Abstract
Objective: To measure the in vitro concentration of potassium ions (K+) on the opposite side of the dentine samples in real-time, using Potassium Ion Selective Electrodes (K+ ISE).
Method: An assembly array was designed and different sections (n=4) of dentine were acid etched in 6% citric acid and ultra-sonicated for five minutes. They were subsequently fixed into a polypropylene tube using impression dental material and immersed into simulated dentine fluid (SDF) which consisted of 0.01 mol/dm3 potassium chloride (KCL) solution. The K+ ISE was placed external to the polypropylene tube, to measure changes in the potassium ion concentration [K+] in the SDF for 60 hrs continuously. Application of potassium nitrate (KNO3) solutions of concentrations between 0.05-3 mol/dm3 were applied to the exposed dentine samples in separate experiments.
Results: Minimal changes in [K+] (<5 mmol/dm3) in the SDF (the opposite side of the sample) were measured by the K+ ISE when applying less than 600 mmol/dm3 of KNO3 solutions to the dentine sections. However, significant changes (P<0.05) in [K+] of 5-25 mmol/dm3 were measured in the SDF when applying a KNO3 solution of concentration more than 600 mmol/dm3 to the dentine samples, indicating greater penetration of K+ through the dentine matrix.
Conclusion: ISE may be used to measure ionic transfer in dentinal tubules although questions arise due to the sensitivity of the ISE and its suitability. However, it was demonstrated that increasing the concentration of an applied KNO3 solution to exposed dentine increased the [K+] in the SDF which was on the opposite side in the dentine disk model and premolar tooth with a cut cavity. This was also the case when the fluid flow was in the opposite direction to diffusion. This study demonstrates that ISE may be suitable for real-time diffusion experiments in dentine for possible future research into dental therapeutics which involve ion exchange.
Keywords
Potassium ion diffusion, Potassium ion concentration, Ion selective electrodes, Potassium nitrate, Dentine
Introduction
The use of Ion Selective Electrodes (ISE) in dental research has become prevalent to measure real-time ionic transfer processes. The method has been used to measure ion releases from restoratives and adhesives to prevent enamel demineralisation by measuring fluoride ion release [1] and the effect of calcium release from enamel in the presence of a solution containing zinc ions [2]. This method has not been used to measure the penetration of ions through the dentine matrix despite some possible limitations when using these electrodes [3]. Therefore, potassium ion concentration [K+] could theoretically be measured using ISE although there are no published in vitro studies where changes in [K+] on the opposite side of the dentine were measured using ISE methodology.
Measurement of ionic processes in dentine may be of interest as it is accepted that the prevailing hypothesis for nerve desensitisation by potassium ions (K+) is a direct diffusion mechanism. It has been demonstrated that K+ does not relieve dental pain by tubular occlusion to prevent dentine fluid flow [4-9] despite this theory remaining an enigma [10]. Potassium toothpaste and mouthwash formulations are effective at reducing dental pain associated with dentine hypersensitivity (DH) in humans [11-13]. A review by Orchardson and Gillam was undertaken to evaluate their efficacy and demonstrated numerous studies in which pain was reduced after the application of potassium-containing desensitisers [14]. Although the concentration of K+ may initiate depolarisation of the nerve fibres, the nerve fibres are unable to repolarise due to an imbalance of sodium and potassium ions [7,8,15]. However, this hypothesis has been criticised by some investigators as these experiments were ‘dissimilar’ to the clinical reality [16], and other investigators have also hypothesised that K+ may directly diffuse to desensitise the intra-dental nerves [17,18].
Stead et al. provided a mathematical framework of potassium diffusion in the clinical environment based on known parameters of the various constituents of the dentine tubule (DT) complex [19]. Furthermore, Stead et al., reported that a one-minute application of 500 mmol/dm3 of K+ increased the concentration of K+ at the inner end of the tubules for 20-30 minutes. However, this does not appear to be the case in the clinical environment, where users of potassium toothpaste would not usually brush each tooth for a minute [19]. According to this model, potassium-containing desensitisers may be able to exert a transient effect and this was demonstrated by Matthews and co-workers in the cut cavity model in humans [11,13].
Despite all these studies, the mechanism of K+ appears to work by a direct diffusion mechanism (simple concentration gradients). Numerous animal studies have also been conducted confirming the effect of K+ on intra-dental nerve activity [6, 20-23]. However, only a few in vitro K+ diffusion experiments have been conducted, purporting to minimal diffusion of K+ through the dentine matrix [24]. The present study was conducted to determine if sufficient diffusion K+ can occur through the DT in vitro and influence [K+] on the opposite side of the sample. This study was conducted to measure dentine permeability to K+ in real-time and whether ISE was suitable for such measurements; if suitable, then further studies can be conducted when K+ is combined with other anions as are the case in potassium-containing dental products.
