DOI: 10.31038/CST.2019442
Abstract
Prostate cancer metastases are commonly seen in the skeleton, lymph nodes, lungs, or liver, and are associated with a poor five-year survival rate. Renal pelvis and ureteral metastasis are exceedingly uncommon and can present with obstructive symptoms or as an asymptomatic mass on imaging. We report the case of a 60-year-old patient who was initially diagnosed with prostate adenocarcinoma and experienced eventual metastasis to the right renal pelvis and proximal ureter. Following the diagnosis, he was started on docetaxel and pembrolizumab as part of a clinical trial protocol. A high index of suspicion and thorough metastatic work-up is necessary when patients with prostate cancer present with symptoms of obstructive uropathy or new visceral disease is identified.
Key Words
Prostate Cancer, Hydronephrosis, Ureteral metastasis, Renal Pelvis Metastasis, Metastatic Prostate Cancer
Introduction
Prostate adenocarcinoma (PCa) is currently the second most common cause of cancer in men worldwide. In 2019, there were an estimated 174,650 new cases and 31,620 deaths attributed to prostate cancer in the United States [1]. Although the incidence of low-risk prostate cancer between 2007 and 2013 decreased to 37% less than that of 2004, the annual incidence of metastatic prostate cancer (mPCa) during the same period increased by 72% [2]. At diagnosis, 12% of prostate cancer cases have spread to regional lymph nodes, and 5% have distant metastases. Distant spread portends a poor prognosis, with a 5-year relative survival rate of only 29.8% [3]. Classically, prostate cancer is associated with skeletal metastases. Visceral organ involvement is most often seen in the lungs and liver, with other sites quite rare. In this light, we report the case of a patient who was diagnosed with prostate adenocarcinoma, with eventual metastasis to the right renal pelvis and proximal ureter.
Case Report
The patient is a 60-year-old African American man who was found to have an elevated prostate specific antigen (PSA) of 34.0 ng/mL during routine screening by his primary care physician in early 2009. He did not follow-up until re-screening in 2011, at which time he was found to have a PSA of 315.86. Transrectal ultrasound guided prostate biopsy at that time revealed high-risk adenocarcinoma of the prostate with a Gleason score 4 + 5 = 9 involving 40% of the submitted tissue (Figure 1). Metastatic workup consisting of bone scan showed a questionable area in the cervical spine, but follow-up magnetic resonance imaging (MRI) revealed simply degenerative disk disease in the cervical spine and no evidence of metastatic disease. He was then started on androgen deprivation therapy with leuprolide and bicalutamide with a PSA nadir of 0.63 ng/mL in April 2012. Later that year, his PSA values began to rise, but restaging scans remained negative for metastatic disease. At this time, he received external beam radiation therapy to the pelvis, prostate, and seminal vesicles to a total dose of 7920 cGy over 4 months, completing in March 2013. Despite an initial improvement, he experienced subsequent increase in his PSA, and antiandrogen withdrawal (bicalutamide) was performed at the end of 2013.
