Abstract
Metallic nanoparticles like iron, zinc, aluminium, copper, and nickel which are used in the removal of dyes and toxic materials from wastewater affect aquatic organisms including fishes. The present study deals with the impact of differentially synthesized copper oxide nanoparticles on haematological and biochemical changes of Common carp Cyprinus carpio. Hyptis suovelens leaf extract was used for the biological synthesis of copper oxide nanoparticles. Copper (II) sulphate pentahydrate is used as a precursor and starch as a capping agent for chemical synthesis. Characterized by using UV-Vis spectroscopy, Fourier transform infrared spectroscopy, Scanning electron microscopy, Energy dispersive X-ray analysis, and X-ray diffraction. Acute toxicity of biologically and chemically synthesized copper oxide nanoparticles was conducted for a period of 96 hrs. Based on the Probit value sub-acute concentrations of biologically and chemically synthesized copper oxide nanoparticles such as 0 (control), 0.23, 0.31, 0.46, 0.93 ppm and 0.018, 0.024, 0.036, 0.072 ppm respectively for a period of 14 days under static condition. Hematological (Red blood corpuscle, white blood corpuscle, Polymorph Neutrophils, lymphocytes, Eosinophils, Hemoglobin) and biochemical parameters (protein, carbohydrate, lipids) in gill, muscle and liver were estimated after exposing the fish for 7 and 14 days. One-way ANOVA was used for the analysis. The mortality was increased with increased concentration of differentially synthesized CuO Nps on Cyprinus carpio. Haematological and biochemical parameters decreased with increased concentration of differentially synthesized CuO NPs when compared to control. Results conclude that the biologically synthesized copper oxide nanoparticles are less toxic than chemically synthesized copper oxide nanoparticles on Cyprinus carpio.
Keywords
Biological, Chemical, Synthesis, Haematology, Biochemical, Cyprinus carpio
Introduction
Nanoparticles are very attractive materials for the manipulation, sensing and detection of biological structures and systems than bulk particles. The principal factors which make nanomaterials different from their bulk counterparts include an increase in their relative surface area and quantum effects, which affect their physical and chemical properties [1]. Nanoparticles are used in many economic sectors including consumer products, the metal industry, transportation, cosmetics, pharmaceuticals, antimicrobial agents and agriculture [2]. Large scale production and use of metal oxide nanoparticles lead to uncontrolled release into the environment. Nanoparticles present in the natural environment includes volcanic dust, glacial ice cores, clays, organic matter, iron oxides and other minerals which may also increase harmful effects to human health [3]. Nanoparticles and metal oxides present in solid wastes, direct or accidental spillages and industrial waste effluent water enter the aquatic ecosystem by rainwater runoff and the waste of nanoparticles synthesizing industry were dumped into landfills may also be washed off into the aquatic environment, because it is one of the final destinations of the released nanomaterials in the environment [4]. Among numerous metal oxide nanoparticles, copper oxide nanoparticles are often present in industrial wastewaters that may become extremely toxic for aquatic animals as their concentration in the water increase. Besides, it is toxic, harmful to organisms living in water, accumulate in the food chain and may affect humans too [5]. Among animal species, fishes are the inhabitants that cannot escape from the detrimental effect of these pollutants. The amount of protein, lipid and carbohydrate in fishes is used for the determination of their nutritive value in fish and haematological analysis of blood parameters are considered physiological indicators of the whole body and therefore are important in diagnosing the structural and functional status of fish exposed to nanoparticles. Haematological parameters such as hematocrit, haemoglobin, and red blood cells are used to assess the functional status of the oxygen-carrying capacity of the bloodstream and have been used as an indicator of metal pollution in the aquatic environment [6]. The biologically and chemically synthesized copper oxide nanoparticles have electrochemical properties, which can be used in the field of medicine, and fish farming. The present study is related to the impact of differentially synthesized copper oxide nanoparticles on haematological, and biochemical analysis of freshwater fish Common carp Cyprinus carpio.
Materials and Methods
For the biological synthesis of copper oxide nanoparticles methanol extracted Hyptis suovelens leaf extract and 1 mM of 100 ml of copper sulphate was used in the ratio of 1:1 under stirring vigorously in a magnetic stirrer for 2 minutes. After stirring the precipitation was achieved. The pH was observed at 7 and kept overnight. Then the CuO precipitate was centrifuged at 3500 rpm for 10 minutes. The centrifugal process continued with water and repeated the centrifuging process for purifying CuO. Then the precipitate was dried at room temperature. Finally, copper oxide nanoparticles were stored in dry tube containers. For chemical synthesis copper (II) sulphate pentahydrate (CuSO4.5H2O) was used as precursor and starch as capping agent. The synthesis preparation was started with the addition of 0.1 M CuSO4.5H2O solution into 120 ml of starch (1.2%) solution with constant stirring under a magnetic stirrer for about 30 min. Then 50 ml of 0.2 M ascorbic acid solution is added under continuous rapid stirring. Subsequently, 30 ml of 1 M sodium hydroxide solution was slowly added to the prepared solution with constant stirring and heating at 80°C for 2 hrs. The colour of the solution turned yellow to Ochre. After the completion of the reaction, the solution was taken from the heat and allowed to settle overnight and the supernatant solution was discarded. Then precipitates were separated by filtration and the filtrate was washed with deionised water and ethanol three times to remove the excess starch which is bound with nanoparticles. Ochre colour precipitates obtained was dried at room temperature. After drying, nanoparticles were obtained and stored in a glass vial for further studies.
For acute toxicity analysis (LC50) healthy fishes of Cyprinus carpio (weight of 7.8 ± 0.02 g and length 6.5 to 7.5 cm) were procured from AM fish farm, Madurai, Tamil Nadu, India and acclimatized to laboratory condition for about 15 days in the fish tank with 50 L aerated dechlorinated tap water. Feeding was given for one hour before the replacement of water. Water (one-third) was changed frequently to remove the excretory wastes. Feeding was stopped 24 hrs before the commencement of the experiment to keep the experimental animals more or less in the same metabolic state. The initial weight and length of the fish were measured. Fish were exposed to biologically and chemically synthesized CuO NPs for a period of 24, 48, 76 and 96 hrs, according to the OECD Test Guideline in static method (The water was not changed during the test). The carp were exposed to biologically and chemically synthesized CuO NPs in different concentrations such as 0 (control), 5, 10, 15, 20 and 25 ppm and 0.5,1, 1.5, 2 and 2.5 ppm respectively for a period of 96 hrs. All the experiments were conducted in a 20 L glass tank containing 10 fish in each concentration. During the experimental period, the mortality was absorbed and statistical Probit analyses using SPSS was carried out to find the LC50 of the CuO NPs.
