Abstract
Objectives: Insects arose in the terrestrial environment. Some species eventually evolved forms that could take advantage of freshwater habitats particularly in the larval stage. The numbers of species capable of surviving in saline is rare (about 5% of all known mosquito species). Most plants do not require sodium but animal however require sodium for some physiological function for example nervous activity. Salinity and certain ions such as sodium and sulphate challenges for insects are important. Other somatically active osmolytes occur in the blood of many insect. The evolutionary advantages of flight for mating and dispersal led more species to retain terrestrial or aerial adult stage. This creates substantial physiological problem for freshwater and terrestrial animals. The insects got around this problem by reducing the amount of sodium in the body to an essential minimum. From a water hardness/salinity perspective, arthropods occupy very soft water up to salt lakes twice as concentrated as seawater.
Materials and methods: To provide authentic information about this novel we use reliable data on academic resources such as Google Scholar, Scopus, Web of Science, Springer, Pro Quest, Wiley Online, Science Direct, Research Gate, PubMed, Sage, and SID.
Results: Only a limited number of species thrive in saline lakes, including some fairy shrimp (e.g., Artemia, Parartemia, and Phallocryptus) and brine and shore flies (Diptera, Ephyridae), such as Ephydra hians.
Discussion: Mechanisms of survival of Ephydra hians will provide a guideline for environmental and ecosphere creatures for environmental adaptation.
Keywords
Ephyda hians, Environment adaptation, Osmoregulation habit
Mini Review
Insects capable of surviving in saline water intensively studied are: Culicidae (mosquitoes) and Ephydridae (brine flies). Brine fly is classified in Phylum: Arthropoda, Class: Insecta, Order: Diptera, Family: Ephydridae, Genus: Ephydra, Species: Ephydra hians. Ephydra hians, commonly known as the alkali fly. Both salinity and desiccation lead to dehydration and osmotic stress, which is a critical problem at the cellular level [1-3]. Therefore, salinity and desiccation stress in insects trigger common physiological mechanisms, mainly aimed at increasing water content (e.g. drinking from the medium), avoiding its loss (e.g. control of cuticle permeability) and maintaining ionic homeostasis (e.g. activity of Malpighian tubules and specialized parts) [1,4-6]. Ecology of Brine fly: Among aquatic insects, members of the shore fly family Ephydridae are well-known for their tolerance of severe environmental conditions. High temperatures and salinities, acid and alkaline pH, anoxia and ephemeral waters are among the factors to which a variety of ephydrids have become adapted. Hot springs, tidal splash pools, salt evaporation ponds, hypersaline desert lakes, and even crude petroleum have been described as larval habitats Collection records for species in the genus Ephydra, which are common in saline waters, indicate a wide range in chemical composition and salinity is tolerated by this group, but that any one species tends to be restricted to a particular type of habitat water chemistry [1,7-10]. The adult flies live 3-5 days, during which they eat algae and lay eggs in the hypersaline water; (Figure 1) lays eggs in saline water (Figure 2). The larvae are important for their capacity to survive in highly saline water (Figure 3). Concretions of calcium carbonate in the Malpigian tubules make the larvae more dense and allow them to stay on the beneath of the water. The haemolymph had a total osmotic concentration of about 300 mOsm. The osmotic concentration of the lake was well over 1500 mOsm. It follows that the larvae must lose water across the cuticle. The larvae drink the external medium. The very high concentration sodium, carbonate and sulphate are present in the lake. The lime gland acts like a kidney, by removing carbonate ions from the blood. Inside the glands carbonate is mixed with calcium to form a limestone (Figures 4 and 5). Saline water larvae drink equal to about their body volume every 10 hours. Table 1 shows different ions in the heamolymph of larvae in comparison to Mono lake water. Eggs of the genus Ephydra are attached to an algae mat. The main food sources of adults and larvae are algae, some bacteria, and protozoa.

