Abstract
MAIT cells are innate like T cells served as; bacterial and fungal sensors through TCR dependent mechanism and viral sensors via cytokine TCR independent mechanism. These mechanisms are along with an overlapping tissue repair mechanisms. The objective of the present opinion paper was to deduce the immune functions and immune features of MAIT cells in; Homeostasis, SARS-COV-2 infection and SARS-COV-2 vaccination as appeared in the current 2020 up to 2022 publications. Single cell transcriptomics, mass transcriptomics, flow cytometery, unsupervised analysis and full immune cell landscapes were the major approaches followed by lymphocyte immunologists. During homeostasis and activation, early antigen specific MAIT cells activation with MRI legand5-OP-RU and non-specific TCR stimulation, it has been found an array of phenotypes as; homeostatic, effector, helper, tissue infiltrating, regulatory and exhausted phenotypes. While, in prolonged stimulation, proliferative, cytotoxic, immune modulating and exhausted phenotypes were identified. In SARS-COV-2 human infection, MAIT cells may be reduced in circulation and enriched in the airways, dys-regulated, or activated then migrate to lungs in the pneumonic COVID-19 pathotype. In vaccinee, however, MAIT cells in mRNA COVID-19 vaccinee was found to be positively correlated with magnitude of humoral and cellular response to vaccine in normal healthy vaccinee but their cytotoxic function post activation is negatively correlated with humoral and cellular immune response to vaccine. MAIT cells were found to be helpful mucosal cellular immune-adjuvant in influenza nasal vaccination strategy. Thus MAIT cell immune functions may be summed up as; microbial immune sensors, immune-pathogenic, immune modulating, mucosal cellular adjuvant to viral protein antigen and intrinsic systemic cellular adjuvant in COVID-19 vaccinee. The evolution mechanisms for MAIT functional phenotypes were suggested.
Keywords
Adjuvant, Activation, cell, cytotoxicity, mucosal, MAIT, TCR dependent, TCR independent, vaccine.
Introduction
MAIT cells are innate-like of recirculating T cells. They can function as bacterial and fungal sensors through TCR dependent mechanism and as viral sensors via cytokine activation mechanisms. These mechanisms are overlapping with their ability to repair tissues. MAIT cells are also found in association with autoimmune, immune mediated and cancer diseases. They are enriched in human; liver, lung, gut with an evident variable existence in peripheral blood of normal healthy individuals. In human viral infections they are of reduced frequencies in blood and enriched in local tissues. The situation in human SARS-COV-2 infection so far concerning the MAIT distribution seems to be not far from that of other viral infections [1- 8]. The aim of the present opinion paper was to deduce the immune functions and immune features of MAIT cells in in; normal human homeostasis, SARS-COV-2 infected and SARS-COV-2 vaccinee.
Investigative Approaches
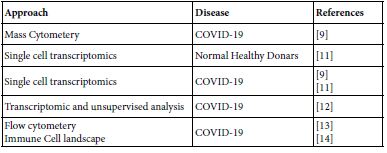
Google mapping the MAIT published contributions all over the world paved the author to the years 2020 up to 2022. The publication covers the areas of biology, molecular biology, pathogenesis in microbial, autoimmune, immune mediated and cancer besides their role in tissue repair. The aiming of the present opinion paper was focused on their role in homeostasis, COVID-19 and COVID-19 vaccinee. Table 1 lists the investigative approaches ensembled in current MAIT-COVID-19 research.
Table 1: Molecular and Immune investigation approaches in COVID-19, homeostasis and COVID-19 vaccinee
MAIT Cell Immunobiology
Ontogeny
The human MAIT cells expressing V alpha 7-CD161hi T cells are generated during gestation and likely share a common prenatal development program. Within cord blood niche the total MAIT cells, V alpha 7+ CD161hi. T cells are forming the minority recognizing MR1; 5Op-RU display a TRAV/TRBV repertoire very similar to adult MAIT cells. During the few weeks of postnatal life only MR1:5-OP-RU reactive to V alpha 7.2+CD161hi T cells aquire memory phenotype Only these cells expand to form adult MAIT pool diluting out other V alpha.7-2+ CD161hi. And V alpha 7-2-CD161hi. Population in a process requiring at least6 years to reach adult level [1].