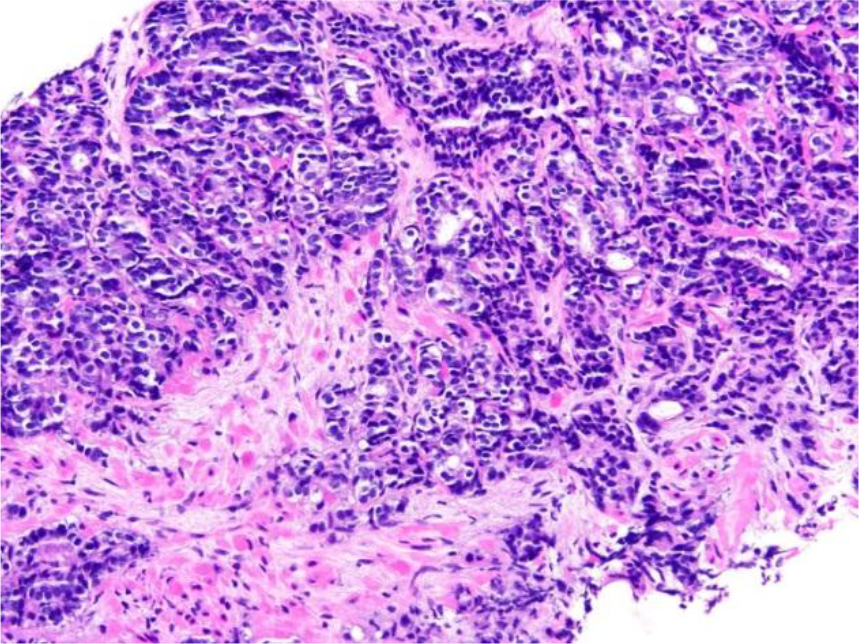
Figure 1. Prostate needle core biopsy with prostatic adenocarcinoma, Gleason score 4+5=9, involving 40% of submitted tissue (original magnification x200)
In 2014, the patient presented to the ED with gross hematuria. Computed tomography (CT) urogram was unremarkable. On cystoscopy, the bladder mucosa showed changes consistent with radiation cystitis. At this time, his PSA was 0.78 ng/mL, where it remained stable for approximately 2 years. When it rose to a level of 7.19 ng/mL in January 2017, he was started on enzalutamide. On restaging scan in July 2017, a new right renal pelvis mass was identified, which was confirmed with a subsequent MRI of the abdomen in September. The 3.7 cm enhancing mass was seen in the right renal pelvis extending and obstructing the proximal right ureter, causing severe right hydronephrosis. A contiguous 1.6 cm nodule was also seen along the inferior aspect of the right renal pelvis (Figure 2). A nephrostomy tube was placed to relieve the hydronephrosis. Ureteroscopic biopsy of this lesion was attempted in early October 2017 but was not feasible due to stenosis of his right distal ureter, likely due to his prior external beam radiation. A right ureteral stent was placed at that time with the goal of removing his nephrostomy tube. He underwent CT guided fine needle aspiration and core biopsy of this right renal pelvis mass on 10/30/17. The biopsy showed poorly differentiated metastatic prostate