Material and Methods
The present study was similar though not identical to previous historical experiments [11, 13, 24]. The objective was to assess whether ISE was suitable for measuring ion penetration in the dentine matrix, in this case, K+ as there was abundant in vivo literature on the effect of K+ ions on intradental nerve activity.
Experimental Design and Setup
The setup is displayed in Figure 1 and a flow diagram of the method is in Figure 2. The setup in Figure 1 was sufficient to undertake in vitro experiments to determine if ISE could measure the penetration of K+ through dentine in real-time. Experiments were also conducted where the flow was in the reverse direction (away from the pulp) and K+ diffusion was towards the pulpal side. The setup in Figure 1 was constructed from an acrylic stage and polypropylene tubing with the K+ ISE. In each case, the dentine model under investigation was fixed into the polypropylene tube using a polyvinyl siloxane impression material (Type 3 Part no. 28418, Extrude Kerr Corporation, USA). It was important to expose the dentine sections on both sides to allow the passage of K+ from the polypropylene tube, through the dentine sample and into the beaker containing simulated dentine fluid (SDF) [25]. The polypropylene tube was then immersed into the beaker containing 40 ml of SDF which in turn was immersed in a water bath (MX07H135-A12E, Polyscience, Illinois, USA) at 25°C to maintain a constant temperature during the experiments. The potassium nitrate (KNO3) solution was placed in the polypropylene tube on top of the dentine sample, 16 cm above the sample, to mimic flow and diffusion in the same direction and this flow was due to gravity. In separate experiments, a volume whose height was 1 cm above the sample was used to mimic flow from the SDF solution into the polypropylene tube and diffusion of K+ in the opposite direction (for practical reasons, it was not possible to increase the height differential more than 1 cm in this direction and only 1 ml in volume was used instead). The assembly was based on the designs of Uddin’s et al. [26] electrochemical cell, as this was sufficient to undertake permeability experiments with a K+ ISE. The beaker containing the SDF was covered with a 3D printed lid (Wanhao Duplicator i3 Plus V2, Dorchester, Dorset, UK) to prevent evaporation of the SDF solution. Also, the K+ ISE (ELIT8031, Nico 2000, Harrow, London, United Kingdom) was placed in the beaker containing the SDF only as it was necessary to measure the changes of [K+] in the external SDF solution which always had an initial concentration of 0.01 mol/dm3 potassium chloride (KCl) solution.
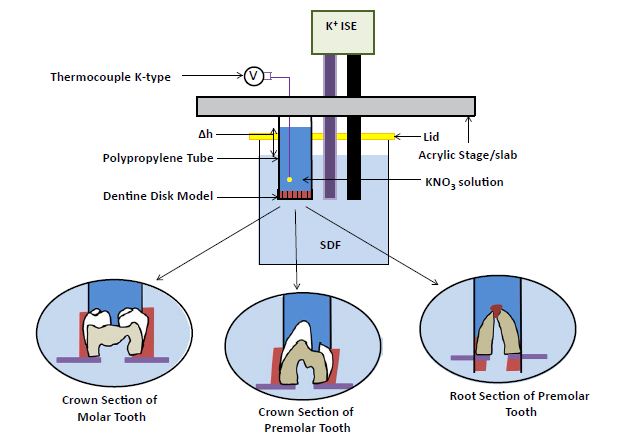
Figure 1: Schematic of the experimental setup in measuring the change in the [K+] in the SDF with K+ ISE in the four dentine models immersed into 40 ml SDF
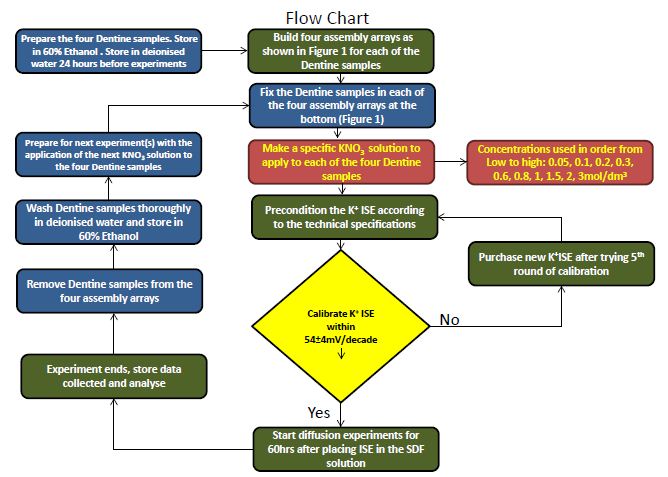
Figure 2: Flow diagram of the Methodology used in the study
Solution Preparation
Experiments were conducted by changing the concentration of the KNO3 solution placed in the polypropylene tube. All chemicals used were obtained from Sigma Aldrich (St Louise, Missouri, US) with at least 99.9% purity. The chemicals used in this investigation were KNO3 salt (CAS number 7757-79-1, Product code: 221295) and KCl salt (CAS number 7447-40-7, Product code: P9333). The concentrations of the KNO3 solution placed in the polypropylene tube for each separate experiment on each of the dentine samples were 0.05, 0.1, 0.2, 0.3, 0.6, 0.8, 1, 1.5, 2, 3 mol/dm3. The concentration of KCl in the SDF was 0.01 mol/dm3, which was a calculated median value of actual values in the published literature. 2.5 mol/dm3 of NaCl was added to the SDF solution as a buffer in the preliminary experiments. It was determined that this was not always necessary due to the electrodes used in this study where K+ ISE had a lithium acetate reference electrode which had an “equi-transferrent filling solution”, i.e., both ions have the same, or nearly the same, mobility – e.g. lithium acetate, or potassium nitrate). These experiments with the ISE were calibrated and conducted following the technical specifications associated with the ISE.