MAIT Cell and Molecular Biology
MAIT cell are forming one subset of T cells with an evident unique characteristics. They function both in innate and adaptive immune responses. MAIT can perform their biologic functions both through TCR dependent and TCR independent manners. Their basic TCR structure composed of TCR alpha chain V alpha33 associated with limited TCR Beta chain repertoire and restricted by the non – polymorphic MHC class related MRI molecule. MAIT cells are innate- like immune cells produce wide range of cytokines. Resting cells are devoid from garnzyme and porins and no-cytotoxic. On activation by either microbial riboflavin through TCR or through non-TCR way by cytokines, they own granzyme and porins and becomes cytotoxic. Such activated MAIT cells allowed to migrate to the inflamed areas and functions therein. So can act as first line defenders against microbial infections. They also contribute in autoimmune and immune mediated diseases. MAIT cells harbor mucosal surfaces of lung, gut and in liver. Variable MAIT cell counts were found in peripheral blood of normal human blood doners. The surface markers of MAIT cells are; C type lectin CD161, integrin and chemokine receptors. The fine structure of the MAIT cellular system composed of g ranzyme type B, TCR alpa and TCR beta but the beta is very limited [5,7,8]. MAIT cell TCR can fine-tune MRI recognition through antigen dependent manner, by which MAIT cell recognition is modulated [4]. MAIT cell are ramified into a number of subsets so far concerned in COVID-19 (Table 2). Comparative view to MAIT and conventional T cells are depicted in Table 3.
Table 2: MAIT cell subsets in COVID-19 and in normal healthy donors
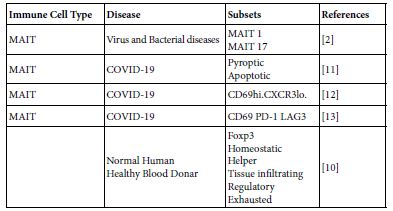
Table 3: Comparative View to MAIT cells And Conventional T cells*
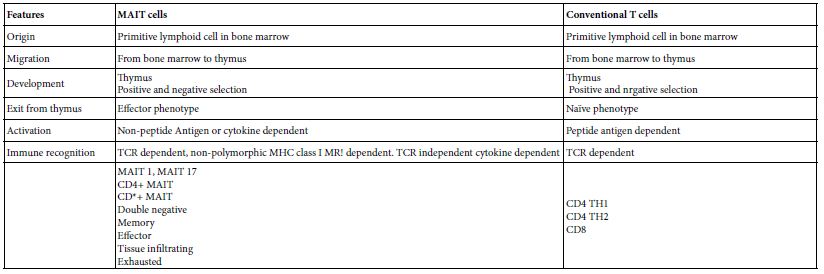
*Based on to Vorkas et al. [10]
To be a functional MAIT cell phenotype there may be a number of suggested sequential steps and several influencing factors in effect leading to the rise of functional MAIT cell phenotype as;
- Primitive lymphoid cell progenitor in bone marrow migrates and homed into thymus.
- Therein thymus homed lymphoid progenitor cells undergoes positive and negative selection processes through the action of thymic factors and cytokines and maturate to naïve MAIT cells.
- Naïve MAIT matured cells undergoes further developmental events mediated by cytokines finalized by evolving of effector MAIT These effector cells leave the thymus.
- On leaving thymus effector MAIT cells migrate through circulation to peripheral tissues and homed therein. By this, two forms of MAIT cells are merging circulatory and tissue resident forms.
- Tissue micro-environmental stimuli are in action within the localized tissue niche.
- Genetic, epigenetic and metabolic reprograming happened.
- Evolving and acquisition of new surface and intracellular receptors or markers.
- Cytokine induction or cell-cell cross-talk are in action
- New functional MAIT phenotype emerged.
Immune Recognition by MAIT Cells
Two immune recognition mechanisms are known to date in MAIT cells. First is the invariant TCR dependent in which MAIT recognize microbial conserved microbial riboflavin derivatives antigens. The process terminated by MAIT cell activation. The second mechanism is TCR independent in which cell mediators the cytokines interacts with their surface receptors on MAIT cells, signal transduced in to the cell interior leading to cell activation. The activated MAIT cell in both mechanisms MAIT cells transformed from resting to activate cells form with acquisition of granzym B and porins. Activated MAIT cells performed immune and non-immune functions. The non- immune functions expressed as affected tissue repair, and the immune functions spans in innate and adaptive immune response arms. The activation by TCR dependent may renders MAIT cells cytotoxic killing virus infected cells. Activation by cytokine may induce MAIT cells to produce IL17A with pathological consequences in the affected tissue lungs [3].