Figure 1: Adults of brine fly (Ephydra hians)
Figure 2: Egg of brine fly (Ephydra hians)

Figure 3: Larvae of brine fly (Ephydra hians)
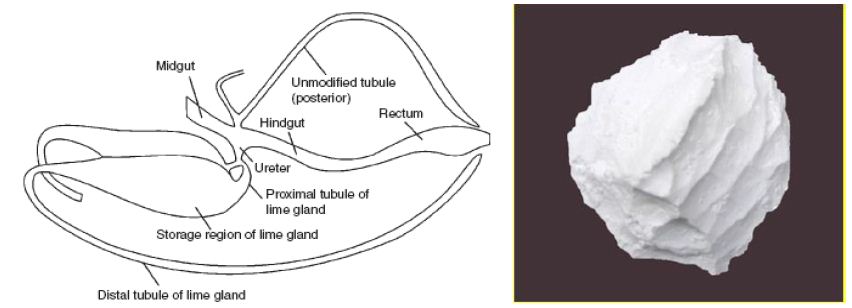
Figure 4: Internal organs of the brine fly (Ephydra hians) with limestone
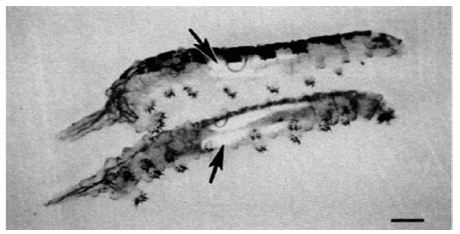
Figure 5: Lime gland of larvae
Table 1: Ionic concentration in the larval haemolymph of Ephydra hians
|
Ion |
Concentration in larval haemolymph |
Concentration in Mono lake water |
| Sodium
Potassium Calcium Magnesium Chloride Sulphate |
135.7 ± 1 6.9 ± 0.4 5.6 ± .0.1 13.2 ± 0.5 120 ± 1.4 0.6 ± 0.1 |
1224 18 ≤1 ≤1 627 151 |
Larvae change to the pupae (Figure 6). Pupae are non-feeding, and sequestered from the aquatic environment, Pupae then to the adults. Adults emerge from water in a large population (Figure 7). Birds understand the time of adult emergence and reach the lake for catching them as a food. Many migrating birds stop at Lake during their trips to feed on the brine fly larvae in the lake water (Figure 8).

Figure 6: Pupae of brine fly (Ephydra hians)

Figure 7: Emerging of brine fly from saline water lake

Figure 8: Emerging of adult of brine flies as a good food source for immigrant birds
In a research conducted on the mosquitoes, they found that Aedes albopictus was the most tolerant species, followed by Anopheles coluzzii, Ae. aegypti, Culex. quinquefasciatus, and An. gambiae, in decreasing order. Cx. pipiens was the least tolerant species [11].
Conflict of Interest
The author declares that there is no conflict of interest.
Acknowledgments
This research is financially supported by Ministry of Health and Medical Education under code number of NIMAD 995633.
References
- Bradley T (2009) Animal Osmoregulation. New York: Oxford University Press.
- Cohen E (2012) Roles of aquaporins in osmoregulation, desiccation and cold hardiness in insects. Entomology, Ornithology and Herpetology 1: 1-17.
- Evans DH (2008) Osmotic and Ionic Regulation: Cells and Animals. Boca Ratón:CRC Press.
- Dow JAT, Davies SA (2006) The Malpighian tubule: rapid insights frompost–genomic biology. Journal of. Insect Physiology 52: 365-378. [crossref]
- Gibbs AG, Rajpurohit S (2010) Cuticular lipids and water balance. In Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology (ed. G. J.Blomquist and A. G. Bagnères), pg: 100-120. Cambridge: Cambridge University Press.
- Larsen E.H, Deaton LE, Onken H, O’Donnell M, Grosell M, et al. (2014) Osmoregulation and Excretion. Comprative Physiology. 4: 405-573.
- Brock,TD, Brock ML (1968) Life in a hot-water basin. Natural History 77: 47-53.
- Brock ML, Wiegart RG, Brock TD (1969) Feeding by Paracoenia and Ephydra (Diptera: Ephydridae) on the microorganisms of hot springs. Ecology 50: 192–200.
- Foley C, White B (1989) Occurrence of Ephydra hians Say (Diptera: Ephydridae) in deep water in Mono Lake, California. Bulletin of South California Academic Science 88: 40-41.
- Herbst D (1988) Comparative population ecology of Ephydra hians Say (Diptera: Ephydridae) at Mono Lake (California) and Abert Lake (Oregon). Hydrobiologia 158: 145-166.
- Kengne P, Charmantier G, Blondeau‐Bidet E, Costantini C, Ayala D (2019) Tolerance of disease‐vector mosquitoes to brackish water and their osmoregulatory ability. Ecosphere 10: 1-14.