Abstract
Peri-operative environments are a hazardous setting for diabetes patients. A systematic review of literature regarding the management of diabetes patients across the peri-operative pathway has been undertaken to assess if the management of patients within this pathway is suitable and effective for patients.
Methods
A database search of Google Scholar, CINAHAL, Embase, OVID, Cochrane Library, Joanna Briggs institute and PUBMED was undertaken from 15th of March 2019 to 30th of March 2019. A total of 57 papers were found and reduced down to 11 final papers that answered the review question and met the inclusion and exclusion criteria. Inclusion criteria were: Full text, English language, human subjects, adult patients only and studies that focused on diabetes care in a section of the peri-operative pathway. Exclusion criteria: children or adults and children, studies that looked a one particular intervention or type of surgery. No date limit was set. PICO tool was used to frame the study question.
Results
Three main themes emerged from the literature. 1. Poor patient outcomes; 2. Longer length of stay (LOS); 3. Lack of adherence to guidance and or protocols and glycaemic control. Elective patients had advantageous outcomes compared to emergency surgical patients. Hyperglycaemia still remained a problem with an increase in other medical complications for diabetes patients. LOS in hospital was found to have increased due to medical complications. Adherence to protocols and guidance was found to be beneficial in monitoring and managing hyperglycaemia. However, this review found that best practice guidance and hospital protocol is not always adhered to. A liberal approach to glycaemic control is beneficial.
Conclusion
This systematic review investigated the management of diabetes patients across the peri-operative pathway. Three main themes emerged from the literature: poor patient outcomes; length of stay; and lack of adherence to guidance and or protocols and glycaemic control. We concluded the peri-operative environment is a hazardous setting for a diabetes patients. Elective patients had slightly more advantageous outcomes than emergency patients. Hyperglycaemia still remains a problem which leads to poor patient outcomes and longer LOS. Adherence to protocols and guidance was found to be beneficial in monitoring and managing hyperglycaemia.
Introduction
The Department of Health and Social Care (DOH) (2001) state that diabetes patients undergoing surgery carry a greater clinical risk than non-diabetes patients. This is due a number of complex factors such as reduced food intake due to a starvation period, and cessation of normal diabetes medications [1]. In addition, the body’s stress response and inhibition of insulin secretion increases the potential for hyperglycaemia [2]. The Association of Anaesthetists of Great Britain and Ireland [3] state that diabetes affects 10–15% of the surgical population, with these patients carrying a greater risk of complication rates, mortality rates and Length of stay (LOS).
Despite these findings, there is very little guidance and research surrounding diabetes management across the peri-operative pathway. There are currently no standardised worldwide guidelines for use by theatre or PACU practitioners [4] and globally, diabetes management during the peri-operative period is widely debated [5]. The aim of this systematic review was to investigate the management of diabetes patients across the peri-operative pathway.
Methodology
A systematic and comprehensive search of databases was carried out between the 15th of March 2019 and the 30th of July 2019. The search involved Google Scholar, CINAHAL, Embase, OVID, Cochrane Library, Joanna Briggs institute and PUBMED. Combinations of key words were inputted into each database. Further restrictions were then applied to reduce the number of papers, such as; English language, full text and used adult human patients as the participants. Studies which examined the care and management of diabetes patients across the peri-operative pathway were included. Studies into specific interventions or surgeries were excluded due to the broadness of the review question. Exclusion criteria: children participants and studies that looked a one particular intervention or type of surgery. No date limit was set.
This review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to ensure systematic transparency of report [6]. After duplications were removed, 57 papers were read to determine their relevance to the review question.