For Sub-acute toxicity analysis glass tanks with 20 L capacity were taken and each glass tank was filled with 15 L of water. In each glass tank, 10 healthy fishes (weight of 7.8 ± 0.02 g and length 6.5 to 7.5 cm) were introduced and different concentrations of biologically synthesized CuO Nps (i.e. 0.23,0.31, 0.46, 0.93 ppm) was added and control was maintained without CuO. Chemically synthesized CuO Nps were exposed in each glass tank for different concentration (i.e.0.018, 0.024, 0.036, 0.072 ppm). The manifestation and survival time of fish was observed in each concentration for 14 days. After the experimental period, the fishes are sacrificed for the analysis of toxicological parameters.
Common carp were exposed to CuO NPs for 14 days. Blood samples were collected from fish in each exposure group and were subjected to complete blood profile analysis. Haematological parameters such as Red blood cells (RBC) (millions/cumm). White blood cells (WBC) (cells/cumm), Polymorph Neutrophils (%), lymphocytes (%), Eosinophils (%), and Hemoglobin (gm/dl).Total protein, carbohydrate and lipid in muscle, gill, liver of Cyprinus carpio was estimated after the 7th and 14th days (7-9]
Fish used in the present research was by the guidelines of the Committee for Control and Supervision of Experiments on Animals [CPCSEA, Ministry of Environment & Forests (Animal Division), Government of India] on the care and use of animals in scientific research and also approved by the Institutional Ethical Committee for Research on Human and Animal Subject (IECRHAS) from The Gandhigram Rural Institute – Deemed to be University, Govt. of India, Gandhigram, Tamil Nadu, India [7-9].
The experimental results are presented in the form of Tables and graphs using Microsoft Excel (Version 2007). One-way ANOVA was used for the analysis using SPSS (Version 2013). The data was input manually and computed. The output results obtained from the software indicate whether is there any differences between the treatments and days. Sum of Variability of sample means (MS), Critical probability value (F) and Probability (Prob.) are also obtained.
Results
The UV-Vis analysis of biologically and chemically synthesized CuO Nps are presented in Figure 1. The sharp bands were observed close to both 249 nm and 264 nm throughout the reaction that indicating the formation of CuO NPs. The FT-IR spectra of the Hyptis suveolens methanol extract were absorbed in different peaks (Figure 2). Several adsorption peaks at 3942.11, 3409.27, 2923.02, 1640.44 and 1055.63 cm-1, like as N-H stretch of amines O-H stretch of Carboxylic group, N-H bending of amines, C-N stretch of aliphatic amines. In biologically synthesized copper oxide nanoparticles, peak values at 3947.47, 3407.04, 2924.51, 1610.08, 1276.10, 1107.36, 817.95, and 616.56 cm-1 (Figure 2a) were observed. A peak at 616.56 cm-1 and 3947.47 cm-1 corresponds to O-H stretch phenolic compounds, N-H stretch of amines, O-H stretch of Carboxylic group, N-H bending of amines, C-H stretch of aliphatic amines, C-Cl stretch of alkaloids and C-H bending of alkanes. In chemical synthesis, copper oxide nanoparticles were carried out to identify the possible functional groups responsible for the reduction of the copper Oxide Nps (Figure 2b). The bands are observed in the sample at 608.07, 1027.54, 1375.48,1108.019, 1009.96, 1108.01, 1634.39, 617.07, 1018.60, 1411. 25, 1099.07, 1625.45 and 617.01 cm-1 were associated with C-O alcohol, N=O Nitro group, C=CO Carbonyl and C-CL alkaline Halide. The SEM images of biologically and chemically synthesized CuO Nps are presented in Figure 3a and 3b. It clearly shows that the particles are small and uniform in size, almost spherical which is free from agglomeration in the biologically synthesized copper oxide nanoparticles. The elemental composition of the biological and chemical synthesized CuO Nps are identified (Figure 4a and 4b) and were 82% and 67% by an Energy Dispersive X-ray Spectroscopy. XRD patterns of biologically and chemically synthesized CuO nanoparticles are presented in Figure 5. In biosynthesized CuO Nps (Figure 5a) a series of diffraction peaks at 2θ of 32.55˚, 35.56˚, 38.75˚, 48.75˚, 53.48˚, 58.34˚, 61.57˚, 65.43˚, 66.27˚, 72.40˚ and 75. 25˚ were assigned to (110), (111), (200), (−202), (020), (202), (−113), (-311), (022), (311) and (004) planes respectively, which are in good agreement with those of powder CuO Nps obtained from the International Centre of Diffraction Data card (JCPDS-80-1916). In chemical synthesized CuO Nps, the XRD diffraction peaks are indexed as 34.014˚ (002), 56.523˚ (111), 69.234˚ (204), 67.002˚ (202) and 68.393˚ (113) (Figure 5b). All diffraction peaks are indexed according to the hexagonal phase of copper oxide nanoparticles ((JCPDS-80-1916) and no characteristic peak impurity phase except copper oxide nanoparticles and revealed the good spherical shape of 65 nm.
