more. (2021) https://www.medicalnewstoday.com/articles/prostate-cancer-in-black-men
- USCS Data Visualizations. (n.d.). Retrieved March 31 (2021) from https://gis.cdc.gov/grasp/USCS/DataViz.html
- Prostate cancer—Symptoms and causes. (n.d.) (2021) Mayo Clinic. from https://www.mayoclinic.org/diseases-conditions/prostate-cancer/symptoms-causes/syc-20353087
- Ng HS, Koczwara B, Roder D, Vitry A (2018) Development of comorbidities in men with prostate cancer treated with androgen deprivation therapy: An Australian population-based cohort study. Prostate Cancer and Prostatic Diseases 21: 403-410. [crossref]
- Roy S, Vallepu S, Barrios C, Hunter K (2018) Comparison of Comorbid Conditions Between Cancer Survivors and Age-Matched Patients Without Cancer. Journal of Clinical Medicine Research 10: 911-919. [crossref]
- Tseng (2011) Diabetes and Risk of Prostate Cancer. Diabetes Care 34: 616-621. [crossref]
- Feng X, Song M, Preston MA, Ma W, Hu Y, et al. (2020) The association of diabetes with risk of prostate cancer defined by clinical and molecular features. British Journal of Cancer 123: 657-665. [crossref]
- DeNoon DJ (2021) Why Does Diabetes Raise Cancer Risk? WebMD. from https://www.webmd.com/diabetes/news/20100616/why-does-diabetes-increase-cancer-risk
- Suissa S, Azoulay L (2014) Metformin and Cancer: Mounting Evidence Against an Association. Diabetes Care 37: 1786-1788. [crossref]
- Saraei P, Asadi I, Kakar MA, Moradi-Kor N (2019) The beneficial effects of metformin on cancer prevention and therapy: A comprehensive review of recent advances. Cancer Management and Research 11: 3295-3313. [crossref]
- Wright JL, Stanford JL (2009) Metformin use and prostate cancer in Caucasian men: Results from a population-based case–control study. Cancer Causes & Control 20: 1617. [crossref]
- What Diabetes Drugs Improve Insulin Sensitivity? (n.d.). (2021) WebMD. from https://www.webmd.com/diabetes/diabetes-drugs-insulin-sensitivity
- Clements A, Gao B, Yeap SHO, Wong MKY, Ali SS, et al. (2011) Metformin in prostate cancer: Two for the price of one. Annals of Oncology 22: 2556-2560. [crossref]
- Hankinson SJ, Fam M, Patel NN (2017) A review for clinicians: Prostate cancer and the antineoplastic properties of metformin. Urologic Oncolog 35: 21-29.
- Nobes JP, Langley SEM, Klopper T, Russell-Jones D, Laing RW (2012) A prospective, randomized pilot study evaluating the effects of metformin and lifestyle intervention on patients with prostate cancer receiving androgen deprivation therapy. BJU International 109: 1495-1502. [crossref]
- Whitburn J, Edwards CM, Sooriakumaran P (2017) Metformin and Prostate Cancer: A New Role for an Old Drug. Current Urology Reports 18: 46.
- Zaidi S, Gandhi J, Joshi G, Smith NL, Khan SA (2019) The anticancer potential of metformin on prostate cancer. Prostate Cancer and Prostatic Diseases 22: 351-361. [crossref]
- Lee MJ, Jayalath VH, Xu W, Lu L, Freedland SJ, et al. (2021) Association between metformin medication, genetic variation and prostate cancer risk. Prostate Cancer and Prostatic Diseases 24: 96-105. [crossref]
- Raval AD, Mattes MD, Madhavan S, Pan X, Wei W, et al. (2016) Association between Metformin Use and Cancer Stage at Diagnosis among Elderly Medicare Beneficiaries with Preexisting Type 2 Diabetes Mellitus and Incident Prostate Cancer. Journal of Diabetes Research e2656814. [crossref]
- Research C (2019) FDA Drug Safety Communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. FDA. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-warnings-regarding-use-diabetes-medicine-metformin-certain
- Beebe-Dimmer JL, Fryzek JP, Yee CL, Dalvi TB, Garabrant DH, et al. (2016) Mesothelioma in the United States: A Surveillance, Epidemiology, and End Results (SEER)–Medicare investigation of treatment patterns and overall survival. Clinical Epidemiology 8: 743-750.
- Daly MC, Paquette IM (2019) Surveillance, Epidemiology, and End Results (SEER) and SEER-Medicare Databases: Use in Clinical Research for Improving Colorectal Cancer Outcomes. Clinics in Colon and Rectal Surgery 32: 61-68. [crossref]
- Ambs A, Warren JL, Bellizzi KM, Topor M, Haffer SC, et al. (2018) Overview of the SEER—Medicare Health Outcomes Survey Linked Dataset. Health Care Financing Review 29: 5-21. [crossref]
- What Is SEER? – National Cancer Institute (nciglobal, ncienterprise). (n.d.). [CgvInfographic]. Retrieved March 31, 2021, from /research/areas/public-health/what-is-seer-infographic
- CMS announces new payment model to improve quality, coordination, and cost-effectiveness for both inpatient and outpatient care | CMS. (n.d.). Retrieved July 5, 2020, from https://www.cms.gov/newsroom/press-releases/cms-announces-new-payment-model-improve-quality-coordination-and-cost-effectiveness-both-inpatient
- Moon M (1996) What Medicare Has Meant To Older Americans. Health Care Financing Review 18: 49-59.
- Fact Sheet: Deciding whether to enroll in Medicare Part A and Part B when you Turn 65. (n.d.). FACT SHEET, 15.
- Brenner H, Arndt V (2016) Long-Term Survival Rates of Patients with Prostate Cancer in the Prostate-Specific Antigen Screening Era: Population-Based Estimates for the Year 2000 by Period Analysis. Journal of Clinical Oncology https://doi.org/10.1200/JCO.2005.11.148
- Stokes ME, Black L, Benedict Á, Roehrborn CG, Albertsen P (2010) Long-term medical-care costs related to prostate cancer: Estimates from linked SEER-Medicare data. Prostate Cancer and Prostatic Diseases 13: 278-284. [crossref]
- He K, Hu H, Ye S, Wang H, Cui R, et al. (2019) The effect of metformin therapy on incidence and prognosis in prostate cancer: A systematic review and meta-analysis. Scientific Reports 9: 2218.
- Bo S, Ciccone G, Rosato R, Villois P, Appendino G, et al. (2012) Cancer mortality reduction and metformin: A retrospective cohort study in type 2 diabetic patients. Diabetes, Obesity and Metabolism. 14: 23-29. [crossref]
- Landman GWD, Kleefstra N, Hateren KJJ, Groenier KH, Gans ROB, et al. (2010) Metformin Associated With Lower Cancer Mortality in Type 2 Diabetes: ZODIAC-16. Diabetes Care 33: 322-326. [crossref]
- Zingales V, Distefano A, Raffaele M, Zanghi A, Barbagallo I, et al. (2017) Metformin: A Bridge between Diabetes and Prostate Cancer. Frontiers in Oncology 7: 243.