adenocarcinoma (Figure 3). By immunohistochemistry, the tumor cells are positive for NKX3.1, PSA and Prostein, while negative for CK7, CK20, PAX8 and GATA3, consistent with mPCa. Following this diagnosis, he was started on docetaxel and pembrolizumab as part of a clinical trial.
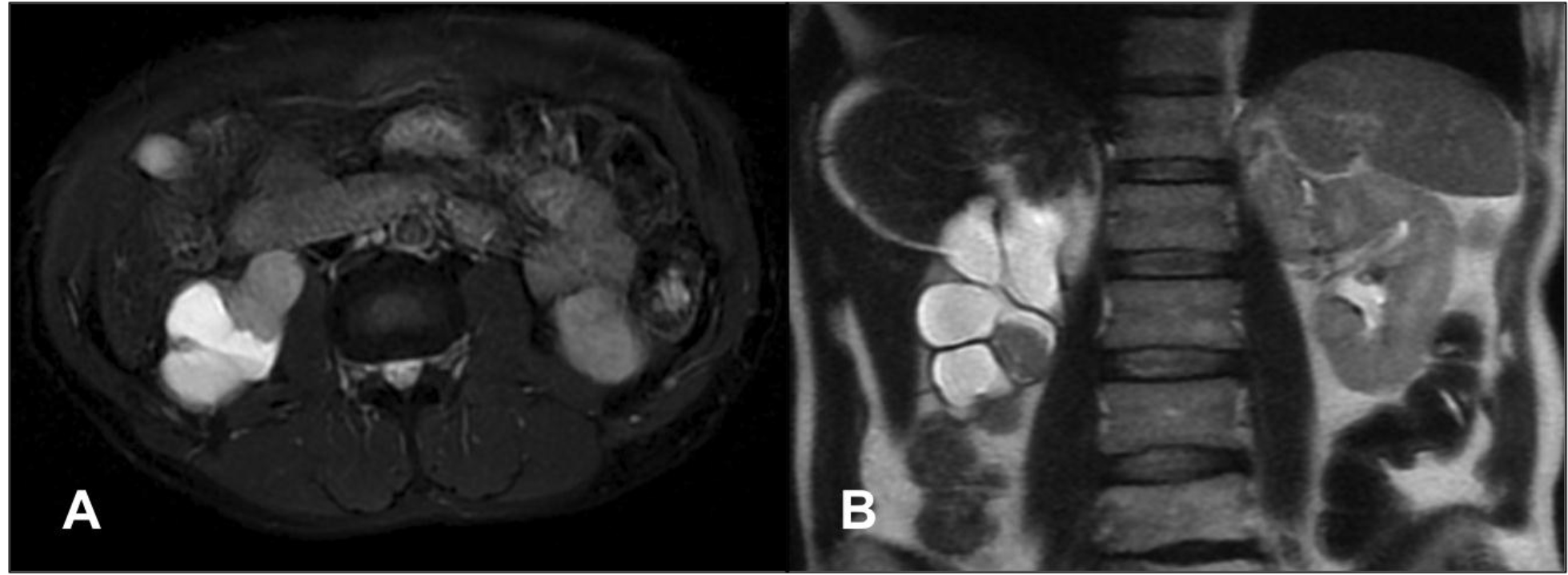
Figure 2. Transverse (A) and coronal (B) images of MRI of the abdomen revealing the right renal pelvis mass causing severe hydronephrosis of the right kidney
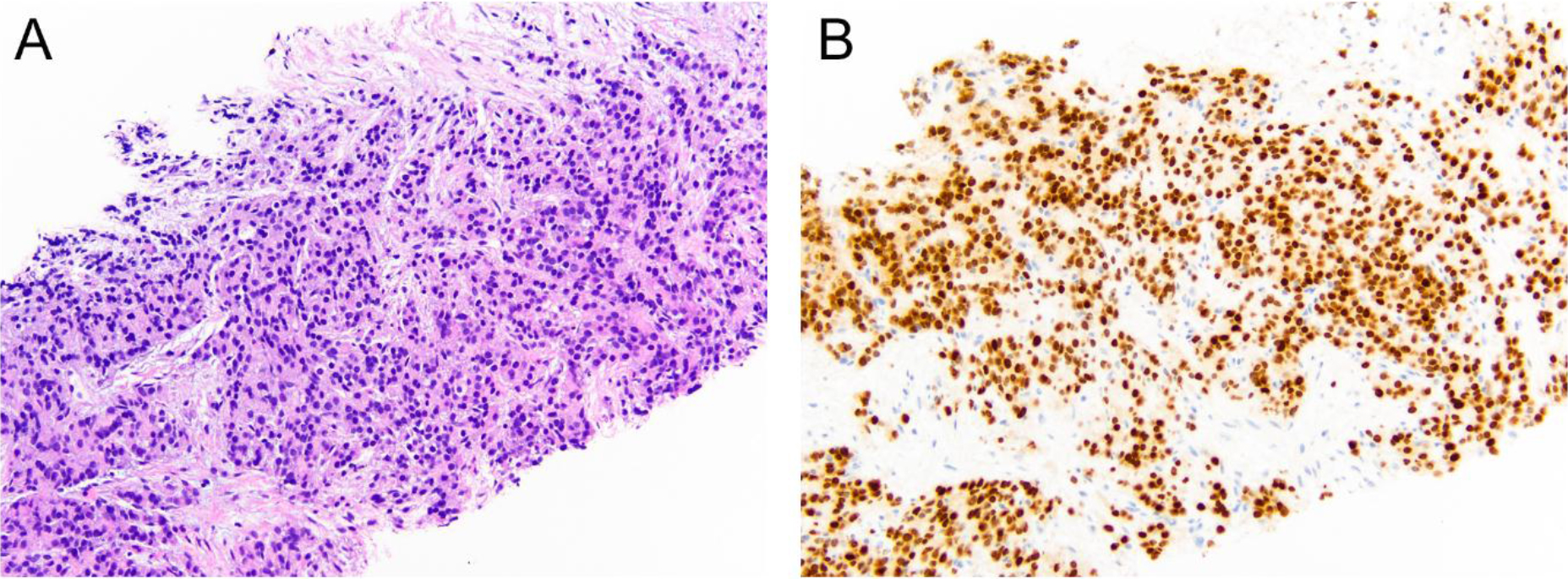
Figure 3. (A) Right renal pelvis core needle biopsy showing mPCa on H&E, confirmed by NKX3.1 immuno histochemical stain (B) (original magnification x200)
Discussion
In 74,826 patients with metastatic prostate cancer from 1998 to 2010, the most common sites of spread were to the bone (84%), distant lymph nodes (10.6%), liver (10.2%), and thorax (9.1%) [4]. At a more local level, prostate cancer can also invade the seminal vesicles, bladder, and rectum, in addition to regional lymph nodes [5]. Renal pelvis and ureteral metastases are quite rare, with less than 50 such cases reported in the literature [6]. The low incidence is hypothesized to be a result of the lymphatic circulation of the ureter is segmental and drains diagonally or transversely, with no continuous longitudinal lymphatic network draining directly from the prostatic region [7]. More commonly, metastases to the ureters and renal pelvis originate from breast, gastric, or colon cancer [8].