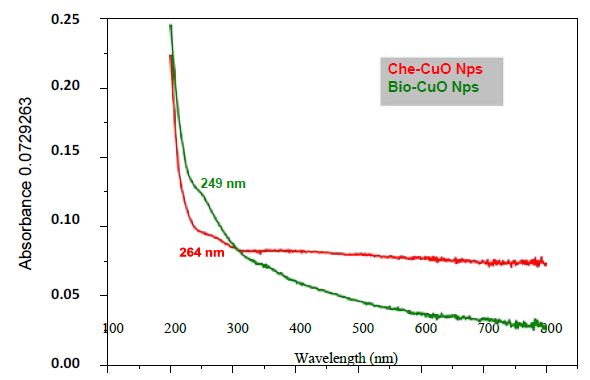
Figure 1: UV-VIS analysis of biologically and chemically synthesized CuO Nanoparticles
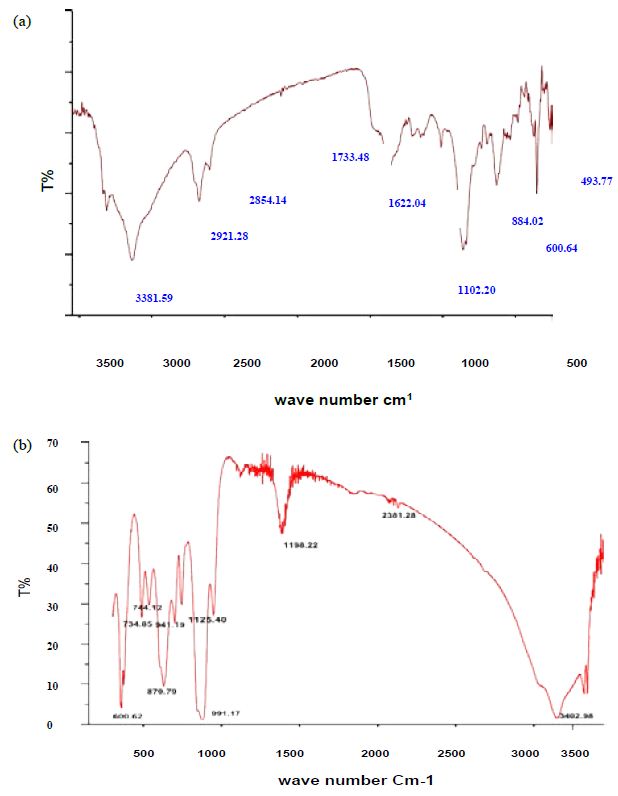
Figure 2: FT-IR analysis of a) biologically and b) chemically synthesized CuO Nps
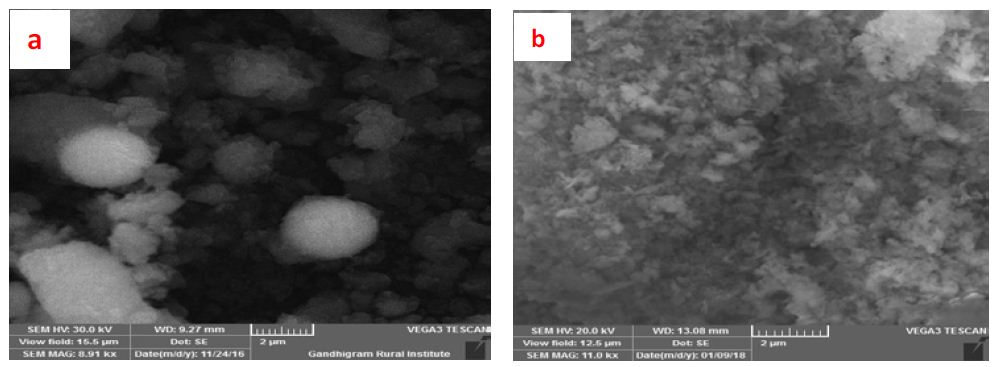
Figure 3: SEM image of biologically (a) and chemically(b) synthesized CuO Nps
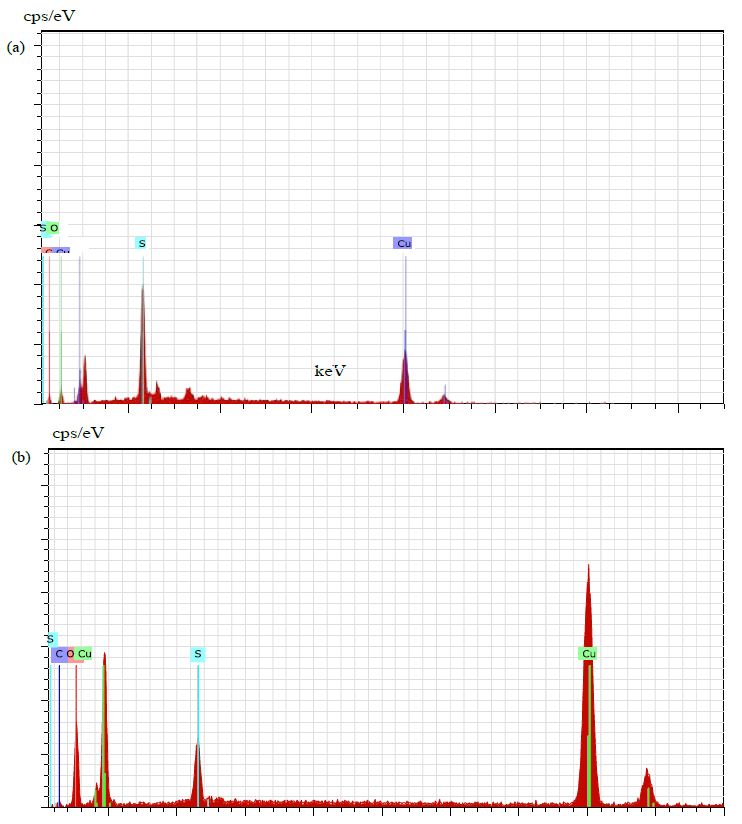
Figure 4: EDAX of biologically (a) and chemically (b) synthesized CuO Nps
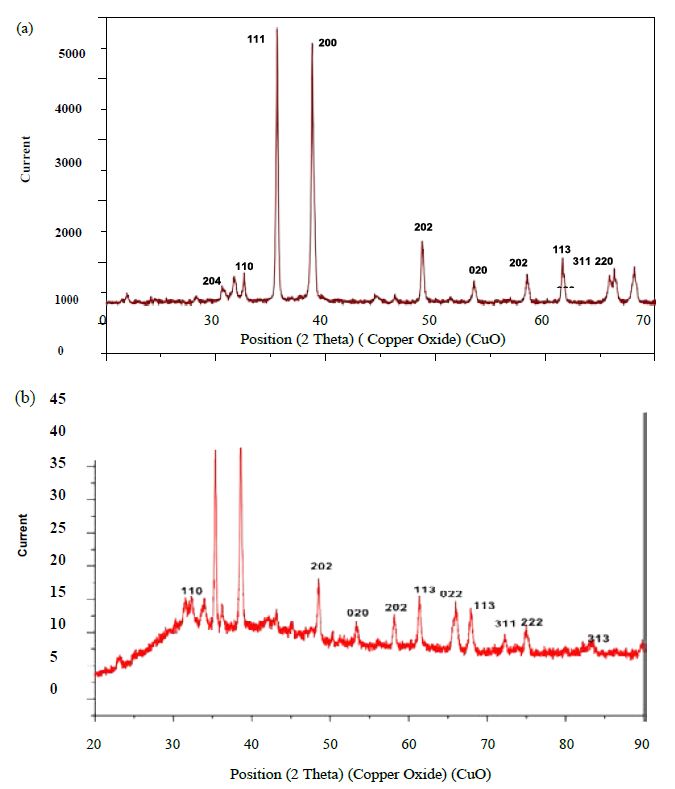
Figure 5: XRD analysis of biologically (a) and chemically (b) synthesized CuO NPs
Acute toxicity tests for biologically and chemically synthesized CuO Nps are presented in Tables 1 and 2. No mortality was observed in the control group during the experiment. Fish mortality increased significantly when the concentration and the time of exposure were increased. Mortality was observed in biologically and chemically synthesized CuO Nps exposed to a dose of 5, 10, 15, 20 and 25 ppm and of 0.5,1, 1.5, 2 and 2.5 ppm for 96 hrs respectively. This indicates an increase in toxicity with exposure duration. Before death, fish exhibited rapid gill movement, nervous manifestations, erratic swimming, loss of equilibrium and inability to remain upright.
Table 1: Probit analysis of biosynthesized CuO NPs on Common carp Cyprinus carpio
|
Probit |
95% Confidence Limits for concentration |
95% Confidence Limits for log concentration |
||||
|
Estimate |
Lower Bound |
Upper Bound |
Estimate |
Lower Bound |
Upper Bound |
|
| LC1 |
1.544 |
0.000 |
4.647 |
0.189 |
-6.854 |
-667 |
| LC2 |
2.123 |
0.000 |
5.550 |
0.327 |
-5.727 |
-744 |
| LC3 |
2.599 |
0.000 |
6.221 |
0.415 |
-5.012 |
-794 |
| LC4 |
3.026 |
0.000 |
6.787 |
0.481 |
-4.475 |
-832 |
| LC5 |
3.424 |
0.000 |
7.291 |
0.535 |
-4.038 |
-863 |
| LC6 |
3.804 |
0.000 |
7.756 |
0.580 |
-3.667 |
-890 |
| LC7 |
4.172 |
0.000 |
8.194 |
0.620 |
-3.342 |
-913 |
| LC8 |
4.532 |
0.001 |
8.613 |
0.658 |
-3.051 |
-935 |
| LC9 |
4.885 |
0.002 |
9.021 |
0.689 |
-2.787 |
-955 |
| LC10 |
5.235 |
0.003 |
9.419 |
0.719 |
-2.544 |
-974 |
| LC15 |
6.972 |
0.029 |
11.403 |
0.843 |
-1.543 |
1.057 |
| LC20 |
8.755 |
0.174 |
13.640 |
0.942 |
-760 |
1.135 |
| LC25 |
10.643 |
0.775 |
16.745 |
1.027 |
-111 |
1.224 |
| LC30 |
12.684 |
2.639 |
22.652 |
1.103 |
421 |
1.355 |
| LC35 |
14.922 |
6.272 |
39.246 |
1.174 |
797 |
1.594 |
| LC40 |
17.411 |
10.015 |
94.151 |
1.241 |
1.001 |
1.974 |
| LC45 |
20.213 |
12.830 |
269.507 |
1.306 |
1.108 |
2.431 |
| LC50 |
23.411 |
15.090 |
823.141 |
1.369 |
1.179 |