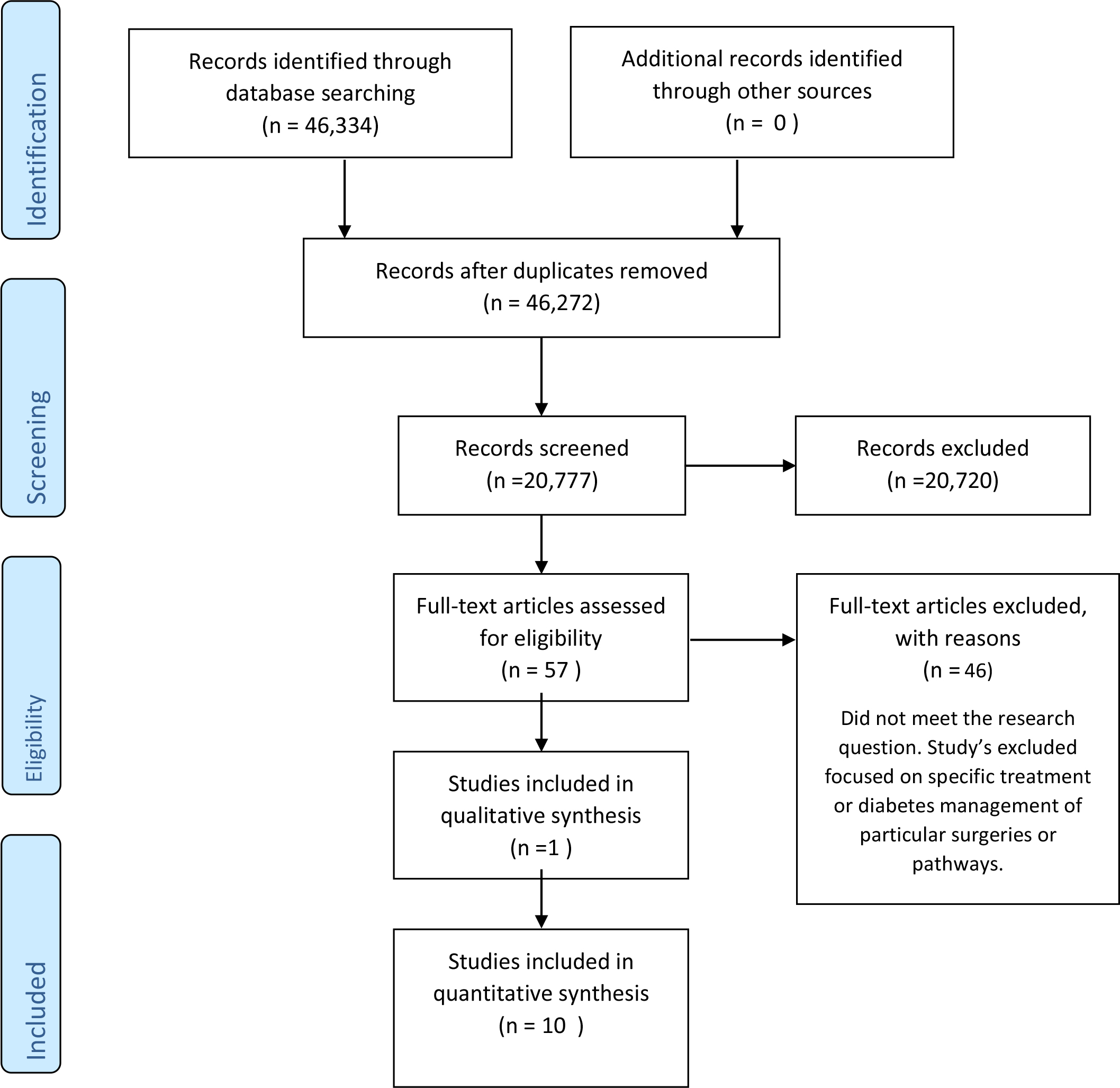
Figure 1. PRISMA flow diagram of Studies included in quantitative synthesis
The Cauldwell, Henshaw and Taylor (2011) framework was utilised for assessing the meaningfulness or generalisability of qualitative and quantitative research in contemporary nursing practice, which enabled a structured approach to the assessment of each study’s quality, validity and reliability (Clarke, 2011). The final 11 papers were RAG (red amber green) rated [7] to reflect the answer to each of the questions from the tool. Dates ranged from 1983- 2019 and included studies from various countries. 9 of the 11 studies focused on the peri-operative period. 1 study focused on intra-operative and post-operative diabetes management. 1 study looks at diabetes management in the pre-operative period. Full text was then read to extract the results from each paper for the formation of themes.
Results and discussion
A systematic review as undertaken to establish the management of diabetes patients across the peri-operative pathway. Three key themes emerged from the review: poor patient outcomes, length of stay (LOS) which were commonly reported jointly and adherence to guidance and or protocols and standards for glycaemic control.
