Abstract
Background: Racial gingival hyper pigmentation is a common esthetic concern, which is aggravated in patients with excessive gingival display, laser ablation is recognized as the most effective, and reliable easy handling technique.
The aim of this study was to evaluate the effect of the Low Level Diode Laser (LLDL) therapy versus surgical abrasion in the treatment of racial gingival pigmentation clinically by assessment of Melanin Pigmentation Index (MPI), Wond Healing Index (WHI), and Visual Analog Scale (VAS).
Subjects and Methods: The present study is randomized, clinical split mouth design, twenty patients of both genders with age ranged from 20 to40 years, all have racial gingival pigmentation score 2 were included in this study, all patients were non pregnant or lactating, free from systemic diseases, or any disease that cause oral pigmentation), all patients were nonsmokers, and had esthetic concern and good oral hygiene, forty sites in twenty patients were divided randomly into two groups (A and B), group A were treated by surgical abrasion at first was performed by large size round carbide bur started from the mucogingival junction toward the free gingival margin, including papillae, the wound covered by non eugenol periodontal dressing, group B: were treated by LLDL without dressing post treatment, the treated site was irradiated for a single time, LLDL was delivered by a 600-μm diameter fiber, 808 nm ± 10 nm wavelength with output power 3 Watt.
Results: There was improvement in MPI in both groups through follow up periods with superiority of LLDL group.
Conclusion: Low level diode laser can be considered as a reliable mean for elimination of melanotic lesion of gingiva and is well tolerated by patients.
Keywords
Racial gingival hyper pigmentation, Low level diode laser, Surgical abrasion
Introduction
Healthy oral mucosa is pink in color, but it can vary from light to dark pink depending on the thickness of epithelium, the amount of melanin and the number of erythrocytes present in the connective tissue vessels and their proximity to the surface. Gingival pigmentation is defined as color change of the gingiva from variable shades of pink to dark brown or black due to genetic factors which is the most important endogenous factor responsible for increased production of melanin by melanocytes, or pathological factors. Some other exogenous factors such as ultra violet radiation (UV), smoking and medications may also increase melanin synthesis [1]. Genetics Racial gingival pigmentation is a common esthetic concern, which is aggravated in patients with excessive gingival display and more common noticed in adults, the attached gingiva is the most common site of involvement in the anterior region of the maxilla and mandible followed by the buccal mucosa, lip corners, lip mucosa, dorsal and ventral surface of the tongue and rarely the floor of the mouth [2] Kauzman [3] were broadly classified the gingival pigmentation into: physiologic and pathologic, all patients except albinos have some degree of physiologic melanin distribution throughout epidermis, which occurs in all races of man without significant difference in oral pigmentation between males and females, the intensity and distribution of racial pigmentation of the oral mucosa is variable, not only between races, but also between different individuals of the same race [4]. Dummettet [5] suggested, that the degree of physiologic pigmentation is partially related to mechanical, chemical and physical stimulation. In darker skinned people oral pigmentation increase, but there is no difference in the number of melanocytes between fair skinned and dark skinned individuals, as it is due to greater melanocyte activity rather than greater number of melanocytes [6,7] and it clinically manifests as multifocal or diffuse melanin pigmentation, which is common in African, Asian and Mediterranean populations, Melanin, a brown pigment, is the most common natural pigment contributing to endogenous pigmentation of gingiva, it is produced by the melanocytes, which are mainly present in the basal and suprabasal layers of the gingival epithelium [8]. Several therapeutic techniques have been proposed and employed for gingival depigmentation, Roshni and Nandakumar [9] classified different gingival depigmentation methods into: methods aimed to mask the pigmented gingival as free gingival graft and acellular dermal matrix allograft, and methods aimed to remove the pigmented gingiva; the chemical methods these methods are no longer in use because of the destructive nature and difficulty in controlling the depth of their penetration and the surgical methods as, scalpel surgical technique, electrosurgery and radiosurgery. cryosurgery, bur abrasion; the first documented case using this technique was reported by Ginwalla [10] it is a relatively simple and versatile technique and requires minimum time and effort, and Lasers therapy which have been introduced to dentistry for more than three decades also been used for treatment of gingival pigmentation, [11] the word LASER is an acronym for light amplification by stimulated emission of radiation. The principle of the laser was the first known in 1917 when physicist Einstein [12] who described the theory of stimulated emission, there are two types of lasers: hard lasers such as carbon dioxide and neodymium-doped yttrium aluminium garnet which offer both hard tissue and soft tissue applications and cold or soft lasers based on the semiconductor diode devices, which are used predominantly for low-level diode laser therapy (LLDT) [11]. Lasers have been proposed as a useful method for the removal of the gingival melanotic pigmentations with many advantages include haemostatic capacity, no need for periodontal dressing, and fewer postoperative complications such as pain, oedema, or infections and a good visualization when compared with conventional surgical methods [13]. Laser light has four types of interactions with the target tissue which depend on the laser wavelength, emission mode, and optical properties of the tissues components such as pigments, mineral content, water content; absorption, transmission of laser energy, reflection, and scattering of the laser light these four phenomena occur together in some degree relative to each other [14]. The absorption of the laser energy by the intended tissue is the first and most desired interaction, different laser wavelengths have different absorption coefficients with the dental tissue components [14]. In general, the longer wavelengths, such as erbium laser has a greater affinity with water and hydroxyapatite, the shorter wavelengths ranging from 500 to 1000 nm are readily absorbed by the pigmented tissue and blood elements. For e.g., the pigment results of hemoglobin has greater affinity for argon laser while melanin absorbs diode and Nd:YAG laser and they have less effect on heamoglobin [15] depending on the wavelength used, some lasers able to penetrate the tissue deeper than others, in contrast other laser has a limited penetration and has effect only on the surface tissue, for example, the (Nd:YAG) which is indicated for bone and hard tissue applications, penetrates 2-5 mm into tissue [16,17] CO2 laser has a limited penetration up to 0.03 to 0.1 mm in the tissue, thus indicated for soft tissue applications, this wavelength provides enough depth to seal the damaged blood, lymphatic vessels and nerve endings resulting in good hemostasis and minimal post-operative morbidity [18] transmission of the laser energy directly through the tissue with no effect on the target tissue, the inverse of absorption, this effect is highly dependent on the wavelength of laser light, reflection, which causes laser light to redirects itself off the surface, having no effect on the target tissue, this reflected light could be dangerous when redirected to an unintended target such as eyes, a caries detecting laser device uses the reflected light to measure the degree of sound tooth structure [14,18]. Scattering of the laser light accompanied with correspondence decrease of laser energy and possibly producing no useful biologic effect, this property can cause unwanted damage as there is heat transfer to the tissue adjacent to the surgical site, however a beam deflected in different