ISE Preparation and Calibration
The K+ ISE was accompanied by a universal reference electrode; a lithium acetate double junction reference electrode (ELIT003n Nico 2000, Harrow, London, United Kingdom). These electrodes were connected to a dual-electrode head (ELIT 201, Nico2000, Harrow, London, United Kingdom). The diameters of these electrodes were 8 mm and had a length of 130 mm and the head was connected to a 4-channel ion analyser (ELIT4, Nico 2000, Harrow, London, United Kingdom) which recorded the voltage between the K+ ISE and the reference electrode in a specific concentration of the potassium-containing solution (KCl). The electrodes were calibrated by making specific concentrations of the KCl solutions and recording the voltage from a data logger (ELIT 4). The calibration of the K+ ISE was conducted from highly concentrated solutions to low concentrated solutions. This was completed by pouring specific volumes of 10, 20, 40, 60, 80 and 100 mmol/dm3 of the KCl solutions in separate beakers and placing the K+ ISE with its Li reference electrode in each of the beakers to obtain a voltage (mV) reading from each beaker. Log concentration against voltage was plotted and the gradient, according to the K+ ISE technical specification, should have been 54±5 mV. The calibration of the ISE was performed before the commencement of the 60 hr experiments for each of the four dentine samples and at each of the concentrations of the KNO3 solutions applied in the polypropylene tube on the exposed areas of the dentine. After each experiment, the electrodes were rechecked against the standard to ensure that the electrodes were in good calibration, which in all cases was within 54±5 mV. The reference electrode and K+ ISE were preconditioned and stored according to the instructions on the ISE technical specification. According to the technical specifications, the lowest concentration change that could have been detected by the K+ ISE was 0.01 mmol/dm3 and the maximum was 100 mmol/dm3. All experiments that were conducted could therefore only measure changes in K+ concentration between 0.01-100 mol/dm3. The calibration of the ISE also occurred in samples of the concentration mentioned above (see Figure 3). Also, to prevent pH changes, a buffer solution was used which involved adding 2 ml of 2.5 mol/dm3 NaCl in the plastic tube and the SDF per 100 ml of solution. In the preliminary experiments, the highest ionic strength of the measuring solution after 60 hrs of KNO3 in the SDF was at times more than 0.01 mol/dm3. However, it was not always very large and the necessity of the NaCl buffer was questionable although was used in all main experiments.
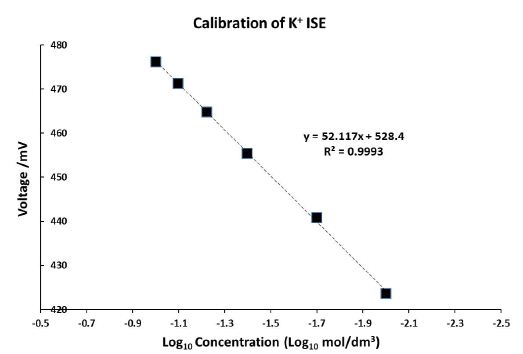
Figure 3: Calibration of the K+ ISE with the gradient 52.117 mV/decade between 0.01 and 0.1 mol/dm3 [K+]
Simulating Flow
Experiments were conducted to simulate fluid flow by changing the volume of the KNO3 solution in the polypropylene tube above the dentine sample [11, 13, 27]. To simulate SDF flow into the polypropylene tube from the beaker, the height Δh (Figure 1) was changed so that it was negative with reference to the volume and height reached by the SDF in the beaker. The volume of the KNO3 solution in the plastic polypropylene tube was 1 ml in this case. The SDF should flow from the beaker into the plastic polypropylene tube whereas the [K+] in the polypropylene tube was greater than that in the SDF. This will simulate the inward diffusion of K+ and the outward fluid flow from the sample. To simulate flow and diffusion direction from the polypropylene tube and through the sample into the SDF, the application of the KNO3 solution at a higher pressure was necessary. The height of the KNO3 solution in the plastic polypropylene tube above the SDF levels Δh was 16 cm, which in this case would have led to flow due to gravity, as was similar to previous experiments conducted [28] although the SDF was not pressurized to simulate pulpal pressure. This effect was undertaken to deliberately ensure that a positive concentration change was measured in the SDF and then compare this to when the flow was reversed i.e., from the SDF through the dentine sample, into the polypropylene tube whilst diffusion occurred from the polypropylene tube, through the dentine sample and then into the SDF. It must be noted this was done simply to measure penetration of K+ through the dentine sample and it was not modelling pulpal pressure. The volume of SDF (containing 0.01 mol/dm3 KCl) in the beaker was 40 ml for the experiments with each of the four dentine sections.