Poor patient outcomes
8 out of 11 studies reported on the outcomes of patients with diabetes. Studies 2, 3,5,6,8,9,10 and 11 discussed surgical outcomes directly related to diabetes management. McCavert, Monem and Dooher, et al [8] found that best practice of glycaemic control, in-line with hospital protocols, saw a 25.4% reduction of peri-operative complications. Overall complications being 29% (out of 69 patients). Elective patients with T2DM were more prone to complications. 5 out of 17 (29.4%) of T2DM elective patients experienced complications; in contrast, only 4 out of 21 (19.0%) of elective patients with T1DM developed a complication such as wound infection or peritonitis. For emergency patients, the rate of complications was slightly higher for those with T1DM (5 out of 14; 35.7%) versus 6 out of 17 patients (35.3%) with T2DM. Complications such as; Wound dehiscence, septicaemia, wound infection, wound infection, confusion, deep vein thrombosis and lower respiratory tract infection were reported as a complication. Frisch, Chandra, Smiley, et al [9] similarly analysed outcomes of mobility contrasting both diabetes and non-diabetes patients. Outcomes such as pneumonia (12.1 vs 5.4%; p=0.001), wound and skin infections (5 vs 2.3%; p<0.001), systematic blood infection (3.6 vs 1.1%; p<0.001), urinary tract infections (4.5vs 1.4%, p<0.001) acute myocardial infarction (2.6 vs 1.2 %; p< 0.001) were reported. Patients who experienced complications had a strong affiliation with high blood glucose levels pre and post-operatively.
“Haemoglobin A1c, often abbreviated as A1C, is a form of haemoglobin (a blood pigment that carries oxygen) that is bound to glucose” [10]. Underwood, Askari, Hurwitz et al [11] linked to various A1C categories to patient outcomes. It showed that, like McCavert et al and Frisch et al, [8, 9] diabetes patients (specifically group A1C ≤6.5%) had a higher incidence of LOS, acute renal failure death within 30 days and wound class (dirty). Groups ≤6.5%, A1C> 8-10% and A1C > 10% was significantly longer compared with the control subjects (p<0.001,p<0.008, and p=0.002, respectively).
Wang, Chen, Li, et al (2019) found that patients over 65-years old, male, high mean post-operative blood glucose (BG), diabetes complications, abnormal kidney function and have underwent general surgery were the highest risk category for poor patient outcomes. The study compared surgery type and patient outcomes. Of the 301 (19.8%) of all patients with diabetes complications, 295, (98.0%) had major vascular complications, 8 (27. %) had diabetes nephropathy, 3 (0.7%) had diabetes retinopathy, 5 (1.7%) had diabetes foot post-operatively. Post-operative adverse events occurred in 118 (7.7%) including 43 (36.4%) delayed extubation caused by surgery-related respiratory failure or muscle weakness. 15 (12.7%) patients had circulatory disorders, 23 (19.5%) had respiratory and circulatory abnormalities. 11 (9.3%) had non-healing of the incision. 15 (12.7%) had infections at other sites. 8 (6.8%) patients with other complications. 3 (2.5%) patients died due to pulmonary embolism and two cases of septic shock. Kotgal, Symods, Hirsch, lrl, et al [12] did not correlate BG management with patient outcomes, but results showed that patients had a greater chance of poorer outcomes with any level of hyperglycaemia versus those who had better diabetes control.
In contrast, Sathya, Davis, Taveria, et al [13] found that stroke, atrial fibrillation and wound infection were the most significant complications from pooled results of 6 studies. Mixed results were noted; 2 pooled results found that the incidence of post-operative stroke was reduced by liberal glycaemic regimes, but pooled results from a further 3 studies suggested that there was no significant difference between the effect of moderate vs strict control on stroke outcomes (odds ratio, 18.5, 95% CI 0.72-4.74, p=0.020). Sathya et al [13] also examined the relationship between atrial fibrillation as a patient outcome and diabetes control. Again, pooled estimates from 2 pooled studies found that moderate versus liberal control had no direct effect on atrial fibrillation as an outcome (Odds ratio 0.54, 95% CI 0.17-1.76, p =0.31). In addition, pooled results from 3 other studies found that there was no significant difference between strict versus moderate control in relation to atrial fibrillation (odds ratio: 0.71, 95% CI0.39-1.30, p=0.27). Wound infection was also not found to have a significant link to the effects of moderate versus glycaemic control from the results of 2 pooled studies.