directions facilitates the curing of the composite resin or when treating an aphthous ulcer [14,18]. There are two laser delivery systems, the first is a flexible hollow waveguide or tube that has an interior mirror finish, the laser energy is reflected along this tube and exits through a hand piece at the surgical end with the beam striking the tissue in a noncontact fashion [17,19]. The second delivery system is a glass fiber optic cable, this cable can be more pliant than the waveguide, has a corresponding decrease in weight and resistance to movement, and is usually smaller in diameter, this fiber system can be used in contact or noncontact mode [19]. There are three different laser emission modes are described: [19] the first is continuous wave, where the beam is emitted at only one power level for as long as the operator depresses the foot switch, eg: diode (used in contact mode with water cooling system) and CO2 (used in no contact mode), the second is termed gated-pulse mode, meaning that there are periodic alternations of the laser energy, much like a blinking light, this mode is achieved by the opening and closing of a mechanical shutter in front of the beam path of a continuous wave emission, e.g. diode laser used in contact mode, the third mode is the free-running pulsed mode, sometimes referred to as “true pulsed”, this emission is unique in that large peak energies of laser light are emitted for a short time span, usually in microseconds, followed by a relatively long time in which the laser is off, the timing of this emission is computer controlled, not mechanically controlled as in a gated pulse device, this mode can be used for thin tissue, as: Neodymium: Yttrium, Aluminum, Garnet (Nd:YAG), Cr: YAG and Er:Cr:YSGG, if the laser is in a pulsed mode, the targeted tissue has time to cool before the next pulse of laser energy is emitted. But in continuous wave mode, the operator must cease the laser emission manually so that thermal relaxation of the tissue may occur, [20] diode lasers known as an injection laser, is a semiconductor device that produces coherent radiation (in which the waves are all the same frequency and phase) in the visible or infrared (IR) spectrum when current passes through it, [21-23] the active medium of the diode laser is a solid state semiconductor made of Aluminum, Gallium, Arsenide, and occasionally Indium, which produces laser wavelengths, ranging from approximately 810 nm to 980 nm, all diode wavelengths are absorbed primarily by tissue pigment (melanin) and hemoglobin, conversely, they are poorly absorbed by the hydroxyapatite and water present in the enamel, these lasers can also stimulate fibroblastic proliferation at low energy levels [23]. The chief advantage of the diode lasers is one of a smaller size, portable instrument, all diode laser beams are carried to the target tissue through quartz glass fibers, this is the simplest and the most effective means of conducting laser energy from its origin in the device to the oral cavity, this fibers are relatively thin between 200 and 600 microns in diameter also, there are disposable fiber-optic tips which can be discarded after use [23]. The diode laser has become the most commonly utilized laser in dentistry it has assumed a prominent role in managing soft tissues, diode lasers can be used in continuous wave mode, used for most surgical procedures such as ablating, incising and excising all types of intraoral soft tissue surgery and pulsed mode where more control over laser output is desirable in removal of coronal pulp adjunct to root canal procedures, tooth whitening, coagulation of extraction sites, disinfecting the periodontal pocket and sulcular debridement therefore it may be useful as an adjunctive means for scaling and root planing due to its bactericidal and detoxification effects, diode laser is highly absorbed by melanin pigment, so it is one of surgical treatment modalities of gingival pigmentation [23]. Moreover, diode lasers can be used with powers well below the surgical threshold for photobiomodulation or biostimulation properties which give the benefit for acceleration of healing, increasing circulation, reducing edema and minimizing pain, increased collagen synthesis, fibroblast proliferation, increased osteogenesis, and anti-inflammatory effect to treat maladies such as recurrent herpes and aphthous stomatitis [22] Materials and methods.
Subjects and Methods, the present study is randomized, clinical split mouth design, twenty patients of both genders with age ranged from 20 to40 years old, all have racial gingival pigmentation score 2 were included in this study and selected from the Outpatient Clinic Department of Oral Medicine and Periodontology, Faculty of Dentistry, Tanta University, all patients were non Pregnant or lactating, free from systemic diseases, or any disease that cause oral pigmentation (e.g. Addison disease), all patients were nonsmokers patients with esthetic concern and with good oral hygiene, the purpose of the study was explained to the patients and informed consents were obtained, forty sites in twenty patients were divided randomly into two groups (A and B), and each site on right or left side of maxilla or mandible were considered from the mesial aspect of central incisor to the distal aspect of first premolar, group A were treated by surgical abrasion (Figure 1 and 2), at first local anesthesia was given (infiltration technique, 1.8 ml mepivacaine 2%) and the depigmentation was performed by large size round carbide bur started from the mucogingival junction toward the free gingival margin, including papillae, bleeding was stopped by applying pressure by a gauze piece on the denuded epithelium, care was taken to include the epithelium at the tip of interdental papilla and the mucogingival junction on the other end without disturbing the marginal gingiva. The wound covered by non eugenol periodontal dressing. Group B: were treated by low level diode laser (LLDL) (Figure 3), without dressing post treatment, patient and operating staff wore special diode-laser protective eye glasses, highly reflective instruments or instruments with mirrored surfaces were avoided as there could be reflection of the laser beam, the treated site was irradiated for a single time, the soft tissue diode surgical laser unit delivered by a 600-μm diameter fiber (Figure 4), 808 nm ± 10 nm wavelength with output power 3 Watt, was used for depigmentation, during the procedure laser ablated the gingival epithelial surfaces little by little to reach the pigments without causing any bleeding for clear visualization, the fiber tip was continuously moved across the site to avoid heat accumulation at any site, the wounds appeared fresh with no bleeding and were no need to apply periodontal dressing. Figures 5 and 6 Patients were instructed to continue good oral hygiene and to avoid eating hot or spicy food for the first 3 days, not to traumatize the area during the healing period which is 4-7 days after treatment and were instructed to use chlorhexidine mouth wash twice daily preferably after meals for one week, no pain medications were generally prescribed, but if needed a mild analgesic anti-inflammatory (sodium diclofenac 50 mg) twice a day was given, however no antibiotics were prescribed. Post-operative assessment: clinical parameters were evaluated using wound healing index (WHI) based on the following scoring system: [24] score A: complete epithelization, score B: incomplete epithelization, score C: ulcer formation, score D: tissue defect or necrosis; melanin pigmentation index (MPI) (Figure 7), based on the following scoring system: [25] score 0: no pigmentation, score 1: solitary units (s) of pigmentation in papillary gingiva without extension between neighboring solitary units, score 2: formation of continuous ribbon extending from neighboring solitary units, and visual analog scale (VAS) [26] is a sheet of paper with horizontal line on it has a left end marked as no symptoms and a right end marked as worst imaginable symptoms, as well as a mark indicating the midpoint, the scores were calculated as follows0 = no pain, 0.1-3.0 cm = slight pain, 3.1-6.0 cm = moderate pain, 6.1-10.0 cm = severe pain (Figure 8), clinical assessment were taken for both groups as shown in Table 1.