2.915 |
Table 2: Probit analysis of chemically synthesized CuO NPs on Common carp Cyprinus carpio
|
Probit |
95% Confidence Limits for concentration |
95% Confidence Limits for log concentration |
||||
|
Estimate |
Lower Bound |
Upper Bound |
Estimate |
Lower Bound |
Upper Bound |
|
| LC1 |
0.053 |
0.011 |
0.088 |
-1.278 |
-1.946 |
-1.054 |
| LC10 |
0.092 |
0.035 |
0.131 |
-1.034 |
-1.458 |
-883 |
| LC15 |
0.105 |
0.046 |
0.144 |
-977 |
-1.341 |
-840 |
| LC20 |
0.0117 |
0.056 |
0.157 |
-931 |
-1.250 |
-805 |
| .LC25 |
0.128 |
0.067 |
0.168 |
-892 |
-1.173 |
-774 |
| LC30 |
0.139 |
0.078 |
0.180 |
-857 |
-1.105 |
-744 |
| LC35 |
0.0150 |
0.090 |
0.193 |
-825 |
-1.044 |
-715 |
| LC40 |
0.0161 |
0.103 |
0.206 |
-794 |
-988 |
-685 |
| LC40 |
0.0172 |
0.116 |
0.222 |
-764 |
-936 |
-655 |
| LC50 |
1.84 |
0.130 |
0.239 |
-735 |
-888 |
-621 |
Haematological parameters of Common carp exposed to differentially synthesized CuO Nanoparticles is presented in Table 3. All the parameters decreased with the increased concentration of differentially synthesized CuO nanoparticles. Impact of differentially synthesized CuO NPs on protein (µg/mg) of Common carp is presented in Figure 6a and 6b and is significant (P<0.05) (Table 4). As the concentration of biologically synthesized CuO, Nps was increased (0.23-0.93 ppm) with exposure periods (7-14 days) decreased protein level in muscle, gill, and liver was observed when compared to the control group. In chemically synthesized CuO Nps, with increased concentration (0.018-0.072 ppm) and exposure periods protein level in muscle, gill, liver decreased when compared to the control group. The impact of differentially synthesized copper oxide NPs on carbohydrate (µg/mg) in Common carp is presented in Figure 7a and 7b and is significant (P<0.05) (Table 4). As the concentration of biologically synthesized CuO Nps increased (0.23-0.93 ppm) for 7-14 days, there is a gradual decrease in the carbohydrate level in muscle, gill, and liver of Common carp. In chemically synthesized CuO Nps also the increase in the concentration (0.018-0.072 ppm) for 7-14 days, level of carbohydrate in muscle, gill and liver decreased when compared to control. The impact of differentially synthesized copper oxide NPs on lipid (µg/mg) in Cyprinus carpio is presented in Figure 8a and 8b and is significant (P<0.05) (Table 4). In biologically synthesized CuO Nps, when the concentration increased (0.23-0.93 ppm) with exposure periods from 7 to 14 days a decrease of lipid level in muscle, gill, and liver when compared to control.