Length of stay
LOS was a significant finding in studies 2, 3, 6 and 8. Although not a complication in itself, LOS was linked to or reported alongside poor patient outcomes.
McCavert et al [8] found that Emergency patients had a significantly longer LOS in hospital than the elective groups. Frisch et al (2010) [9] also reports that diabetes patients had a higher rate of complications than non-diabetes counterparts (p=0.105). Patients with diabetes were found to have a greater LOS (and LOS in ICU) than non- diabetes patients. It was also noted that African American patients were not at an increased risk of mortality than other races. No other study compared likelihood of surgical outcomes and race.
Patients with diabetes were also more likely to have greater complications including LOS. Underwood et al, 2014 [11] however, reported that patients with A1C levels >6.5-8% had a similar LOS to the control group. Patients with higher A1C ≤6.5 up to greater than 10% had a significantly longer LOS compared to control subjects. This was the most significant difference of the various A1C groups compared in the study. Higher A1C level was more significant than any other variable such as a diabetes patient’s race, gender or type of surgery in relation to LOS. Longer LOS in the hospital was found by Hommel et al [14] to be associated with higher dissatisfaction of patients regarding patient centred-ness in their assessment of results.
Lack of adherence to guidance and or protocols and glycaemic control
The third key theme that emerged from the literature was adherence to guidance, such as hospital protocols and national guidelines and glycaemic control. This theme was disused in studies 1,2,5,7 and 10.
McCavert et al [8] studied both elective and emergency surgical patients. 60% of elective patients with T1DM were not treated according to hospital protocol. Elective patients who were treated according to protocol had a complication rate of 6.3 %. For emergency surgical patients, 7.3% of T1DM patients who were treated as per protocol developed a complication. 12.3% of scheduled blood glucose measurement were not completed. 11.1% of T1DM elective patients did not have their blood glucose checked, and 6.8% of emergency T1DM patients. For T2DM, blood glucose was not checked in 17.4% of elective patients and 12.7% in emergency cases.
Similarly, Coan, Schlinkert, Brandon et al [15] note that capillary BG was taken in 89% of cases in the pre-operative area, and only52% of patients had a HBA1C. Intra-operatively, 33% of patients had a BG check, and the post- operative figure was 87%. 90% of pre-operative BG was point of care (POC), and 4% was venous sampling. Intraoperatively, 10% of patients had POC BG values, 16% had POC blood gas sampling. In the PACU, 86% of BG were obtained by POC and 1% was venous. Similarly, Jackson, Patvardhan et al (2015) reported that only 71% of patients had a HBA1C recorded pre-operatively and 56% intra-operatively via CBG. 73% of patients had a CBG performed in recovery (PACU) contrary to national guidance. Hommel, Van Gurp, Tack et al’s [16] quality indicators suggest that best-practice involved measuring BG 4 hours pre-operatively, every 2 hours intra-operatively, and 1 hour post-operatively. Hommel et al [14] reported that in relation to patient satisfaction and person centeredness, 20% of 362 patients were not informed about intra-operative BG level and its effect. 15% were also not informed that insulin was administered during surgery. This correlated to overall low score from patients’ involvement in the survey. Sathya et al [13] report that patients undergoing a liberal target for glycaemic control had significantly better post-operative outcomes (less or no complications) than other groups. No difference with wound infection or atrial fibrillation were found. Bibble (1983) commented from the 3 case studies that protocols for glycaemic control were directed towards managing ‘average’ diabetes patients rather than complex ones, making guidance non-beneficial.
Future recommendations would be to undertake extensive quantitative and qualitative research across the peri-operative pathway with staff who have direct responsibility for diabetes patients undergoing surgery. The views and attitudes of staff members regarding diabetes management may shed light on the barriers as to why this is still a problem despite being highlighted by several studies seen in this review since 1983. Any further research conducted needs to be influential on practice in order to drive change.