Dentine Sample Preparation
Ethics approval was obtained (Ethical code: QMRECC200014/17) to enable the investigators to acquire human teeth from the Royal London Dental Hospital Tooth Bank. The dentine samples used were 1) dentine disks; 2) a mandibular premolar tooth with a crown section and a cut cavity; 3) a root section from the same mandibular premolar tooth and 4) a crown section of a molar tooth with a cut cavity. 0.5 mm thick mid-coronal dentine disks were sectioned from human mandibular premolar teeth using a diamond saw (Struers Accutom-5, Willich, Germany) and initially acid etched in 6% citric acid for two minutes to remove the smear layer. A mandibular premolar tooth was sectioned into two pieces: the crown section and the root section; a 2 mm diameter cavity was made from the occlusal surface of the crown section using a dental bur until the dentine was exposed from the mid coronal section; the roots were sectioned using a diamond saw (Struers Accutom-5, Willich, Germany) so that the tubules were exposed on the mid coronal sides. The whole crown section was acid etched in 6% citric acid for two minutes. The cementum from the roots section used in experimentation was removed by polishing with a polishing paper (P-1000) and both were acid etched in 6% citric acid for two minutes. The apex of the root was sealed with a polyvinyl siloxane impression dental material (Type 3 Part no. 28418, Extrude Kerr Corporation, USA). The root of a maxillary molar tooth was removed and sectioned along the cervical region and a crown section was treated in the same way that the crown section of the premolar tooth was and having the same diameter of the cavity. All samples were ultra-sonicated in deionised water for five minutes after acid etching and stored in 60% ethanol and immersed in deionised water for 24 hours before experimentation. All samples were also thoroughly rinsed in deionised water before the start of each experiment. After each experiment, the samples were thoroughly washed with deionised water and stored in 60% Ethanol and then immersed again in deionised water for 24 hours before the next set of experiments. Experiments were also conducted on the application of low to high concentrations of the KNO3 solutions.
Statistical Test Method
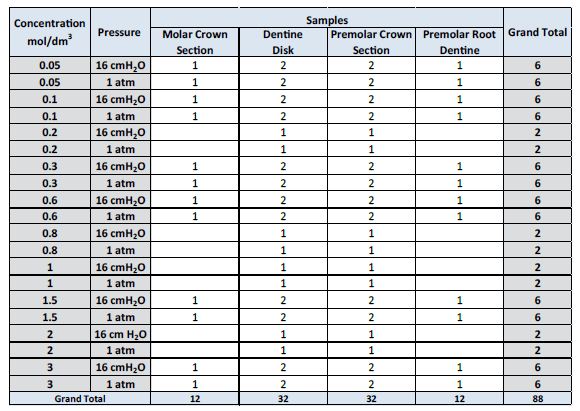
88 experiments were conducted and all experiments were categorised into two groups; Group 1 (experiments conducted for applications of the KNO3 solution to the four samples being <0.6 mol/dm3) and Group 2 (≥0.6 mol/dm3). This grouping was undertaken as it was reported in the literature; that 0.5 mol/dm3 of KNO3 relieved sensitivity (DH) and most dental products containing K+ have these concentrations [14]. Therefore, it was hypothesised that the ISE should be able to measure the difference between these two groups and that the larger the concentration of KNO3 applied, the larger the change in concentration in the SDF. The statistical test to determine the statistical difference between Group 1 and 2 used was a two-tailed Student T-test assuming unequal variance. 32 experiments were conducted with the dentine disk, 32 experiments were conducted with the crown section of a premolar tooth, 12 experiments were conducted with the premolar root section and 12 experiments were conducted with the molar crown section with a cut cavity. Table 1 displays the breakdown of each of these experiments conducted.
Table 1: Breakdown of experimentation conducted with each of the four dentine samples.
Results
Before the commencement of each experiment, the K+ ISE was calibrated. When plotting Log10 concentration as a function of voltage, the gradient was always 50-54 mV/decade, in agreement with the technical specifications of the K+ ISE. Otherwise, the K+ ISE was recalibrated or replaced with a new K+ ISE; the K + ISE was replaced after 5 failed attempts of recalibration, which only occurred once in all experiments conducted. Figure 3 demonstrates an example of the calibration of the ISE where it can be observed that the gradient was 52.117 mV/decade. Also, Figure 4 displays the real-time measurement of the potassium ion concentration in the SDF after diffusion has occurred for two different concentrations of KNO3 applied to the dentine disk model over 60 hrs.