MAIT Cell Immune Functions
MAIT cells of human and mice are programed in the thymus to seed and reside in barriers tissues. Therein local cues possibly modulate the MAIT transcription program so that MAIT cells isolated from different organs express distinct gene sets [15]. Hence MAIT cells seems to display specific properties according to the organ from which they recovered, suggesting that their function is related to the tissue they recovered from [16]. MAIT cells express both antimicrobial and wound healing molecules. So that they are well aquinted to contribute both of these overlapping phases of immune responses to infections according to lymphokine milieu and legend availability [2]. The assembly of granzym B and porins within the basic biology structure of MAIT cells renders them capable for direct infected cell cytotoxicity. MAIT cells hold the position of microbial sensor via microbial metabolite and first line defenders within the inflamed tissue niche against the tissue invasive microbial infections. In human pulmonary tissue microenvironment MAIT cells produce IL17A cytokine that implicated in viral infection including SARS- COV-2. They may contribute to immune protection against some virus human infection like influenza virus [7,8].
Homeostasis
In an experimental setting, peripheral blood was collected for 30 blood healthy donors from whom PBMC were separated by cell separation media Ficoll prep. Washed PBMC were subjected to an early and prolonged activation with 5-OP-RU or anti CD3/CD28 in presence of IL2 or Il2/TGFB and examined by single cell transcriptomics and flow cytometery. It was evident that CD4+MAIT cells are associated with expression of co-stimulatory receptors IL2 signaling and memory markers, whereas CD8+ MAIT cells are defined by granzym modulated cytotoxicity and type IFN signaling. CD4-CD8-expression on MAIT cells may define distinct functional subpopulation. The landscape of MAIT cell clusters during homeostasis and early activation have shown that MAIT cell may respond earlier than conventional T cells. CD4+ and CD8+ MAIT cell subsets are transcriptionally distinct and can adopt similar program to innate lymphocyte and conventional T cells. MAIT cells up-regulate Foxp3 prolonged activated MAIT cell expand homeostatic subpopulation and inducing; proliferative, cytotoxic, regulatory and exhausted phenotypes [10].
MAIT Cells and Human Diseases
MAIT cells decreased in blood stream of tuberculus patients but appeared to be accumulated in lungs suggesting that they are recruited to the infected lung tissue contributing to tissue defects. Migration of blood MAIT cells to the infected tissue as activated and expanded clones in gut of typhoid patients. MAIT cells could be involved in autoimmune and immune mediated diseases. After activation MAIT cells could act doubly on targets of other immune cells in immune mediated and autoimmune diseases as reduced in blood, increase in the affected tissues and are in altered and dys-regulated forms [5].
MAIT Cells in Viral Infections
At resting state in human being MAIT cells are characterized by lack of granzyme B and low perforin expression. These two key proteins are required for cytotoxic activity. While once MAIT cells activated they can rapidly induce granzyme B and granzyme K. Pateint with human influenza A virus infection has shown significantly increased MAIT cell counts with an evident cytotoxic function as compared to healthy controls. Influenza infection is capable of inducing MAIT cells to up-regulate antiviral IFN-g and cytolytic granzyme B in a TCR independent pathway, requiring IL18 and potentially other mediators from accessory cells include monocyte and macrophages clinical significant of these findings is a matter of debate [8].
Measles McV initially infects macrophages or conjunctival epithelial cells then migrate to regional lymph nodes where it infects lymphocytes and spread systematically through the lympho-reticular system and generation of viremia of secondary nature. McV utilize CD46-nectin 4 and CD150 to enter host cells. While nectin 4 is used as a receptor on epithelial cell, CD150 is used to infect immune cells leading to apoptosis of CD15 memory cells, resulting in immune amnesia. Human PBMC MAIT cells were highly expressing CD150. The rapid virus induced apoptosis in infected immune cells is likely share in induction of immunosuppression associated with measles virus disease [8,17,18].
MAIT in SARS-COV-2 Infections
In 24 moderate and severe SARS-COV-2 cases, It was found that total and CD4, CD8 and double negative MAIT cell counts in circulation is reduced but with marked activation state. Meanwhile MAIT cells were enriched in the airways, on recovery MAIT cell counts restored to normal [12]. MAIT cell frequencies in 208 COVID-19 patients were investigated for MAIT cell profiles. They were of reduced counts in peripheral blood with appearance of; active, cytokine producing and CD4CD8 MAIT cell phenotypes. The activated MAIT cells were migrating to lungs and enriched therein in the affected pulmonary tissues [14]. In other study, 13 moderate and severe COVID-19 patients PBMC were mapped for MAIT cell profiles, they were decreased in circulation and activated, their activation initiate chemotaxis, apoptosis and found involved in the virus immune responses and possibly engaged in immune tissue damage [11]. MAIT cell reduction and functionally impaired in peripheral blood of; 23 mild, 22 severe, 6 asymptomatic patients and 44 were controls. Together with appearance of IFN stimulated gene up-regulated HLA-DRlo-monocytes and enrichment of suppressive macrophages [9]. Kim et al. [12], have been studied 50 severe and 50 normal control subjects, they were showing that MAIT cell reduction in number but activated in circulation and they were inversely correlated with disease severity and mortality. The activated MAIT cells migrate to lung wherein stimulate macrophage. MAIT cells contribute to the worsening of inflammation in severe pneumonic lungs (Table 4).