Table 3: Hematological parameters of Common carp Cyprinus carpio exposed to biologically (a) and Chemically (b) synthesized CuO NPs.
|
Parameters |
Treatments |
a |
b |
||
|
Experimental Period (Days) |
Experimental Period (Days) |
||||
|
7 |
14 |
7 |
14 |
||
|
Red Blood Cells (Million/cumm) |
T0 (Control) |
0.20 |
0.05 |
0.47 |
0.30 |
| T1 |
0.17 |
0.01 |
0.25 |
0.19 |
|
| T2 |
0.10 |
0.005 |
0.15 |
0,007 |
|
| T3 |
0.07 |
0.005 |
0.05 |
0.04 |
|
| T4 |
0.02 |
0.004 |
0.03 |
0.02 |
|
|
White Blood Cells (Cells/cumm) |
T0 (Control) |
10000 |
8100 |
9500 |
7100 |
| T1 |
9200 |
7800 |
9000 |
6900 |
|
| T2 |
8000 |
5100 |
7100 |
5000 |
|
| T3 |
5200 |
3150 |
5200 |
2000 |
|
| T4 |
3100 |
2000 |
4100 |
1200 |
|
|
Polymorphic Neutrophils (%) |
T0 (Control) |
60 |
35 |
52 |
48 |
| T1 |
54 |
32 |
46 |
38 |
|
| T2 |
32 |
30 |
42 |
25 |
|
| T3 |
30 |
25 |
20 |
19 |
|
| T4 |
20 |
18 |
10 |
7 |
|
|
Lymphocytes (%) |
TO |
65 |
42 |
49 |
38 |
| T1 |
63 |
32 |
40 |
34 |
|
| T2 |
45 |
30 |
24 |
20 |
|
| T3 |
32 |
29 |
15 |
10 |
|
| T4 |
19 |
18 |
05 |
02 |
|
|
Eosinophils (%) |
T0 |
0.70 |
0.35 |
0.6 |
0.40 |
| T1 |
0.60 |
0.29 |
0.41 |
0.30 |
|
| T2 |
0.39 |
0.25 |
0.38 |
0.10 |
|
| T3 |
0.31 |
0.24 |
0.12 |
0.05 |
|
| T4 |
0.21 |
0.19 |
0.002 |
0.001 |
|
|
Haemoglobin(gm/dl) |
To |
0.7 |
0.50 |
0.47 |
0.31 |
| T1 |
0.6 |
0.45 |
0.25 |
0.19 |
|
| T2 |
0.5 |
0.30 |
0.15 |
0.07 |
|
| T3 |
0.3 |
0.20 |
0.05 |
0.04 |
|
| T4 |
0.26 |
0.19 |
0.03 |
0.02 |
|
Table 4: ANOVA of a) biologically and b) chemically synthesized CuO NPs on protein, carbohydrate and lipid in Muscle, Gill and Liver of Common carp Cyprinus carpio.
|
Biochemical Parameters |
Source of variation |
SS |
Df |
MS |
F |
PROB>F |
|
|
Protein |
a |
Different concentration
Exposure period Error Total |
600555 319371 66537.4 586464 |
4 6 24 34 |
150138.7 386561.9 27772.39 |
5.406043 13.91893 |
0.003002 8.79 S |
|
b |
Different concentration
Exposure period Error Total |
879498.7 2498362 643437.1 4021297 |
4 6 24 34 |
219874.7 416393.6 26809.88 |
8.201255 15.53135 |
0.000257 3.26 s |
|
|
Carbohydrate |
a |
Different concentration
Exposure period Error Total |
24158.99 33680.29 16192.63 74031.92 |
4 6 24 34 |
6039.748 5613.382 674.6931 |
8.951846 8.319904 |
0.000143 6.13 s |
|
b |
Different concentration
Exposure period Error Total |
17890.6 26671.92 11201.2 55763.72 |
4 6 24 34 |
4472.651 4445.319 466.7166 |
2.776289 2.508189 |
0.000154 2.16 s |
|
|
Lipid |
a |
Different concentration
Exposure period Error Total |
0.202657 32.19387 11.85176 44.24828 |
4 6 24 34 |
0.050664 5.365645 0.493823 |
0.102596 10.86552 |
0.980507 7.41 s |
|
b |
Different concentration
Exposure period Error Total |
0.326944 32.19435 12.09109 44.61239 |
4 6 24 34 |
0.081736 5.365725 0.503796 |
0.16224 10.6506 |
0.955403 8.74 s |
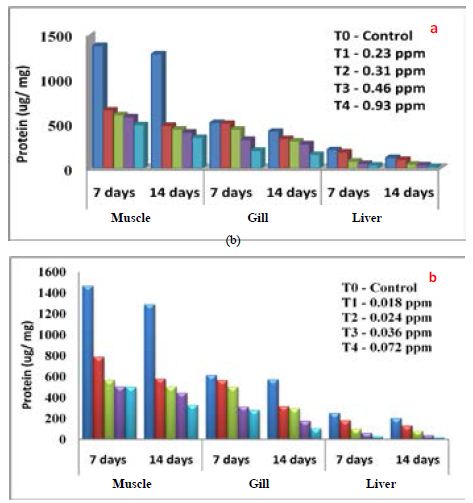
Figure 6: Impact of a) biologically and b) chemically synthesized CuO NPs on protein of Common carp Cyprinus carpio
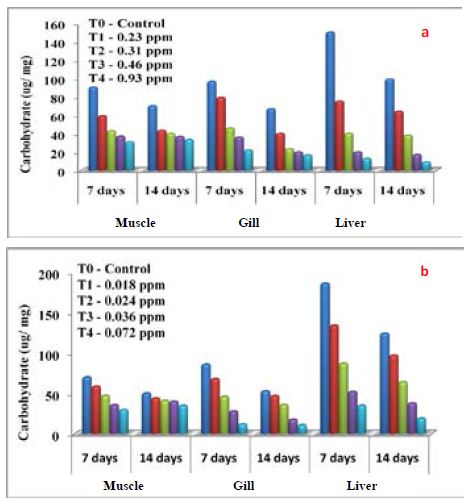
Figure 7: Impact of a) biologically and b) Chemically synthesized CuO NPs on carbohydrate of Common carp Cyprinus carpio
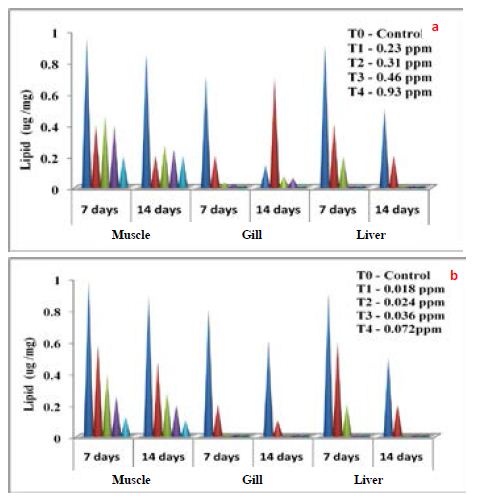
Figure 8: Impact of a) biologically and b) Chemically synthesized CuO NPs on lipids of Common carp Cyprinus carpio
Discussion
The UV-Vis analysis of biologically and chemically synthesized CuO Nps shows the sharp peaks at 249 nm and 264 nm, this peak indicates the formation of CuO NPs. Similarly, Sutradhar Prasanta et al. [10] reported that a strong absorption peak at 269 nm for CuO Nps synthesized by using tea leaf and coffee extraction and between 200-300 nm for CuO Nps synthesized from C. papaya leaves extract. Raja Naika et al. [11] reported that the synthesis of copper oxide nanoparticles (CuO Nps) using Gloriosa superba L. plant extract and UV-vis spectra, the formed CuO Nps dispersed in water exhibiting the maximum absorption peaks at about 380 nm. Jayalakshmi and Yogamoorthi [12] reported that the synthesized copper oxide particles are subjected to UV-spectroscopic analysis and obtained a single peak but broad at 263 nm indicating the presence of oxides of copper metal. Pulicherla Yugandhar et al. [13] reported the synthesis of copper oxide nanoparticles (CuO NPs) using fruit extract of Syzygium alternifolium, peak manifested at 285 nm in UV-Vis analysis confirms the synthesis of CuO NPs. In biologically and