Conclusion
This systematic review examined the management of diabetes patients across the peri-operative pathway. Three main themes emerged: poor patient outcomes; longer length of stay; and lack of adherence to guidance and or protocols and glycaemic control. We concluded the peri-operative environment can be a hazardous setting for diabetes patients. Elective patients had slightly more advantageous outcomes than emergency patients. Hyperglycaemia still remains a problem which leads to poor patient outcomes and longer LOS. Adherence to protocols and guidance was found to be beneficial in monitoring and managing hyperglycaemia.
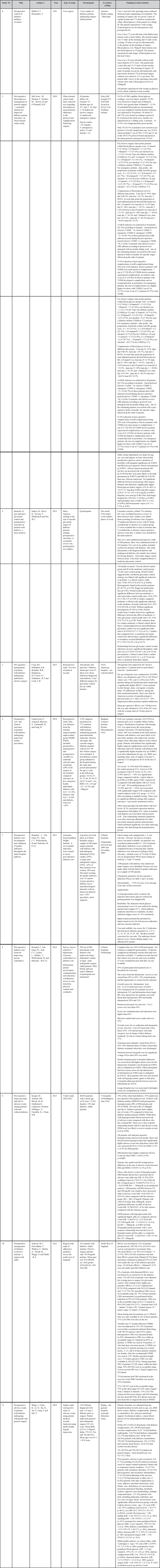
Table 1. Characteristics of studies
References
- McAnulty GR, Robertshaw HJ, Hall GM (2000) Anaesthetic Management of patients with diabetes mellitus. British Journal of Anaesthesia 85: 80-90.
- Dagogo-Jack S, Alberti KGMM (2002) Management of Diabetes Mellitus in Surgical Patients. Diabetes Spectrum 15: 44-48 [online].
- Association of Anaesthetists of Great Britain and Ireland. (2015) Peri-operative management of the surgical patient with diabetes 2015. Anaesthesia 70: 1427-1440.
- Coan KE, Apsey HA, Schlinkert RT, Stearns, JD Cook, CB (2014) Managing diabetes mellitus in the surgical patient. Diabetes management 4: 515-526.
- Duggan EW, Carlson K, Umpierrez GE (2017) Perioperative Hyperglycaemia management: an update. Anesthesiology 126: 547-560.
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, et al. (2015) Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement. Syst Rev 4: 1.
- Webster V, Webster M (2019) How to Use RAG Status Ratings to Track Project Performance. [online]. Available at: https://www.leadershipthoughts.com/rag-status-definition/ (Accessed 12 June 2019).
- McCavert M, Mone F, Dooher M, Brown R, O’Donnell ME (2010) Peri-operative blood glucose management in general surgery-a potential element for improved diabetes patient outcomes- An observational cohort study. International Journal of Surgery 8: 494-498.
- Frisch A, Chandra P, Smiley D, Peng L, Rizzo M, (2010) Prevalence and clinical outcome of hyperglycaemia in the peri-operative period in noncardiac surgery. Diabetes care 33: 1783-1788.
- Stöppler MC (2019) Shiel WC (eds.) Haemoglobin A1c Test (HbA1c) [online]. Available at: https://www.emedicinehealth.com/hemoglobin_a1c_hba1c/article_em.htm#facts_and_definition_of_hemoglobin_a1c_hba1c (Accessed 19 June 2019).
- Underwod P, Askari R, Hurwitz S, Chamarthi B, Garg R (2014) Preoperative A1C and clinical outcomes in patients with diabetes undergoing major noncardiac surgical procedures. Diabetes care 37: 611-616.
- Kotagal M, Symons RG, Hirsch IB, Umpierrez GE, Dellinger EP, et al. (2015) Perioperative hyperglycaemia and risk of adverse events among patients with and without diabetes. Annals of surgery 261: 97-103.
- Sathya B, Davis R, Taveria T, Whitlach H, WU WC (2013) Intensity of peri-operative glycaemic control and postoperative outcomes in patients with diabetes: a meta-analysis. Diabetes research and clinical practice 102: 8-15.