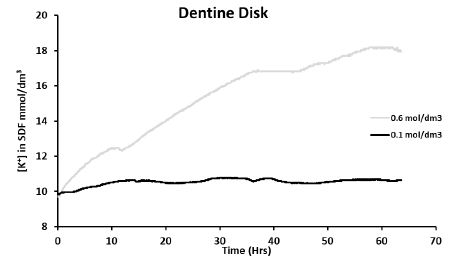
Figure 4: Preliminary experiments: real-time measurement of [K+] in SDF when applying 0.6 mol/dm3 and 0.1 mol/dm3 in separate experiments to the dentine disk model at 16 cmH2O
In Figure 4, it can be observed that there was a large increase in the [K+] in the SDF (initially containing [K+] ≈ 0.01 mol/dm3) when applying the KNO3 solution of 0.6 mol/dm3 (compared to 0.1 mol/dm3) at 16 cmH2O to the dentine disk. The pressure, at which the KNO3 solution was applied, also appeared not to affect the change in [K+] in the SDF.
For all experiments conducted, statistically significant changes (P<<0.05), were measured in the [K+] in the SDF when concentrations of the KNO3 solution applied to any of the samples were more than 0.6 mol/dm3 (equivalent to 600 mmol/dm3). This was most statistically significant for the dentine disk model where the mean [K+] in the SDF for Groups 1 and 2 were 0.106 mmol/dm3 and 13.615 mmol/dm3 respectively after 60 hrs. In the premolar crown tooth, the mean for Group 1 and 2 were -0.415 mmol/dm3 and 4.063 mmol/dm3 respectively after 60 hrs and there was a significant difference between the two groups (P=0.0002). In the premolar root section, the mean measurement for Group 1 concentration in the SDF and Group 2 concentration in the SDF were -2.351 mmol/dm3 and 8.149 mmol/dm3 respectively, with a significant difference between the two groups (P=0.041). However, when applying the KNO3 solutions of concentrations more than 0.6 mol/dm3 to the molar crown section, there were no statistically significant differences observed between Groups 1 and 2 (P=0.1). In addition, the [K+] in the SDF was not significantly different (P=0.96) when the KNO3 solution was applied to the four samples at 16 cmH2O or atmospheric pressure.
Figures 5a and 5b show that there was significant diffusion of K+ through the dentine disk and the premolar crown sections of dentine when applying larger (more than 0.6 mol/dm3) concentrations of the KNO3 solution. This resulted in an increase in the [K+] in the SDF between 4 and 20 mmol/dm3 for the dentine disk and between 3 and 10 mmol/dm3 for the premolar crown section. However, it must be acknowledged that at times there was no increase in [K+] in the SDF, for example, when applying 2 mol/dm3 of KNO3 at 16 cmH2O to the premolar crown section which only increased the [K+] in the SDF by 0.6 mmol/dm3 (Figure 5b). This was also the situation when applying the 3 mol/dm3 KNO3 solutions to the premolar root dentine in Figure 5c (which produced a change in [K+] in the SDF of 1-1.5 mmol/dm3) and applying 0.6 mol/dm3 and 3 mol/dm3 KNO3 solutions to the crown section of molar tooth (Figure 5d). However, the application of concentrations of the KNO3 solution less than 0.6 mol/dm3 did not produce any significant changes in the [K+] in the SDF for all four samples. Occasionally there was a decrease in the [K+] in the SDF which was unexpected and could only be attributed to electrode drift. In the dentine disk, there was generally an increase in [K+] in the SDF of about 0-2.5 mmol/dm3. In the premolar crown section of the tooth, this increase was only between 0-0.8 mmol/dm3. However, it can be observed from Figures 5c and 5d for the premolar root dentine and a molar with a cut cavity that there was a reduction in the [K+] in the SDF at applications of KNO3 less than 0.6 mol/dm3. The results in the Dentine disk model and the Premolar with a cut cavity could not be reproduced in the molar tooth with a cut cavity or in the root section.