Table 4: Role of MAIT cells in SARS-COV-2 human pneumonia*
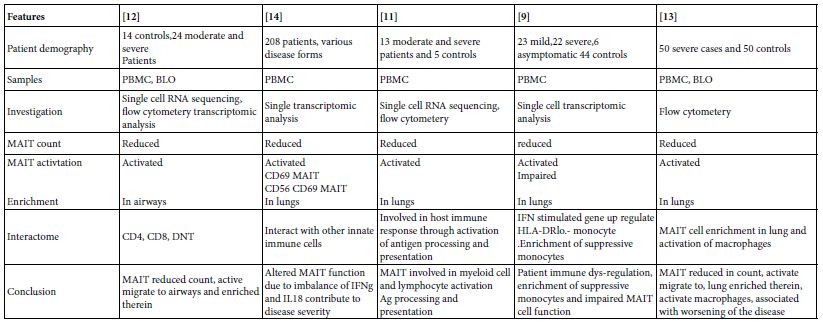
*The infection of MAIT cells by SARS-COV-2 virus is rather unclear till date
MAIT Cells in SARS-COV-2 Vaccinee
At the days seven and 21 post mRNA COVID-19 vaccination of; 42, 42, and 24 normal, immune compromised patients. From whom peripheral blood were sampled. PBMC were separated and mapped for MAIT cells. The MAIT cell frequencies remained stable overall the normal Subject vaccinee, immune compromised vaccinee. There were a positive correlation between the size of MAIT cell compartment and the vaccine induced adaptive immune response to srs-cov-2 spike protein. The pre-vaccinee and post-vaccinee levels of MAIT cells correlated positively with magnitude of SARS-COV-2 spike specific antibodies and CD4 T cells in both vaccinee groups. In normal healthy donors the levels of MAIT cell activation is negatively correlated with the spike antigen specific immune responses. Hence, MAIT cell compartment is involved in the early stages of primary adaptive immune to vaccine and may be they are important in the vaccine induced immunity [19].
The protective immune response to respiratory pathogens including influenza virus are initiated by mucosal immune system. The development of safe and effective mucosal vaccine has greatly been impaired due to lack of a properiat mucosal adjuvant. MAIT cells when co-administered with influenza A haeagglutinin protein induced protective immunity in mice and the resultant antibodies were of predominant IgA type. The heamagglutinin immune primed mice were found on influenza A live virus challenge immune protected. MAIT cell in the study act as a cellular adjuvant. Their adjuvanicity was mediated by CD40L dependent activation of DCs and subsequent priming of CD4+ follicular T cells. Hence MAIT cells acts as cellular mucosal adjuvant in a mucosal vaccine strategy [20]. The comparative role of MAIT cells in SARS-COV-2 infection and vaccination was depicted in Table 5.
Table 5: Comparative view to the role of MAIT cells in SARS-COV-2 infection and in vaccination*
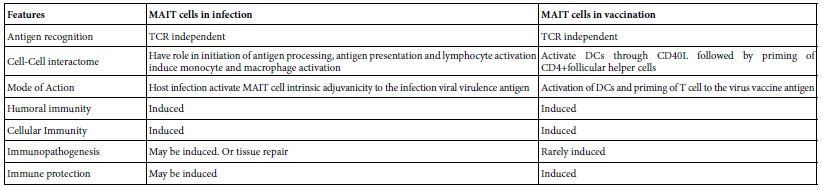
*Based on Boulus [19], Pankurest et al. [20], Shi et al. [11].
Conclusions
MAIT cells are functioning in; Homeostasis, infection, vaccination, tissue repair, autoimmune diseases, immune mediated diseases and cancer. In SARS-COV-2 infections, MAIT displayed number of immune functions as; Chemo-attractant, initiation of antigen processing, antigen presentation in antigen presenting cells as well as activation of lymphocytes. During the adaptive immune responses to SARS-COV-2 virus infection it acts early in the adaptive immune response as an intrinsic cellular adjuvant to the immune cells involved in the immune response. While in vaccination with influenza haemagglutinins it was found that MAIT cell activate DCs through DC40L and prime follicular CD4+ helper T cells by this it may acts as mucosal cellular vaccine adjuvants. MAIT cells may adopt; pyroptic, apoptotic, active tissue infiltrating and exhausted phenotypes. During SARS-COV-2 severe infection, MAIT cell count in circulation dropped down but resume active phenotype then migrates to air ways then to lungs therein they expand and worsen the patients state. On recovery, however, MAIT cells restore their normal distribution both in circulation and mucosal tissues. The evolution of MAIT functional phenotype was suggested.