- Hommel I, Van Gurp PJ, Tack CJ, Lifers J, Mulder J, et al. (2014) Peri-operative diabetes care: room for improving the person centredness. Diabetes Medicine 32: 561-568.
- Coan KE, Schlinkert AB, Beck BR, Haakinson DJ, Castro JC, et al (2013) Perioperative management of patients with diabetes undergoing ambulatory elective surgery. Journal of diabetes science and technology 7: 983-989.
- Hommel I, Van Gurp PJ, Tack CJ, Wollersheim H, Hulscher MEJL (2015) Perioperative diabetes care: development and validation of quality indicators throughout the entire hospital care pathway. British Medical Journal BMJ 25: 525-534.
- Coan, K.E., Schlinkert , A.B., Beck, B.R., Haakinson, D.J ., Castro, J.C., Schlinkert, R.T., Cook, C.B (2013) Perioperative management of patients with diabetes undergoing ambulatory elective surgery. Journal of diabetes science and technology. 7 (4) pp: 983-989.
- Department of Health and Social Care (2008) National service framework for diabetes [online]. Accessed: https://www.gov.uk/government/publications/national-service-framework-diabetes [Accessed 15 March 2019].
- Diabetes UK (2010) Key statistics on diabetes. Available at: https://www.diabetes.org.uk/resources-s3/2017-11/diabetes_in_the_uk_2010.pdf (Accessed 15 March 2019).
- Diabetes UK (2018) Diabetes Prevalence 2018. Available at: https://www.diabetes.org.uk/professionals/position-statements-reports/statistics/diabetes-prevalence-2018 (Accessed 29 May 2019).
- Diabetes.co.uk (2019) ISO Standards for Blood Glucose Meters [online]. Available at: https://www.diabetes.co.uk/blood-glucose-meters/iso-accuracy-standards.html (Accessed 19 June 2019).
- Gandhi, G.Y., Nutthall, G.A ., Mullany C.J., Schaff H.V., Williams B.A .,Schrader L.M., Rizza R.A and McMahon M.M (2005) Intraoperative hyperglycaemia and peri-operative outcomes in cardiac surgery patients. Mayo clinic. Proc. 80 (7) pp: 862-866.
- Godby, M.E (2019) Control Group Science. [online]. Available at: https://www.britannica.com/science/control-group (Accessed 08.06.2019).
- Gov.uk (2019) Ethnicity facts and figures: UK population by ethnicity. [online]. Available at: https://www.ethnicity-facts-figures.service.gov.uk/uk-population-by-ethnicity (Accessed: 04.06.2019).
- Herman, W.H (2010) Are there clinical implications of racial differences in HbA1c? Yes, to not consider can do great harm! Diabetes Care. 2016; (39) pp: 1458–146 [online]. Available at: http://care.diabetesjournals.org/content/diacare/39/8/1458.full.pdf (Accessed 11 June 2019).
- Kang, H (2013) The prevention and handling of the missing data. Korean Journal of Anesthesiology. 64(5)pp: 402–406. [online]. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3668100/ (Accessed 19 June 2019).
- Kendall, J.M (2018) Designing a research project: randomised controlled trials and their principles. Emergency Medicine Journal. 20 (0) pp: 164-168. [online]. Available at: https://emj.bmj.com/content/20/2/164.info (Accessed 19 June 2019).
- Kumar PR ., Bhansali A., Ravikiran M., Bhansali , S., Dutta., Thakur, J.C., Sachdeva, N., Bahdada, S.K and Walia, R (2010)Utility of glycated hemoglobin in diagnosing type 2 diabetes mellitus: a community-based study. Journal of endocrinology and metabolism. 95 pp: 2832–2835.
- Leung, V and Ragbir-toolsie (2017) Perioperative management of patients with diabetes. Health serv insights .:2017 10. [online]. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5692120/ (Accessed 29 May 2019).
- Moher, D, Liberati, A.,Tetzlaff J and Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Medicine.6:(7). [online]. Available at: http://prisma-statement.org/PRISMAStatement/FlowDiagram.aspx (Accessed 20.03.2019).
- National Institute of Diabetes and Digestive and Kidney diseases (2019) Diabetes overview : what is Diabetes? [online]. Available at:https://www.niddk.nih.gov/health-information/diabetes/overview/what-is-diabetes (Accessed 08 June 2019).