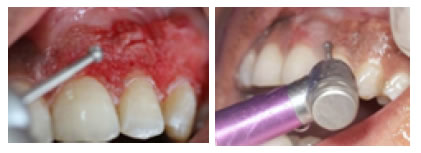
Figure 1. Rotary gingival abrasion by large rose head bur (Group A).
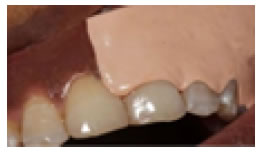
Figure 2. Application of periodontal pack after surgical abrasion.

Figure 3. Showing the diode laser divece laser (elexxion claros nanocompactclass IV dental, 600 µm optic fiber, Radolfzell,Germany).

Figure 4. 600 µm optic fiber.
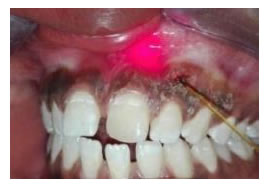
Figure 5. Applicalion of LLDL (group B).
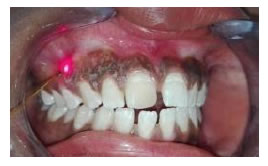
Figure 6. Depithlzation and carbonization of gingiva.
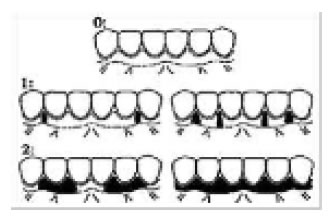
Figure 7. Scores of MPI.
|
Table 1: Comparison of MPI along the follow up periods in group A. |
||||||||
|
Melanin pigmentation index |
At the baseline |
2 Weeks |
3 Months |
χ2 |
MCp |
|||
|
No. |
% |
No. |
% |
No. |
% |
|||
|
Score 0: |
0 |
0.0 |
17 |
85.0 |
2 |
10.0 |
61.494* |
<0.001* |
|
Score1: |
0 |
0.0 |
3 |
15.0 |
10 |
50.0 |
||
|
Score 2: |
20 |
100.0 |
0 |
0.0 |
8 |
40.0 |
||
|
Sig. bet. periods |
MCp1<0.001*, MCp2<0.001*, MCp3<0.001* |
|
|
|||||
χ2: Chi square test for comparing along the follow up periods.
MCp: p value for Monte Carlo for Chi square test for comparing between the three periods.
p1: p value for comparing between baseline and 2 Weeks.
p2: p value for comparing between baseline and 3 Months.
p3: p value for comparing between 2 Weeks and 3 Month.
* Statistically significant at p ≤ 0.05.
Results
No patient had marked complications or infection during the healing of the gingiva, regarding melanin pigmentation index assessment in group A, there was a significant reduction in the gingival pigmentation from the baseline (before treatment) (100% +ve score 2) to 2 weeks post treatment and (15%+ve score 1) and the rest of cases were free from pigmentation, but at 3 months post treatment there was increase in the recurrence rate of pigmentation as (40%+ ve score 2 & 50% +ve score 1 and 10% free of pigment) (Table 2, Figure 9), however in group B at 2 weeks post laser treatment the melanin gingival pigmentation appear in 5% of cases (score1) only and the rest of cases remain free (score 0), and at 3 months post treatment 65% of cases remain free of repigmentation (score 0) as (30% score1, 5% of cases score 2 and the rest of cases remain score 0). In Table 3 and Figure 10 by comparing the results of MPI through the both groups there was statistically significant improvement in MPI in group B than group A showed in Table 2 and Figure 11.
|
Table 2: Comparison of MPI along the two studied groups at the follow up periods. |
||||||
|
Melanin pigmentation index |
Group A |
Group B |
χ2 |
MCp |
||
|
No. |
% |
No. |
% |
|||
|
At the baseline |
|
|
|
|
|
|
|
Score 0 |
0 |
0.0 |
0 |
0.0 |
– |
– |
|
Score 1 |
0 |
0.0 |
0 |
0.0 |
||
|
Score 2 |
20 |
100.0 |
20 |
100.0 |
||
|
2 Weeks |
|
|
|
|
|
|
|
Score 0 |
17 |
85.0 |
19 |
95.0 |
1.111 |
0.605 |
|
Score 1 |
3 |
15.0 |
1 |
5.0 |
||
|
Score 2 |
0 |
0.0 |
0 |
0.0 |
||
|
3 Months |
|
|
|
|
|
|
|
Score 0 |
2 |
10.0 |
13 |
65.0 |
14.728* |
0.001* |
|
Score 1 |
10 |
50.0 |
6 |
30.0 |
||
|
Score 2 |
8 |
40.0 |
1 |
5.0 |
||
χ2: Chi square test for comparing between group A and group B.
MCp: p value for Monte Carlo for Chi square test for comparing between group A and group B.
*Statistically significant at p ≤ 0.05.
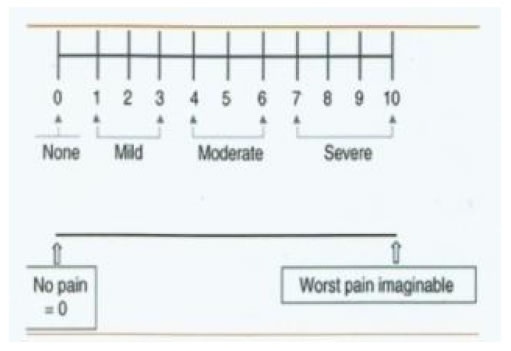
Figure 8. Recording the pain score by VAS.