References
- Ben Youssef G, Tourret M, Salon M, Ghazarian L, Houdouin V, et (2018) Ontogeny of human mucosal associated T cells and related T cell subsets. J Exp Med 215: 459-479. [crossref]
- Legoux F, Salou M, Lantz O (2020) MAIT cell development and functions: the microbial connections. Immunity 53: 710-723. [crossref]
- Lopez-Sagaseta J, Dulberger CL, Crook JE, Parks CD, Luoma AM, et (2013) The molecular basis of mucosal associated invariant T cell recognition of MRI proteins. PNAS E1771-E1778. [crossref]
- Eckle SBG, Birinskaw RW, Kostenks L, Corbett AJ, Hamish EGMC et al. (2014) A molecular basis of underpinning the T cell receptor heterogeneity of mucosal associated invariant T J Exp Med 211: 1585-1600. [crossref]
- Toubal A, Nel I, Lotersztaji S, Lehuen A (2019) Mucosal associated invariant T cells and disease. Nat Rev Immunol 19: 663-657. [crossref]
- McCarthy C, O’Donnell D, Kelly NEW, Shea DO, Hogan AE, et (2021) Covid-19 severity and obesity;Are MAIT a factor. Lancet 5: 445-447. [crossref]
- Ussher JE, Willberg CB, Klenerman P (2018) MAIT cells and J Immunol Cell Biol 96: 630-641. [crossref]
- Long Y, Hinks TSC (2021) MAIT cells in respiratory viral infections in mouse and human. Crit Rev Immunol 41: 19-35. [crossref]
- Yang Q, Wen Y, Qi F, Gao X, Chen W, et al. (2022) Suppressive monocyte impair MAIT cell response via IL10 in patients with severe covid-19. J Immunol 207: 1848- 1856. [crossref]
- Vorkas CK, Krishna C, Li K, Aube J, Fitzgerald DW, et al. (2022) Single cell transcriptional profiling reveals signatures of helper, effector, and regulatory MAIT cells during homeostasis and J Immunol 208: 1042-1056. [crossref]
- Shi J, Zhou J, Zhang X, Hu W, Zhao JF, et (2021) Single cell transcriptomic profiling of MAIT cells in patients with covid-19. Front Immunol 12: 700152. [crossref]
- Parrot T, Gorin JB, Ponzetta A, Maleki KT, Kammann T, et al. (2020) MAIT cell activation dynamics associated with covid-19 disease severity. Sci Immunol 5: eab1670. [crossref]
- Kim TO, Park KJ, Cho YN, Jin HM, Jo YG, et (2022) Alterd distribution ,activation and increased IL17 production of mucosal associated invariant T cells in patient with acute respiratory distress syndrome. Thorax 77: 865-872. [crossref]
- Flament H, Rouland M, Beaudoin L (2021) Outcomes of sars-cov-2 infection is linkedto MAIT cell activation and Nat Immunol 22: 222-235.
- Saluo M, Nicol B, Garcia A, Baron D, Michel L, et al. (2016) Neuropathogenic phenotype and functional analysis of mucosal associated invariant T cells in multiple seclerosis. Clin Immunol 166-167: 1-11. [crossref]
- Matzinger P, Kamala T (2011) Tissue based class control; the other side of Nat rev Immunol 11: 221-230. [crossref]
- DeVenes RD, McQuaid S, Van Amerongen G, Yuksel S, Verburgh RJ, et al. (2012) Measles immunosuppression lessons from maqaque Plos Pathog 8: e1002885. [crossref]
- Rudak PT, Richardson CD, Haeryfar SMM (2021) Measles virus infects and program MAIT cells for J Infect Dis 223: 667-672. [crossref]
- Boulouis C, Kammann T, Cupio A, Parrot T, Gao Y, et (2022) MAIT compartment characteristic associated with immune response magnitude and sars-cov-2 vaccine. Mol Med 28: 54. [crossref]
- Pankhurst TE, Buick KH, Lange JL, Marshall AJ, Button KR, et al. (2022) MAIT cells activate dendritic cells to promote T follicular helper cells differentiation and humoral immunity. BioRxiv 486638.