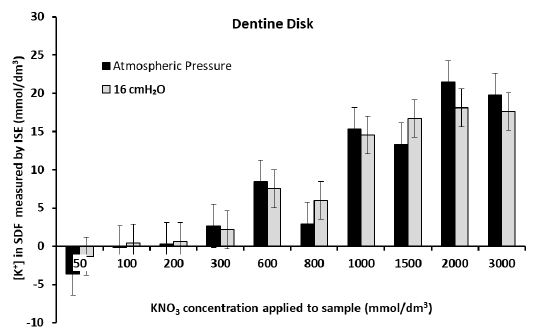
Figure 5a: Measurement of the final [K+] in the SDF after 60 hrs for each application of KNO3 solution to Dentine Disk
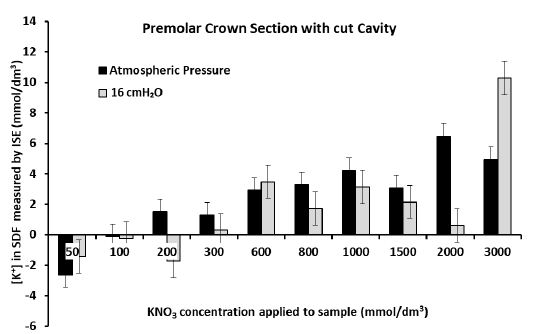
Figure 5b: Measurement of the final [K+] in the SDF after 60 hrs for each application of KNO3 solution to the crown section of a premolar tooth with a cut cavity
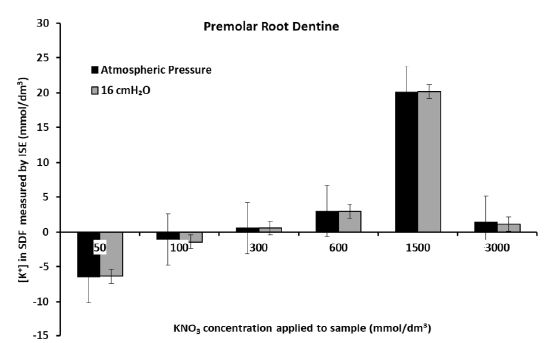
Figure 5c: Measurement of the final [K+] in the SDF after 60 hrs for each application of KNO3 solution to the premolar root dentine section
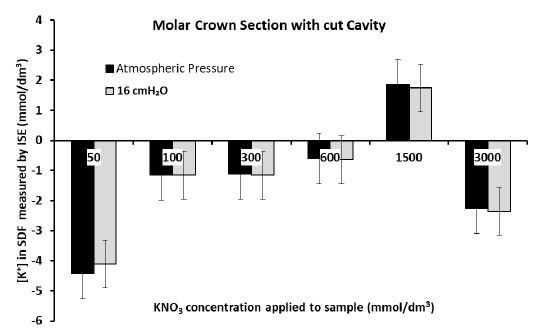
Figure 5d: Measurement of the final [K+] in the SDF after 60 hrs for each application of KNO3 solution to the crown section of a molar tooth with a cut cavity
Discussion
This study aimed to determine whether K+ ISE was suitable for the detection of potassium ion concentration [K+] changes on the opposite side of the dentine samples in real-time. Experiments were conducted to deliberately measure a positive change in [K+] in which both flow and diffusion were in the same direction. The flow was then reversed in the opposite direction to K+ diffusion in separate experiments. Changes in the [K+] in the SDF were measured electrochemically using a K+ ISE. It can be observed in Figure 5a-d that the pressure at which the KNO3 solution was applied to the dentine does not affect the changes in [K+] in the SDF. The diffusion of K+ also does not appear to be affected by the flow rates. This may be consistent with the in vivo observations of Ajcharanukul et al., [11] and Noparatkailas et al., [13] despite the modelling of the oral environment not being the main objective in this study. If the oral environment were modelled, the SDF would have to be enclosed in a chamber at 20 cmH2O above atmospheric pressure [24]. The experiments conducted simply demonstrate that a concentration gradient leads to the penetration of K+ through the dentine matrix as measured by the ISE method in real-time instead of taking a measurement every 60 seconds for 20 minutes [24]. However, application of a KNO3 solution of less than 0.6 mol/dm3 applied to the dentine disk and premolar with a cut cavity appeared to on average produce insignificant diffusion of K+, producing less than 10 mmol/dm3 increase in the [K+] of the SDF. It appears that when applying 0.6 mol/dm3 or more of a KNO3 solution to the dentine disk and a crown section of a premolar tooth for 60 hrs, this produced significant K+ diffusion in the SDF and concentrations changes were more than 10 mmol/dm3. However, this was not observed with the root dentine model, or in the crown section of a maxillary molar tooth and the present study could not reproduce this in these models possibly because of the biological variation, the possible preparation method or even the small sample sizes used in the study. In fact, the results of the premolar tooth and the dentine disk were not reproducible in the crown section molar tooth and the root dentine may also be due to the variation in the orientation within the DT in the samples used in the experiments.