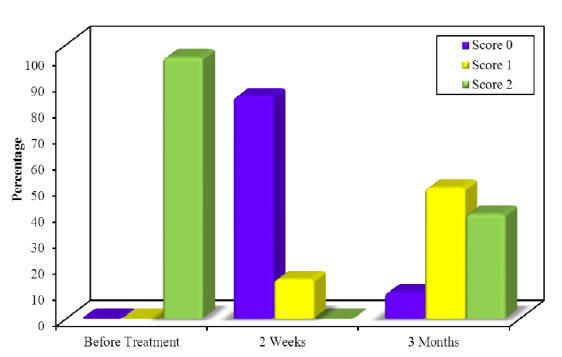
Figure 9. Comparison of MPI along the follow up periods in group A.
|
Table 3a: Study assessment and the times of assessment. |
|||||
|
Clinical assessment |
Before treatment |
Immediately post treatment |
After 2 weeks |
After 1 month |
After 3 month |
|
Clinical photographs |
+ |
+ |
+ |
+ |
+ |
|
Melanin pigmentation score MPI |
+ |
– |
+ |
– |
+ |
|
Wound healing score WHI |
– |
+ |
– |
+ |
+ |
|
Pain score VAS |
– |
+ |
+ |
– |
+ |
|
Table 3b: Comparison of MPI along the follow up periods in group B. |
||||||||
|
Melanin pigmentation index |
At the baseline |
2 Weeks |
3 Months |
c2 |
MCp |
|||
|
No. |
% |
No. |
% |
No. |
% |
|||
|
Score 0 |
0 |
0.0 |
19 |
95.0 |
13 |
65.0 |
64.446* |
<0.001* |
|
Score 1 |
0 |
0.0 |
1 |
5.0 |
6 |
30.0 |
||
|
Score 2 |
20 |
100.0 |
0 |
0.0 |
1 |
5.0 |
||
|
Sig. bet. periods |
MCp <0.001*, MCp <0.001*, MCp =0.044* 1 2 3 |
|
|
|||||
χ2: Chi square test for comparing along the follow up periods.
MCp: p value for Monte Carlo for Chi square test for comparing between the three periods.
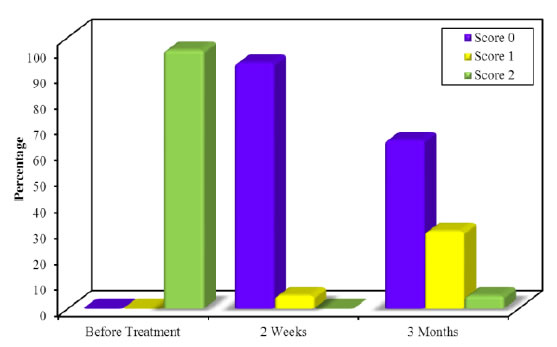
Figure 10. Comparison of MPI along the follow up periods in group B.
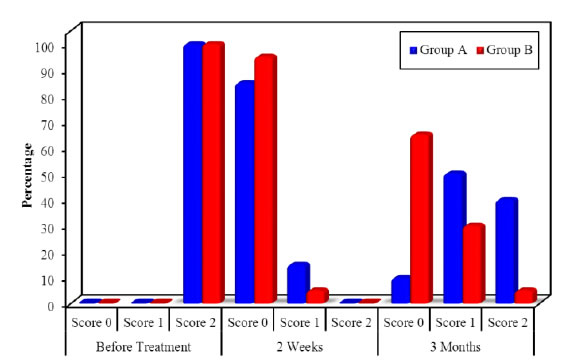
Figure 11. Comparison of MPI along the two studied groups at the follow up periods.
|
Table 4: Comparison of WHI along the two studied groups in the follow up periods. |
||||
|
Wound healing |
Group A |
Group B |
||
|
No. |
% |
No. |
% |
|
|
At baseline |
|
|
|
|
|
Score A |
0 |
0.0 |
0 |
0.0 |
|
Score B |
0 |
0.0 |
0 |
0.0 |
|
Score C |
20 |
100.0 |
20 |
100.0 |
|
Score D |
0 |
0.0 |
0 |
0.0 |
|
1 Month |
|
|
|
|
|
Score A |
20 |
100.0 |
20 |
100.0 |
|
Score B |
0 |
0.0 |
0 |
0.0 |
|
Score C |
0 |
0.0 |
0 |
0.0 |
|
Score D |
0 |
0.0 |
0 |
0.0 |
|
3 Months |
|
|
|
|
|
Score A |
20 |
100.0 |
20 |
100.0 |
|
Score B |
0 |
0.0 |
0 |
0.0 |
|
Score C |
0 |
0.0 |
0 |
0.0 |
|
Score D |
0 |
0.0 |
0 |
0.0 |
|
Table 5: Comparison the mean value of VAS scores along the two studied groups at the follow up periods. |
||||
|
Evaluation of pain |
Group A |
Group B |
Z |
p |
|
At the baseline |
|
|
|
|
|
Min.-Max. |
0.50-6.0 |
0.50-2.50 |
3.572* |
<0.001* |
|
Mean ± SD. |
3.33 ± 1.56 |
1.57 ± 0.57 |
||
|
Median |
3.50 |
1.50 |
||
|
After 2 week |
|
|
|
|
|
Min. -Max. |
0.0-1.0 |
0.0-0.0 |
2.762* |
0.006* |
|
Mean ± SD. |
0.30 ± 0.38 |
0.0 ± 0.0 |
||
|
Median |
0.0 |
0.0 |
||
|
After 3 months |
|
|
|
|
|
Min. -Max. |
0.0-0.0 |
0.0-0.0 |
0.0 |
1.000 |
|
Mean ± SD. |
0.0 ± 0.0 |
0.0 ± 0.0 |
||
|
Median |
0.0 |
0.0 |
||
Regarding Pain score (VAS)
Inter group comparison of VAS at both groups (A and B): The results showed a statistically significant reduction in the mean value of VAS in group B as compared to group A at the baseline (P=0.001) and at two weeks post treatment (P=0.006). However there was statistically non-significant difference in the mean value of pain score between both groups at three month post treatment (P=0.317), with superiority of diode laser (Tables 4, 5 and Figures 12-14).
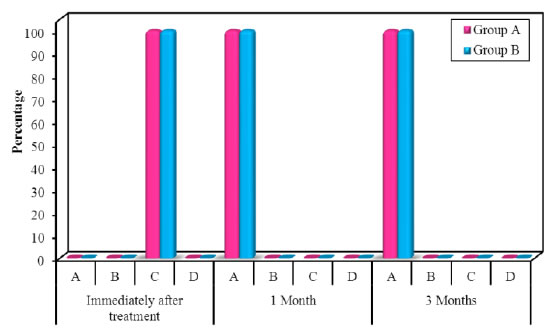
Figure 12. Comparison of WHI along the two studied groups in the follow up periods.