chemically synthesized copper oxide nanoparticles, FT-IR peaks correspond to O-H stretch phenolic compounds, N-H stretch of amines, O-H stretch of Carboxylic group, N-H bending of amines, C-H stretch of aliphatic amines, C-Cl stretch of alkaloids, C-H bending of alkanes and these peaks are similarly reported by Abbas Eslami et al. [14]. Vijay Kumar et al. [15] reported spectral peaks proposing the occurrence of bands relevant to amide NAH stretching (3444 cm-1), alkane CAH stretching (2926 cm-1) anhydride C-O bending (1880 cm-1) and C-O stretching (1087 cm-1). The SEM image of biologically and chemically synthesized CuO Nps clearly shows that the particles are small and uniform in size, almost spherical which is free from agglomeration that indicates the spherical and rectangular shape of synthesized copper oxide nanoparticles. Gultekin Demet Demirci et al. [16] reported that the copper nanoparticle was synthesized by using the water extract of Erzincan Cimini grape (Vitis vinifera). Nithya et al. [17] and Hemalatha and Maheshwari [18] reported the synthesis of CuO nanoparticles from Aloe barbadensis has a well-defined morphology and are nearly spherical. Saranyaadevi et al. [19] reported the synthesis of Cu NPs by the plant extract of Capparis zeylanica. Synthesized Cu Nps were obtained by Scanning Electron Microscopy (SEM) analysis and showed that the spherical and relatively uniform shape of the copper nanoparticles was confirmed in the range of 60-100 nm. Javad Karimi and Sasan Mohsenzadeh [20] reported that the formation of copper nanoparticles from the Aloe vera flowers extract as well as their morphological dimensions in the SEM study demonstrated that the average size was 40 nm and was spherical. Suresh et al. [21] reported that the SEM image of tea decoction stabilized copper nanoparticles prepared from copper sulfate salt and the image show clear spherical morphology with an average particle size around 5 nm. Mina Sorbiun et al. [22] reported the green synthesis of highly crystalline CuO nanoparticles (NPs) by oak fruit hull (Jaft) as a reducing and stabilizing agent and observed that most of the CuO nanoparticles are in nanometre scale and are mostly of quasi-spherical shape. The elemental composition of the biological and chemical synthesized CuO Nps was identified as spherical and sizes were 82% and 67% by an Energy Dispersive X-ray Spectroscopy. Alwin David et al. [23] reported the presence of copper and oxygen signal peaks in the EDX spectrum confirms CuO Nps which are synthesized by using Momordica charantia leaf extract. Vanathi et al. [24] also reported that Aloe barbadensis mediated copper oxide nanoparticles were spherical and elemental compositions were confirmed by EDX. Ayesha Khan et al. [25] reported that the synthesis of Cu NPs by chemical reduction method and ascorbic acid as reducing agents at low temperature (80°C). EDX spectroscopy is applied to quantify the elemental composition of the synthesized Cu nanoparticles in 83. 75%. Pulicherla Yugandhar et al., (2018) synthesized copper oxide nanoparticles (CuO NPs) using fruit extract of Syzygium alternifolium and the EDX analysis of nanoparticles showed 34.32 and 31.54% of copper oxide. The crystalline structure and size of biologically and chemically synthesized CuO nanoparticles were analyzed by using XRD analysis, a series of diffraction peaks and plane values showing hexagonal phase (JCPDS-80-1916) and 67 nm and 65 nm in size respectively. Similarly, Srivastava Sanjay et al. [26] reported that the lattice parameters of a unit cell of CuO are found as 4.691 Å, b=3.432 Å, c=5.138 Å and the peak positions with 2 theta values of 29.4, 36.8, 42.1, 61.9 and 77.6 are indexed with 110, 111, 200, 220 and 222 planes of biologically synthesized CuO NPs by using alga (Bifurcaria bifurcata) extract. Abboud et al. [27] reported that the CuO Nps synthesized by the hydrothermal method is indexed as monoclinic phased CuO NPs by comparison with a Joint committee on Powder Diffraction Standards (JCPDS) card files No. 80-1916. Abbas Eslami et al., reported that the XRD patterns of all the diffraction peaks are in good agreement with the standard diffraction data for CuO (JCPDS 45-0937), no characteristic peaks were observed for other oxides (such as Cu2O or Cu2O3) and the average crystallite size estimated by Debye-Scherer equation was about 71 nm.