When the flow was from the SDF to the solution in the polypropylene tube with the dentine disk model and the premolar crown section tooth model, there was no question of diffusion occurring. However, in the case of flow from the polypropylene tube to the SDF and diffusion in the same direction, it is questionable whether this is a diffusion mechanism or just simply a flow due to gravity and there appears to be no evidence as to which was the dominant mechanism. However, K+ ISE was still able to measure changes in the [K+] of the SDF. Occasionally there was a decrease in [K+] in the SDF which could have been attributed to the drift in the electrodes, although this was minimal (<3 mV/day) and there was no evidence suggesting that this was due to the electrodes used in the experiments. Applications of the KNO3 solutions of concentrations 0.6 mol/dm3 and higher increased the [K+] in the SDF, although this was not observed in the molar tooth with a cut cavity and to some extent in the root dentine as previously mentioned. Therefore, another explanation is that there was a local concentration build-up of KNO3 in the crown sections and the root sections of the dentine tubules although there was no evidence of this.
It can also be stated that these experiments support the DT that 5-6 mol/dm3 of KNO3, as would be present in most potassium-containing dental products, appears to produce significant diffusion of K+ compared to concentrations less than 5-6 mol/dm3. Potassium-containing desensitisers relieve pain associated with DH [14], and the present study demonstrates the significance of having 5 mol/dm3 or more of [K+] in dental products. However, it must be noted that the experimental setup does not represent the oral environment as it was difficult to model the oral environment with the current setup, although it may be possible with an entirely different setup where the SDF was in a pressurised chamber to mimic the pulpal pressure. It must also be acknowledged that the ISE did not measure the [K+] in the nerves per se as the present study was an in vitro study, which according to Fick’s law is where there would have been a higher concentration of K+ within the nerve membrane and the surrounding area rather than in the dentinal fluid within the DT. However, this is an assumption and may therefore be impossible to measure. Furthermore, there are challenges in conducting these types of experimentation with a K+ ISE since the KNO3 solution was applied on top of the sample. In future experimentation, with an inductively coupled plasma mass spectrometry (ICP analysis) could be used as this would allow for very low concentrations of K+ to be detected after K+ had diffused into the sample through the DT into a much smaller volume of SDF. It was not clear whether the final experimental results in this study appear to be contrary to the in vitro diffusion experiments of Miller et al., who measured the penetration of K+ in combination with other ingredients through acid-etched dentine disks in a 2-chamber diffusion cell and concluded that there was ‘very’ limited diffusion of potassium in both etched and untreated dentine disks [14, 24] but this can be explained by the different parameters used and the enclosure of a second chamber that mimicked pulpal pressure. The setup used by Miller et al., may present challenges to the real-time monitoring of K+ diffusion using ISE. However, Miller et al., was taking samples every 60 seconds for 20 minutes and then measured the [K+]. The use of ISE was beneficial in that the readings were taken automatically, and the concentration could be measured over longer periods. However, it can be observed from Figure 4 that the penetration of K+ through the dentine disk model is minimal which agreed with Miller et al., [24]. Further research with K+ ISE is recommended, such as measuring [K+] in the saliva following brushing the teeth with potassium desensitisers in vivo and measuring whether diffusion is affected by different anions such as citrates, tartrates, and oxalates, which could also have an occluding action. Potassium Ion Selective Electrodes (K+ ISE) were able to measure the changes in the potassium ion concentration ([K+]) in the SDF which was an indicator of the [K+] in the dentine tubules (DT). Other anions in combination with other ingredients (as possible mixtures) may also affect the penetration of K+ (and possibly other ions), such was the case when Miller et al. [24] treated dentine disks multiple times with sensitive tartar control. In this situation, experiments with K+ ISE may be useful also to test whether nitric oxide may act as a secondary messenger which could be measured using the ISE method. Further research however should be conducted to evaluate the influence of any confounding factors in a suitable model mimicking the oral environment.
Conclusion
- ISE real-time measurements may be suitable for permeability measurements of the dentine matrix to different ions.
- K+ ISE was able to demonstrate that 5 mol/dm3 KNO3 applied to dentine significantly affected the concentration of K+ on the opposite side of the sample except for in the premolar root section and crown section of a molar tooth.
- KNO3 solution of concentration less than 5 mol/dm3 appeared to produce insignificant diffusion in all dentine models.
- K+ ISE demonstrated the significance of having 5 mol/dm3 or more in dental products containing K+.
- The pressure of KNO3 (volume of KNO3 and the height reached above the sample) applied to the models of dentine does not appear to affect the diffusion result i.e., its ability to affect the concentration on the opposite side of the sample.
- Both the Dentine disk and premolar tooth models appeared to be much more reliable models when undertaking these experiments. However, further research will be needed if root sections and crown sections with a cut cavity are used.
- Further research is required to mimic the oral environment and change other relevant parameters.