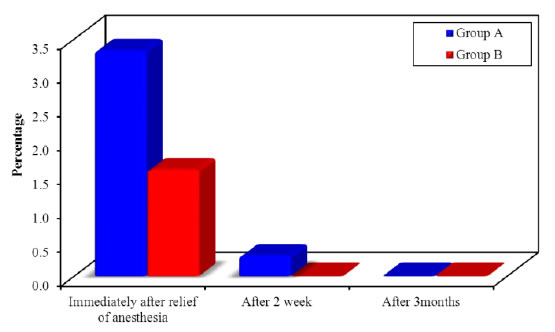
Figure 13. Comparison of the mean value of VAS scores along the two studied groups at the follow up periods.
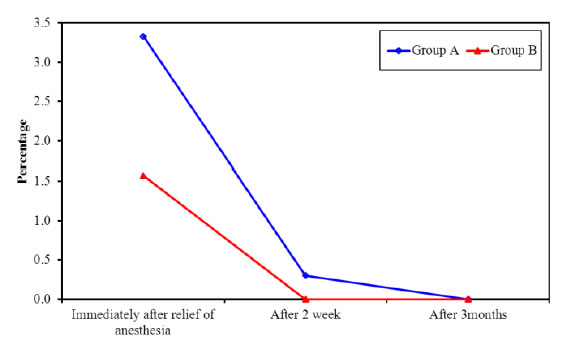
Figure 14. Comparison of the mean value of VAS scores along the two studied groups at the follow up periods.
Discussion
Gingival abrasion technique was selected in present study as it is relatively simple, safe, non-aggressive method and easy to perform, it causes less discomfort, economical as no sophisticated equipment required and is esthetically acceptable to the patients [27,28] moreover in case of recurrence, the procedure can be done repeatedly in the same areas without clinical limitations or permanent damage, [16] the diode laser is a solid state semiconductor laser that typically uses a combination of gallium (Ga), arsenide (Ar), and other elements such as aluminum (Al) and indium (In) to change electrical energy into light energy; of wavelength range from 810 nm to 900 nm, which is poorly absorbed in water, but has an affinity for hemoglobin, melanin and other pigment, resulting in much more selective destruction and far less damage to normal gingival tissue than does with CO2 laser (100) thermal effects of diode laser are attributed to ‘hot tip’ effect caused by accumulation of heat at the end of fiber, these effects result in the production of a thick coagulation layer on the treated surface, [29] Soliman [30] and Sathyanarayanan and Hari [31] reported that diode laser is a useful safe mean, and is the preferred laser for treatment of the pigmented areas of the gums when no other short Lasers are available, the present study included patients who were medically free from any systemic disease associated with pathological hyper pigmentation or improper delayed wound healing as uncontrolled diabetes, and autoimmune diseases, and non-treated periodontal disease to avoid the interference with wound healing process as these diseases affect the inflammatory mediators production, [32] also pregnant women were excluded as the hormonal changes during pregnancy and lactation causes increase of melanin production which can affect the recurrence time of pigmentation and there is no a lot of information provide to be sure that the use of laser during pregnancy and lactation is safe, [33] smokers patients were excluded, to avoid the masking of the results by smoker melanosis as smoker’s melanosis is the most common lesions in smokers’ mouth and 30% of Caucasian heavy smokers have oral melanin pigmentation [34] the MPI was assessed at the baseline (before treatment), 2 weeks and 3 months post treatment, and the results showed a significant improvement in MPI scores in both groups along the follow up periods with superiority of diode Laser as In group A, the recurrence of gingival pigmentation started at two weeks post treatment in 15% of cases and increased gradually till three months as appeared in 90% of cases but In group B, the recurrence of pigmentation started at two weeks post treatment in 5% of cases and increased gradually till three months post treatment as appeared in 35% of cases; the recurrence of melanin pigments which was observed at the end of three months in both groups (A and B), could be a result of the ongoing process of repigmentation, but this repigmentation was not as pre-treatment level in intensity of color, may be due to the lesser production of pigments, and so the intensity of pigmentation may increase with time and may reach to pre-treatment level as it depends on the racial background of the patient, the present results was consistent with the findings of Bergamaschi [35] who demonstrated that permanent results cannot be offered when gingival depigmentation procedures are performed for cosmetic reasons, repigmentation also may due to the fact that the melanocytes have a reproductive self-maintaining system of cells, and they when locally depleted, they repopulate and keratinocyte-derived growth factors “Fibroblast Growth Factor-β” act as a mitogen, these cells lack desmosomes and possess long dendritic processes that extend between keratinocytes, melanin is synthesized in the melanocytes in small structures called melanosomes, these melanosomes are injected into the keratinocytes by the dendritic processes, all individuals, whether lightly or darkly pigmented, have the same number of melanocytes in any given region of the mucosa, but it has been observed that cells which contain melanin are present in connective tissue in the case of individuals who have a very high melanin pigment score, these cells are actually macrophages that have engulfed the melanin pigment, [36] reappearance of pigmentation indicates that the melanocytes may have repopulated the treated sites from adjacent tissues, and these may be explained by Soliman [30] who discussed the use of soft-tissue diode laser in the treatment of oral racial pigmentation and found that some underlying melanocytes were not sufficiently affected by the heat to be destroyed which causes repopulation of melanocytes at treated areas causing repigmentation of gingiva (Figures 15-18).
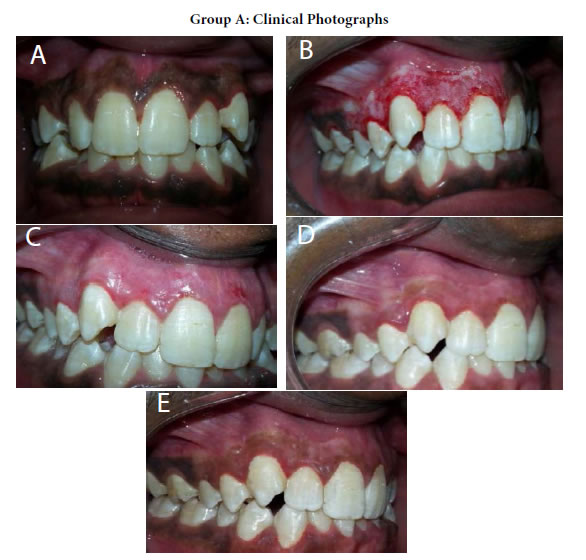
Figure 15. (a) Preopertive clinical photograph show patient has score2 of MPI at the baseline. (b) The site immediatelyposttreatment. (c) MPI (score 0) at two weeks post treatment. (d) Complete epthilization and keratinzation of gingivaat one month. (e) Core 2 ofMPI at three months post treatment.