Haematological parameters can be useful for the measurement of physiological disturbances in stressed fish and thus used as a reliable indicator for toxicological research, environmental monitoring and as indicators of disease and stress [28]. In this study, haematological parameters such as RBC, WBC, polymorphic, neutrophils, lymphocytes, eosinophil, and haemoglobin levels in Cyprinus carpio decreased significantly (P<0.05) with increasing concentration of biologically and chemically synthesized CuO NPs. Similar results were also reported in fish Cyprinus carpio exposed to sublethal concentration of ammonia for 35 days [29], in rainbow trout (Oncorhynchus mykiss) exposed to CuSO4 [30]. Mayilathal and Thamizhselvi [31] reported that the blood parameters like total RBC count, WBC count, haemoglobin content and hematocrit in the blood of C. carpio exposed to LC50 value of lead nitrate (4.45 ppt) are decreased with exposure periods. The decrease in RBC count, and Hb levels may act as indicators of acute anaemia. John et al. [32,33] suggested that in toxicological experiments the decrease in RBC count, Hb and Hct levels, lysing of RBC due to toxicant stress may also lead to a reduction in Hb values in the fish, it leads to alteration in the selective permeability of the membrane. Kori-Siakpere and Oghoghene [34] reported that an increase in the number of white blood cells is a normal reaction of fish to substances that alter their normal physiological processes. But in this present study, WBC count was significantly decreased with an increase in the concentration of CuO Nps, it may due to the toxic effect of nanoparticles and stress caused on the cell production activity of the spleen. Keerthika et al. [35] reported that the haematological parameters decreased with the increased concentration of iron oxide nanoparticles in Labeo rohita.
The protein, carbohydrate and lipid in muscle, gill, and liver of Cyprinus carpio decreased as the concentration of differentially synthesized CuO NPs when compared to the control group. Sevcikova et al. [36,37] reported a decreased total glycogen content of the fish tissues viz., gill, muscle when exposed to CuO and TiO2 Nps in Common carp and Zebrafish respectively. Mehibeen Javad and Nazurausmani [38] reported that the decreased total protein, albumin and globulin level when exposed to Cu NPs in Cyprinus carpio fish tissue viz., liver and muscle. Abdel-Tawwab Mohsen et al. [39] reported that the total protein and lipid decreased significantly by increasing Zinc NPs concentrations and exposure period. This decrease may be due to Zn exposure which causes significant alteration in the protein secondary structure by decreasing the α-helix and increasing the β-sheet content of the gill tissues of carp Rohu (Labeo rohita). Similarly, Rajeshwari and Sevarkodiyone [40] reported that the carbohydrate, lipids and protein content in Cyprinus carpio fish tissue viz., muscle and liver were exposed to sublethal concentrations of cadmium Nps for various exposure periods.
Conclusion
From the present study, it was concluded that the biologically synthesized copper oxide nanoparticles are less toxic than chemically synthesized copper oxide nanoparticles on Cyprinus carpio. This information may provide great benefit in the field of nanotechnology, biomedical and aquaculture industries.
Acknowledgment
The authors thank the Department of Biology, The Gandhigram Rural Institute – Deemed to be University, Dindigul, Tamil Nadu, India for giving laboratory facility.
Conflict of Interest
The authors declare no conflict of interest.
Authors Contribution
Muthuswami Ruby Rajan: The research work was formulated and the guidance was given to the second author for execution. Jayaram Mekala: Done experiments related to the biological and chemical synthesis of CuO nanoparticles, characterization using UV-Vis, FT-IR, SEM, EDX, and XRD, collection of fish, estimation of haematological, and biochemical parameters.
Abbreviations
ppm: Parts per million; mg: Milligram; mM: Milli Molar; cu mm: Cubic Millimeter; g/dl: Grams Per Decilitr; UV-Vis: Ultraviolet-Visible Spectroscopy; FTIR: Fourier Transform Infrared Spectroscopy; SEM: Scanning Electron microscopy; EDX: Energy Dispersive X-Ray Spectroscopy; XRD: X-Ray Diffraction; CuO: Copper oxide; NPs: Nanoparticles; ANOVA: Analysis of Variance;%: Percentage; SS: Sum of square Variation; df: Degree of Freedom; MS: Variability of Sample Mean; F: Critical Probability Value; Prob: Probability; S: Significant; Ө: Theta; JCPDS: Joint Committee on Powder Diffraction Standards; KeV: Kiloelectron Volts; LC50: Lethal concentration; WBC: White Blood Cell; RBC: Red Blood Cell; nm: nanometer; °C: Degree Celsius.