References
- Dionysopoulos D, Koliniotou-Koumpia E, Helvatzoglou-Antoniades M, et al. (2016) In Vitro Inhibition of Enamel Demineralisation by Fluoride-releasing Restorative Materials and Dental Adhesives Oral Health Prev Dent 14(4): 371-380. [crossref]
- Huang WT, Shahid S, Anderson P (2018) Validation of a Real-Time ISE Methodology to Quantify the Influence of Inhibitors of Demineralization Kinetics in vitro Using a Hydroxyapatite Model System Caries Research 52(6): 598-603. [crossref]
- Sokalski T, Ceresa A, Zwickl T, et al. (1997) Large improvement of the lower detection limit of ion-selective polymer membrane electrodes J Am Chem Soc 119(46): 11347-11348. [crossref]
- Greenhill JD, Pashley DH (1981) The effects of desensitizing agents on the hydraulic conductance of human dentin in vitro J Dent Res 60(3): 686-698. [crossref]
- Pashley DH, Galloway SE (1985) The effects of oxalate treatment on the smear layer of ground surfaces of human dentine. Arch Oral Biol 30(10): 731-737. [crossref]
- Peacock JM, Orchardson R (1995) Effects of potassium ions on action potential conduction in A- and C-fibers of rat spinal nerves. J Dent Res 74(2): 634-641. [crossref]
- Kim S (1988) Method of desensitizing hypersensitive dentin employing compositions containing potassium chloride. Google Patents.
- Markowitz K, Bilotto G, Kim S (1991) Decreasing intradental nerve activity in the cat with potassium and divalent cations Arch Oral Biol 36(1): 1-7. [crossref]
- Miglani S, Aggarwal V, Ahuja B (2010) Dentin hypersensitivity: Recent trends in management J Conserv Dent 13(4): 218-224. [crossref]
- McCormack K, Davies R (1996) The enigma of potassium ion in the management of dentine hypersensitivity: is nitric oxide the elusive second messenger?. Pain 68(1): 5-11. [crossref]
- Ajcharanukul O, Kraivaphan P, Wanachantararak S, et al. (2007) Effects of potassium ions on dentine sensitivity in man Arch Oral Biol 52(7): 632-639. [crossref]
- Markowitz K (2010) Pretty painful: Why does tooth bleaching hurt? Med Hypotheses 74(5): 835-840. [crossref]
- Noparatkailas S, Wanachantararak S, Vongsavan N, et al. (2009) The effect of applying potassium chloride solutions at atmospheric pressure on the sensitivity of dentine in man Arch Oral Biol 54(1): 50-54. [crossref]
- Orchardson R, DG Gillam (2000) The efficacy of potassium salts as agents for treating dentin hypersensitivity J Orofac Pain 14(1): 9-19. [crossref]
- Kim S, (1986) Thermal stimuli in dentinal sensitivity Endod Dent Traumatol 2(4): 138-140. [crossref]
- Sena FJ, (1990) Dentinal permeability in assessing therapeutic agents Dent Clin North Am 34(3): 475-490. [crossref]
- Markowitz K, Kim S (1990) Hypersensitive teeth. Experimental studies of dentinal desensitizing agents Dent Clin North Am 34(3): 491-501. [crossref]
- Pashley DH, (1986) Dentin permeability, dentin sensitivity, and treatment through tubule occlusion J Endod 12(10): 465-474. [crossref]
- Stead WJ, Orchardson R, Warren PB (1996) A mathematical model of potassium ion diffusion in dentinal tubules Arch Oral Biol 41(7): 679-687. [crossref]
- Bishop GH (1932) Action of nerve depressants on potential J Cell Physiol 1(2): 177-194. [crossref]
- Orchardson R (1978) The generation of nerve impulses in mammalian axons by changing the concentrations of the normal constituents of extracellular fluid J Physiol 275: 177-189. [crossref]
- Orchardson R (1978) An electrophysiological investigation of the sensitivity of intradental nerves in the cat to changes in the ionic composition of extracellular fluid Arch Oral Biol 23(6): 471-475. [crossref]
- Peacock JM, Orchardson R (1999) Action potential conduction block of nerves in vitro by potassium citrate, potassium tartrate and potassium oxalate J Clin Periodontol 26(1): 33-37. [crossref]
- Miller S, Gaffar A, Sullivan R, et al. (1994) Evaluation of a new dentifrice for the treatment of sensitive teeth J Clin Dent 5: 71-79. [crossref]
- Coffey CT, MJ Ingram, AM Bjorndal (1970) Analysis of human dentinal fluid Oral Surg, Oral Med, Oral Pathol 30(6): 835-837. [crossref]
- Uddin A, Yemenicioglu S, Chen CH, et al. (2013) Integration of solid-state nanopores in a 0.5 mum CMOS foundry process Nanotechnology 24(15): 155501. [crossref]
- Brannstrom M, Johnson G (1970) Movements of the dentine and pulp liquids on application of thermal stimuli. An in vitro study Acta Odontol Scand 28(1): 59-70. [crossref]
- Ciucchi B, Bouillaguet S, Holz J, et al. (1995) Dentinal fluid dynamics in human teeth, in vivo J Endod 21(4): 191-194. [crossref]