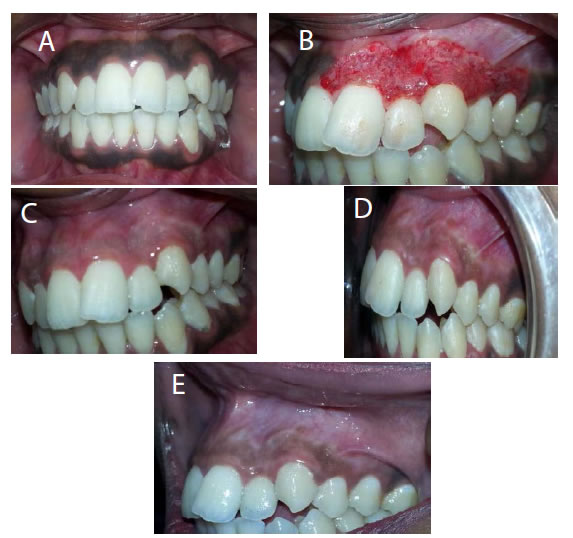
Figure 16. (a) Preopertive clinical photograph show patient has score 2 of MPI at the baseline. (b) The site immediatelyposttreatment. (c) MPI (score 0) at two weeks post treatment. (d) Complete epthilization and keratinzation of gingivaat one month post treatment. (e) MPI (score 2) at three months post treatment.
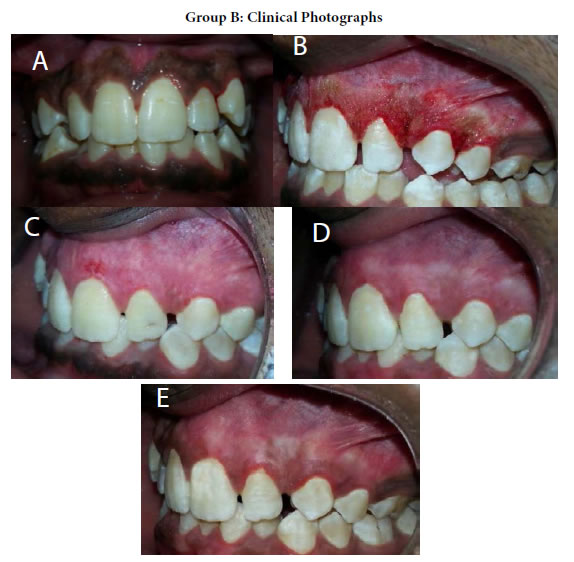
Figure 17. (a) Preopertive clinical photograph show patient has score 2 of MPI at the baseline. (b) the site immediatelypost application of LLDL. (c) MPI (score 0) at two weeks post treatment. (d) complete epthilization and keratinzation of gingivaat one month post treatment. (e) MPI (score 1) at three months post treatment.
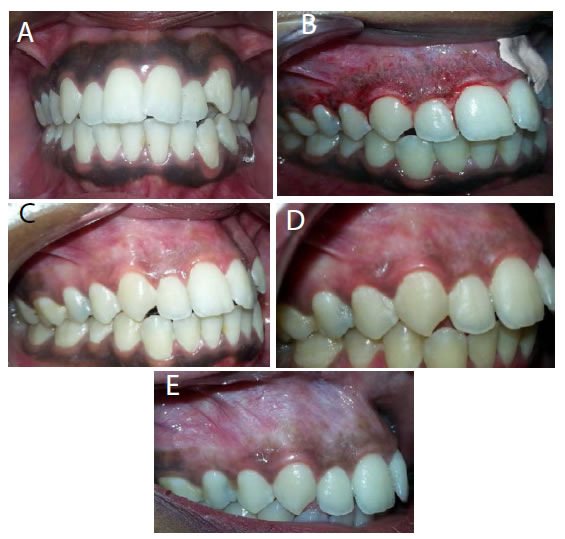
Figure 18. (a) Preopertive clinical photograph show patient has score 2 of MPI at the baseline. (b) The site immediatelypostapplication of LLDL. (c) MPI (score 0) at two weeks post treatment. (d) Complete epthilization and keratinzation of gingivaat one month post treatment. (e) MPI (score 1) at threemonths post treatment.
Furthermore, Azzeh [37] who stated that because of the presence of rete pegs in oral epithelium, the ablation of the epithelium at the same level may not remove all melanocytes which repopulate the treated area and cause repigmentation [38,39]. The clinical findings of the present study were in agreement with results of the study carried by Sathanarayanan and Lyer Mahajan [31,39] also, Abdullah and Al- shmaah study [40]. However, the results were not in agreement with the clinical results of the study conducted by Bakutra [41] they showed that at twelve months post treatment in all sites; repigmentation was observed with different grades of Hedin index, and there pigmentation in surgical stripping is significantly lesser compared to laser ablation, as lesser numbers of melanocytes were found on immune histological examination at twelve months postoperatively in sites treated by scalpel, the difference in the results may be due to different in the race of patients or difference in the degree and intensity of gingival pigment before treatment. Regarding WHI assessment the healing of the depigmented gingiva was uneventful irrespective of the techniques used, however the abrasion treated sites healed faster compared to LLDL treated sites, but this did not reach a statistically significant level and also it was necessary to cover the exposed lamina propria with a periodontal pack for seven days, although the healing of diode laser wounds were slower than that of surgical abrasion wounds, a sterile inflammatory reaction occurs after laser use, [42] evaluation of both procedures on thirty days post treatment revealed restoration of normal features of the gingiva without any scar formation, which supported by the results of Moritz [27] study as they reported a bactericidal effect of LLDL moreover it has the unique property of being able to remove a thin layer of epithelium [9,43]. The LLDL wounds were initially covered by a carbonized layer then after 24 hour were covered by a thick white fibrinous layer which is a normal characteristic of a laser wound during the first several days of healing due to the relatively thick coagulation layer on the treated surface produced by the “hot tip” of the diode laser fiber optic [44]. Gingival recession and loss of papilla were not observed. At one month post treatment the gingiva showed a normal appearance with pink color irrespective of the techniques used. The delayed healing of LLDL wounds may be due to the fact that the usual mechanism of LLDL which lead to ablation or decomposition of biological cellular materials is thermal, the thermal ablation means that the energy delivered by the laser interacts with irradiated material by an absorption process, yielding rapid raise in the intercellular temperature and pressure lead to cellular rupture, necrosis and delayed healing [45,46] also it leads to release of vapors and cellular debris termed the laser plume, which creates locally sterile condition, resulting in a reduction of bacteremia [47]. The results of the present study were in agreement with the clinical results of the study conducted by [41,47-50]. The post-operative experience of pain is a complex phenomenon, influenced by psychological, environmental and physical factors, VAS score a reliable and practical method to assess pain in clinical settings, sensitive to treatment effect and the its data derived can be analyzed using parametric statistical techniques [26]. In this study, there was a statistically significant improvement in VAS scores in both treatment groups, as in group A all patients suffer from pain ranged from mild to moderate, immediately post treatment, however pain gradually decreased till disappear completely after two weeks in all patients except two cases still complained from mild pain, only with external stimuli, like food chewing and this may be due to individual variation in pain threshold, but in group B only slight or no pain was recorded, however, the pain had reduced considerably at two weeks post treatment. Laser depigmentation procedure as compared to abrasion