- National Health Service NHS (2016) Diabetes [online]. Available at: https://www.nhs.uk/conditions/diabetes (Accessed 15 March 2019).
- National Health Service NHS (2017) Next steps on the five-year forward view [online]. Available at: https://www.england.nhs.uk/wp-content/uploads/2017/03/NEXT-STEPS-ON-THE-NHS-FIVE-YEAR-FORWARD-VIEW.pdf (Accessed 15 March 2019).
- New York University NYU libraries (2019) Health (Nursing, Medicine, Allied Health): Search Strategies: Framing the question (PICO) Guide to locating health evidence. [online]. Available at: https://guides.nyu.edu/c.php?g=276561&p=1847897 (Accessed 15 June 2019).
- Pal Singh, A (2015) Bone and Spine : What is Hierarchy of Evidence? [online]. Available at : https://boneandspine.com/what-is-hierarchy-of-evidence/ (Accessed 01 July 2019).
- Pimentel M.P.T., Choi, S ., Fiumara, K., Kachalia, A and Urman, R.D (2017) Safety Culture in the Operating Room: Variability Among Perioperative Healthcare Workers. Journal of patient safety. [online]. Available at: https://www.ncbi.nlm.nih.gov/pubmed/28574955 (Accessed 12 May 2019).
- Preston, N. Gregory, M (2012) Patient recovery and post-anaesthesia care unit (PACU). Anaesthesia and intensive care medicine. 13: (12) pp: 591–593. [online]. Available at: https://www.anaesthesiajournal.co.uk/article/S1472-0299(12)00234-2/fulltext [Acessed:15.03.2019].
- Public health England press release (2016) 3.8 million people in England now have diabetes. [online]. Available at: https://www.gov.uk/government/news/38-million-people-in-england-now-have-diabetes (Accessed 15 March 2019).
- Quesada, I ., Tudurı´,E ., Ripoll, C and Nadal, A (2008) Physiology of the pancreatic a-cell and glucagon secretion: role in glucose homeostasis and diabetes. Journal of Endocrinology. (199) pp: 5–19 [online]. Available at: https://joe.bioscientifica.com/view/journals/joe/199/1/5.xml (Accessed 08 June 2019).
- Royal College of Nursing RCN (2019) Peri-operative Care. [online]. Available at: https://www.rcn.org.uk/library/subject-guides/perioperative-care (Accessed 08 June 2019).
- Sargis, R.M (2015) An Overview of the Pancreas: Understanding Insulin and Diabetes. [online]. Available at: https://www.endocrineweb.com/endocrinology/overview-pancreas (Accessed 12 June 2019).
- Shuttleworth, M and Wilson, L.T (2019) Scientific Control Group. [online]. Available at: https://explorable.com/scientific-control-group (Accessed 26 June 2019).
- Smiley, DD,. Umpierrez, GE. (2006) Perioperative glucose control in the diabetes or nondiabetes patient. South Med J. 99 (6) pp: 580-9; quiz 590-1. Available at: https://www.ncbi.nlm.nih.gov/pubmed/16800413 (Accessed 15 March 2019).
- Swenne, C.L and Alexandrén, K (2012) surgical team members’ compliance and knowledge of basic hand hygiene. Journal of infection and prevention control. 2 (3) pp: 114-119. [online]. Available at: https://pdfs.semanticscholar.org/cd6d/e40147cef4e4010ddb1912fb8c3f3fd00345.pdf (Accessed 12 June 2019).
- World Health Organisation WHO (2018) Diabetes. Available at: https://www.who.int/news-room/fact-sheets/detail/diabetes (Accessed 29 May 2019).
- Ziemer, DC,., Kolm. P., Weintraub W.S., Vaccarino. V., Rhee, M.K., Twombly, J.G., Narayan, K.M., Koch, D.D and Philips, L.S (2010) Glucose-independent, black-white differences in hemoglobin A1c levels: a cross-sectional analysis of 2 studies. Ann Intern Med. 152 pp:770–777 [online]. Avalible at: https://www.ncbi.nlm.nih.gov/pubmed/20547905 (Accessed : 04 June 2019)