Before the development of advanced imaging and ureteroscopic techniques, ureteral metastases were most often incidental findings on autopsies [9]. Approximately 85% of these ureteral metastases were asymptomatic at diagnosis and most of them are identified in patients with known prostate cancer [10]. In symptomatic patients, the symptoms mimic other renal pelvic and ureteral tumors, with flank pain and hematuria being the most common. The diagnosis can be made via a traditional ureteroscopic evaluation and biopsy [11]. However, this was not feasible in our patient due to ureteral stenosis. Zhang et al. reported their experience with a similar patient having a distal ureteral stricture which prevented a biopsy done via an endoscopic approach [12]. Although a nephroureterectomy was performed in that patient given the concern for urothelial carcinoma of the ureter, the final pathology revealed mPCa. This highlights the importance of establishing a pathologic diagnosis from a biopsy when there is a possibility that a ureteral lesion is of metastatic origin.
The low incidence of mPCa to the ureters and renal pelvis is hypothesized to be a result of the lymphatic circulation of the ureter, which is segmental and drains diagonally or transversely, with no continuous longitudinal lymphatic network draining directly from the prostatic region.7 One proposed mechanism for metastasis involves dissemination of malignant cells to the retroperitoneal lymph nodes near the ureter via the periureteral lymphatic pathway [13]. Yonneau et al. further added that the secondary tumor develops from the adventitia prior to invading the ureteric wall [14]. This differs from the proposed mechanism for prostate cancer metastasis to the renal parenchyma, which is attributed to the spread of tumor emboli in the arterial system to the highly vascular kidneys [5]. Singh et al. mention preceding genitourinary tract instrumentation, including urethral catheterization or cystoureteroscopy, as a risk factor for urothelial seeding [15]. Thus, in patients with pCa and clinical signs such as hydronephrosis or symptoms such as flank pain and hematuria, workup for ureteral metastases may be warranted, especially when there is a history of prior urinary tract instrumentation.
In 2004, Milbank et al. described percutaneous resection to manage symptomatic obstruction from mPCa to the renal pelvis in two patients, a technique that resulted in complete relief of symptoms and obstruction [16]. Open operative intervention is not ideal in a patient population with metastatic disease, and angioinfarction is of unlikely benefit as these lesions are not of a parenchymal origin. Chronic internal stenting or nephrostomy tube placement are viable low-risk options, but they can compromise patients quality of life [17]. While the two patients in Milbank’s report ultimately died of mPCa, percutaneous resection may be a worthwhile consideration when performed by experienced surgeons in carefully selected patients.
Despite progression to castration resistance, a subset of patients may not harbor detectable metastasis by traditional imaging techniques, and therefore are categorized as having non-metastatic castration resistant prostate cancer (M0 CRPC). This was initially the case with our patient. Similar patients with M0 CRPC are at high risk for progression to metastatic disease, as 15% to 33% develop metastasis within 2 years [18]. In 2017, the American Society of Clinical Oncology issued a provisional clinical opinion that stated patients with radiographic evidence of metastases and minimal symptoms should be offered enzalutamide or abiraterone plus prednisone after weighing potential harms, benefits, costs, and patient preferences [19]. However, newer positron emission tomography (PET) imaging-based tracers were not used in these studies, and it is possible that a subset of these men had metastases that were not detectable by the limits of conventional CT imaging and nuclear bone scan [18]. This may have been the case in our patient, who eventually developed metastasis to the renal pelvis within a year of starting enzalutamide.