method had significantly lesser postoperative pain immediately after surgery (P<0.05), the increased pain perception associated with the abrasion method might be attributed to the fact that it is a more intrusive surgical procedure involving blood loss and a wide open surgical wound, which cause discomfort postoperatively since it heals by secondary intention, and slight pain after using LLDL may be attributed by formation of protein coagulum on the wound surface serving as a biological barrier, also laser irradiation seals the sensory nerve endings and inhibits the transfer of pain signals and has analgesic effects [51] caused by a disruption of Na+-K+ pump in the cell membrane, resulting in a loss of impulse conduction or simply due to an ablation of the nerve endings because of protein coagulum formation [51]. Also, in the present study, a low power of LLDL was used(1.02 W)as higher power (>2.5 W) would manifest as discomfort and pain during the post-operative follow up periods, moreover could delay the healing time, therefore, as a rule, a low-power setting (≤2.5 W) was used during the procedure [43]. The results were in agreement with the results of the study conducted [41,52].
Conclusion
Both, LLDL and surgical abrasion were safe for treatment of oral racial pigmentation, and they showed significant clinical improvement in Melanin Pigmentation Index (MPI) with superiority of low level diode lasers until 3 months follow up without any post-surgical problem. LLDL documented as there was minimal postoperative pain and discomfort, ease of the procedure and more acceptable for patients as compared to surgical abrasion.
Recommendation
Multiple sessions of LLDL is recommended in some patients, removal of gingival melanin pigmentation should be performed cautiously and the adjacent teeth should be protected, since inappropriate application may cause gingival recession, damage to underlying periosteum and bone, delayed wound healing, as well as loss of enamel. Further studies are required for standardized laser parameters (as wave length and frequency of used laser) in the management of the different scores of gingival pigmentation.
References
- Brenner M, Hearing VJ (2008) Modifying skin pigmentation – approaches through intrinsic biochemistry and exogenous agents. Drug DiscovToday Dis Mech 5: 189-199. [crossref]
- Dummett CO, Barens G. (1971) Oromucosal pigmentation: An updatedliterary review. J Periodontol 42: 726-736. [crossref]
- Kauzman A, Pavone M, Blanas N, Bradley G (2004) Pigmented lesions of the oral cavity: Review, differential diagnosis, and case presentations. J Can Dent Assoc 70: 682-683. [crossref]
- Ozbayrak S, Dumlu A, Ercalik-Yalcinkaya S (2000) Treatment of melanin-pigmented gingiva and oral mucosa by CO2 laser. Oral Surg Oral Med Oral Pathol Oral 90: 14-15. [crossref]
- Dummett CO, Sakamura J, Barrens G (1981) Attitudes towards normal pigmentation of the oral mucosa. Quintessence Int 10: 115-22. [crossref]
- Esen E, Haytac MC, Oz IA, Erdoğan O, Karsli ED (2004) Gingival melanin pigmentation and its treatment with the CO2 laser. Oral Surg Oral Med Oral Pathol Oral RadiolEndod 98: 522-527.
- Gorsky M, Buchner A, Fundoianu-Dayan D, Aviv I (1984) Physiologic pigmentation of the gingiva in Israeli Jews of different ethnic origin. Oral Surg Oral Med Oral Pathol 58: 506-509. [crossref]
- Ciçek Y, Ertas U (2003) The normal and pathological pigmentation of oral mucous membrane: A review. J Contemp Dent Pract 4: 76-86. [crossref]
- Roshni T, Nandakumar K (2005) Anterior esthetic gingival depigmentation and crown lengthening: report of a case. J ContempDent Pract 3: 139-147. [crossref]
- Ginwalla TM, Gomes BC, Varma BR (1966) Surgical removal of gingival pigmentation. J Indian Dent Assoc 38: 147-150.
- Dederich DN, Bushick RD (2004) ADA council on scientific affairs, division of science; Journal of the American Dental Association. Lasers in dentistry: separating science from hype. J Am Dent Assoc 135: 204-212.
- Einstein A (1917) Strahlungs- emission and absorption of radiation in quantum theory. Verhandlungen der Deutschen Physiklischen Gesellschaft.Concept and theory of stimulated light emission. 18: 318-323.
- Lee KM, Lee DY, Shin SI, Kwon YH, Chung JH, Herr Y (2011) A comparison of different gingival depigmentation techniques: ablation by erbium:yttrium- aluminum-garnet laser and abrasion by rotary instruments. J Periodontal Implant Sci 41: 201-207. [crossref]
- Kumar M, Grishmi M, Girish N, Harish B (2017) State of the art laser technology in dentistry. Heal Sci 3: 3-6.
- (1996) Lasers in periodontics. J Periodontol 67: 826-830.
- Coluzzi DJ (2004) Fundamentals of dental lasers: science and instruments. Dent Clin North Am 48: 751-70. [crossref]
- Rossmann JA, Cobb CM (2000) Lasers in periodontal therapy. Periodontal 9: 150- 164.
- Sulieman M (2005) An overview of the use of lasers in general dentist practice: Laser physics and tissue interaction. Dent Updat 32: 228-236. [crossref]
- Aoki A, Sasaki KM, Watanabe H, Ishikawa I (2004) Lasers in nonsurgical periodontal therapy. Periodontol 36:59-97.
- Clayman L, Kuo P (1997) Laser in Maxillofacial Surgery and Dentistry. New York: Thieme 1-9.
- Stirban A, Gawlowski T, Roden M (2013) Vascular effects of advanced glycation endproducts: Clinical effects and molecular mechanisms. Mol Metab 3: 94-108. [crossref]
- White JM, Goodis HE (1993) Thermal laser effects on intraoral soft tissue, teeth and bone in vitro In: Proceedings of the third international congress on lasers in dentistry international society for lasers in dentistry; 1993: University of Utah Printing Services 189-90.
- John JG (2015) Diode Lasers: A Primer. AGD 1-10.
- Tal H, Oegiesser D, Tal M (2003) Gingival depigmentation by erbium: YAG laser: Clinical observations and patient responses. J Periodontol 74: 1660-1667. [crossref]
- Takashi H, Tanaka K, Ojima M, Yuuki k (2005) Association of melanin pig mentation in the gingiva of children with parents who smoke pediatrics 116: 86-90. [crossref]
- Wewers ME and Lowe NK (1990) A critical review of visual analog scales in the measurement of critical phenomena. Research in Nursing and Health 13:227-236. [crossref]
- Moritz A, Gutknecht N, Doertbudak O, Goharkhay K, Schoop U et al. (1997) Bacterial reduction in periodontal pockets through irradiation with a diode laser: A pilot study. J Clin Laser Med Surg 15: 33-37. [crossref]
- Farnoosh AA (1995) Treatment of gingival pigmentation and discoloration for esthetic purposes. IntJPeriodontics Restorative Dent 10: 312-319. [crossref]
- Kravitz ND, Kusnoto B (2008) Soft-tissue lasers in orthodontics: an overview. AmJ Orthod Dentofacial Orthop 133: 110-114. [crossref]
- Soliman MM, Al Thomali Y, Al Shammrani A, El Gazaerly H (2014) The use of soft tissue diode laser in the treatment of oral hyper pigmentation. Int J Health Sci 8: 133-1340.
- Sathyanarayanan C, lyerH (2014) A comparative study between conventional method and diode laser in treatment of gingival hyperpigmentation. International Journal of laser dentistry 4: 8-19. [crossref]
- Agrawal A, Gang TB, Rusiñol AE (2014) Recognition functions of pentameric C-reactive protein in cardiovascular disease. Mediators Inflamm 2014: 319215. [crossref]
- Mcleod SD, Ranson M, Mason RS (1994) Effects of estrogens on human melanocytes in vitro. J Steroid Biochem Mol Biol 49: 9-14.
- Axell T, Hedin CA (1982) Epidemiologic study of excessive oral melanin pigmentation with special reference to the influence of tobacco habits. Scand J Dent Res 90: 434-442. [crossref]
- Bergamaschi O, Kon S, Doine AI, Ruben MP (1993) Melanin repigmentation after gingivectomy: A 5-year clinical and transmission electron microscopic study in humans. IntJ Periodontics Restorative Dent 13: 85-92. [crossref]
- Ashri N, GaziM (1990) More unusual pigmentations of the gingiva. Oral Sur Oral Med Oral Pathol 70: 445-449. [crossref]
- Azzeh MM (2007) Treatment of gingival hyperpigmentation by erbium- doped:yttrium, aluminum, and garnet laser for esthetic purposes. J Periodontol 78: 177-184.42. AshriN, GaziM (1990) Moreunusual pigmentations of the gingiva Oral Sur Oral Med Oral Pathol 70:445-449. [crossref]
- Dummett CO (1946) Physiologic pigmentation of the oral and cutaneous tissues in the Negro. J Dent Res 25: 421-432. [crossref]
- Mahajan G, Kaur Ht, Jain S, Kaur N, Kaur NS, et al. (2017) Compare the gingival melanin repigmentation after diode laser application and surgical removal done by scraping with Kirkland knife. IndianSociety of Periodontology.
- Abdullah A B and Al-shmaah AZ (2014) The Use of ErCrYSGG versus diode laser in gingival melanin de-pigmentation, Clinical study. IJERSTE 3: 2319-7463.
- Bakutra G, Shankarapillai R, Mathur L, and Manohar B (2017) comparative evaluation of diode laser ablation and surgical stripping technique for gingival depigmentation: A clinical and immunohistochemical study. International Journal of Health Sciences11: 51-58.
- Kaarthikeyan G, Jayakumar ND, Padmalatha O, Varghese S, Kapoor R (2012) Pain assessment using a visual analog scale in patients undergoing gingival depigmentation by scalpel and 970 nm diode laser surgery. J Laser Dent. 20: 20-23.
- Pick R, Colvard M (1993) Current status of laser in soft tissue dental surgery. J Periodontol 64: 589-602. [crossref]
- Ojha A, Srivastava1 V (2015) Gingival depigmentation with diode laser, electrosurgery and scalpel: a comparative report of 2 cases TMU. J Dent 2: 1.
- Coluzzi DJ (2004) Fundamentals of dental lasers: Science and instruments. Dent Clin North Am 48: 751-770. [crossref]
- Simşek Kaya G, YapiciYavuz G, Sümbüllü MA, Dayi E (2012) A comparison of diode laser and Er: YAG lasers in the treatment of gingival melanin pigmentation. Oral Surg Oral Med Oral Pathol Oral Radiol 113: 293-299. [crossref]
- Rossmann JA, Gottlieb S, Koudelka BM, McQuade MJ (1987) Effects of CO2 laser irradiation on gingiva. J Periodontol 58: 423-425. [crossref]
- (2016) Comparative evaluation of two surgical techniques using conventional scalpel method and diode laser for treatment outcome of depigmentation: 6 months follow- up study. J Dent Lasers 10: 2-9.
- Grover HS, Dadlani H, Bhardwaj A, Yadav A, Lal S (2014) Evaluation of patient response and recurrence of pigmentation following gingival depigmentation using laser and scalpel technique: A clinical study. J Indian Soc Periodontol 18: 586-592. [crossref]
- Lagdive S, Doshi Y, Marawar P (2009) Management of gingival Hyper pigmentation using surgical blade and diode laser therapy: a comparative study. J Oral Laser Applications9: 41-47. [crossref]
- Schroeder HE (1969) Melanin containing organelles in cells of the human gingival I. Epithelial melanocytes. J Periodont Res. 4: 1-18. [crossref]
- Suragimath G, Lohana MH, Varma S (2016) A split mouth randomized clinical comparative study to evaluate the efficacy of gingival depigmentation procedure using conventional scalpel technique or diode laser. J Lasers Med Sci 7: 227-232. [crossref]