Conclusions
A small portion of men with advanced prostate cancer may develop metastasis to atypical sites, such as the renal pelvis and ureter. Thus, oncologists must have a high index of suspicion when prostate cancer patients are found to have ureteral/renal pelvis masses causing hydronephrosis. Biopsy of the metastatic site should be considered if extirpative surgery, or a change in hormonal/systemic therapy, is being considered [20]. While a considerable amount of research has been devoted to the treatment of mPCa, the response of these treatments in the setting of metastatic disease to the renal pelvis and ureter is not clear.
Funding: This work is supported by a grant from the National Cancer Institute (P30CA072720).
Disclosure: EA Singer receives research support from Astellas/Medivation.
References
- Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, CA Cancer J Clin 69: 7–34.
- Weiner AB, Matulewicz RS, Eggener SE, Schaeffer EM (2016) Increasing incidence of metastatic prostate cancer in the United States (2004–2013). Prostate Cancer Prostatic Dis 19: 395–397.
- Institute NC. (2018) Cancer stat facts: prostate cancer. Surveillance, Epidemiology, and End Results (SEER) Program Research Data (1973–2015).
- Gandaglia G, Abdollah F, Schiffmann J (2014) Distribution of metastatic sites in patients with prostate cancer: A population-based analysis. Prostate 74: 210–216.
- Khan F, Mahmalji W, Sriprasad S, Madaan S (2013) Prostate cancer with metastases to the kidney: a rare manifestation of a common disease. BMJ Case Rep.
- Morales I, Bassa C, Pavlovic A, Morales C (2016) Ureteral Metastasis Secondary to Prostate Cancer: A Case Report. Urol Case Rep 5: 4–5.
- Zhang T, Wang Q, Min J (2014) Metastasis to the proximal ureter from prostatic adenocarcinoma: A rare metastatic pattern. Can Urol Assoc J 8: E859–861.
- Haddad FS (1999) Metastases to the ureter. Review of the world literature, and three new case reports. J Med Liban 47: 265–271.
- Schneider S, Popp D, Denzinger S, Otto W (2012) A rare location of metastasis from prostate cancer: hydronephrosis associated with ureteral metastasis. Adv Urol 656023.
- Hulse CA, O’Neill TK. (1989) Adenocarcinoma of the prostate metastatic to the ureter with an associated ureteral stone. J Urol 142: 1312–1313.
- Otta RJ, Gordillo C, Fernandez I (2015) Ureteral metastasis of a prostatic adenocarcinoma. Can Urol Assoc J 9: E153–155.
- Zhang D, Li H, Gan W (2016) Hydronephrosis associated with ureteral metastasis of prostate cancer: A rare case report. Mol Clin Oncol 4: 597–598.
- Chung HS, Kim MS, Cho YH (2017) A rare presentation of metastatic prostate cancer, initially a suspect for urothelial cell carcinoma of the ureter: a case report. BMC Urol 17: 37.
- Yonneau L, Lebret T, Herve JM, Barre P, Lugagne PM, et al. (1999) [Isolated ureteral metastasis of prostatic adenocarcinoma. Apropos of a case]. Prog Urol 9: 118–121.
- Singh G, Tiong HY, Kalbit T, Liew L (2009) Urothelial metastasis in prostate adenocarcinoma. Ann Acad Med Singapore 38: 170–171.
- Milbank AJ, Savage SJ, Angermeier KW, Ng CS, Streem SB (2004) Metastatic cancer to the renal pelvis: a novel approach to management. Urology 64: 807–809.
- Farber N, Salib A, Shinder B, Modi P, Elsamra S, Singer E (2017) American College of Surgeons Evidence-Based Decisions in Surgery: Management of Malignant Upper Urinary Tract Obstruction. Committee on Surgical Palliative Care.
- Ritch C, Cookson M (2018) Recent trends in the management of advanced prostate cancer. F1000Res 7.
- Virgo KS, Basch E, Loblaw DA (2017) Second-Line Hormonal Therapy for Men With Chemotherapy-Naive, Castration-Resistant Prostate Cancer: American Society of Clinical Oncology Provisional Clinical Opinion. J Clin Oncol 35: 1952–1964.
- Mottet N, Bellmunt J, Bolla M (2017) EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 71: 618–629.