References
- Nel A, Xia T, Madler L, Li N (2006) Toxic Potential of Materials at the Nanolevel. Sci 311: 622-627. [crossref]
- Jovanović B, Palić D (2012) Immunotoxicology of non-functionalized engineered nanoparticles in aquatic organisms with special emphasis on fish-review of current knowledge, gap identification, and call for further research. Aqua Toxicol 118: 141-151. [crossref]
- Hasan K, Fatih A, Mert G, Sevdan Y, Mehmet A, et al. (2016) A comparative toxicity study between small and large size zinc oxide nanoparticles in tilapia (Oreochromis niloticus): Organ pathologies, osmoregulatory responses and immunological parameters. Chemosphere 144: 571-582. [crossref]
- Hamed GF, Halina BD, Hadi J, Soleiman H, Neda M, et al. (2017) The protective role of vitamin E on Oreochromis niloticus exposed to ZnO NP. Ecotoxicol Environ Saf 145: 1-7. [crossref]
- Pourkhabbaz A, Mohammad EK, Vahed K, Mohammad HH (2011) Effects of water hardness and Cu and Zn on LC50 in Gambusia holbrooki. J Chem Speci Bioavail 23: 224-228.
- Rajendra S, Ramrao P, Shivdas N (2014) Biochemical Analysis to Study the Nutritive Value of the Fish Catla Catla after Exposure to the Phytotoxin from Lasiosiphon Eriocephalus. Int Conf Trends in Econ Hum Manag 203-204.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein Measurement with the Folin Phenol Reagent. J Biol Chem 193: 265-275. [crossref]
- Sadasivam S, Manickam (1991) Biochemical methods (Third edition), New Age International (P) limited , publishers.
- Folch J, Lees M, Stanley SGH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J of Biochem 226: 497-509. [crossref]
- Sutradhar P, Mitali S, Debasish M (2014) Microwave synthesis of copper oxide nanoparticles using tea leaf and coffee powder extracts and its antibacterial activity. J Nanostru Chem 4: 1-6.
- Raja NH, Lingaraju K, Manjunath, Danith Kumar K, Nagaraju G, et al(2015) Green synthesis of CuO nanoparticles using Gloriosa superba extract and their antibacterial activity. J Taibah Uni for Sci 9: 7-12.
- Jayalakshmi, Yogamoorthi A (2014) Green synthesis of copper oxide nanoparticles using aqueous extract of flowers of Cassia alata and particles characterization. Int J Nanomat Biostruct 4: 66-71.
- Pulicherla Y, Thirumalanadhuni V, Yagani JR, Narasimha G, Savithramma N, et al. (2018) Cost Effective, Green Synthesis of Copper Oxide Nanoparticles Using Fruit Extract of Syzygium alternifolium (Wt.) Walp., Characterization and Evaluation of Antiviral Activity. J Cluster Sci 29: 743-755.
- Abbas E, Nafise MJ, Seyed GH, Maryam A (2017) Synthesis and Characterization of CuO Nanoparticles by the Chemical Liquid Deposition Method and Investigation of its Catalytic Effect on the Thermal Decomposition of Ammonium Perchlorate. Central Euro J Energetic Mater 14(1): 152-168.
- Vijay Kumar PPN, Shameem U, Pratap K, Kalyani RL, Pammi SVN (2015) Green synthesis of copper oxide nanoparticles using Aloe vera leaf extract and its antibacterial activity against fish bacterial pathogens. Bio Nano Sci 1-12.
- Gultekin DD, Hayrunnisa N, Azize AG, Nurhan HK (2017) Biosynthesis and characterization of copper oxide nanoparticles using cumin grape Vitis vinifera cv. Int J Sec Metabolite 4: 77-84.
- Nithya KP, Yuvasree N, Neelakandeswari N, Rajasekaran K, Uthayarani M, et al. (2014) Preparation and Characterization of Copper Oxide Nanoparticles. Int J Chemical Tech Res 6: 2220-2222
- Hemalatha S, Maheshwari M (2017) Green synthesis, characterization and antibacterial studies of CuO nanoparticles from Eichhornia crassipes. Rasayan J Chem10: 838-843.
- Saranyaadevi K, Subha V, Ernest Ravindran RS, Renganathan S (2014) Synthesis and characterization of copper nanoparticle using Capparis zeylanica leaf extract. Int J Chem Tech Res 6: 4533-4541
- Javad K, Sasan M. (2013) Rapid, green, and eco-friendly biosynthesis of copper nanoparticles using flower extract of Aloe vera. Nano-Metal Chem 45: 895-898.
- Suresh Y, Annapurna S, Singh AK, Bhikshamaiah G (2014) Green Synthesis and Characterization of Tea Decoction Stabilized Copper Nanoparticles. Int J Innovat Res Sci Engin Tech 3: 11265-11270.
- Mina S, Ebrahim SM, Ali R, Asemeh MM (2018) Biosynthesis of metallic nanoparticles using plant extracts and evaluation of their antibacterial properties. J Nanochem Res 3: 1-16.
- Alwin DS, Immanuel RS, Vignesh KS (2017) Biosynthesis of copper oxide nanoparticles using Momordica charantia leaf extract and their Nat Confer on Recent Innov in Sci Tech Engin pp. 333-340.
- Vanathi P, Rajiv P, Rajeshwari S. (2016) Synthesis and characterization of Eichhornia-mediated copper oxide nanoparticles and assessing their antifungal activity against plant pathogens. J Mat Sci 39: 1165-1170.
- Ayesha K, Audil R, Rafia Y, Ren C.(2016) A chemical reduction approach to the synthesis of copper nanoparticles. Int J Nano Let 6: 21-26.
- Srivastava S, Mahendra K, Arvind A, Sudhanshu KD (2013) Synthesis and Characterisation of Copper Oxide nanoparticles. IOSR J Appl Phy 5: 61-65.
- Abboud Y, Saffaj T, Chagraoui A, El Bouari A, Brouzi K, et al. (2014) Biosynthesis, characterization and antimicrobial activity of copper oxide nanoparticles (CuO NPs) produced using brown alga extract Bifurcaria bifurcata. Appl Nanosci 4: 571-576.
- Shaluei F, Hedayati A, Jahanbakhshi A, Kolangi H, Fotovat M (2013) Effect of subacute exposure to silver nanoparticle on some hematological and plasma biochemical indices in silver carp (Hypophthalmichthys molitrix) Human Experi Toxicol 32: 1-8. [crossref]
- Thangam Y, Perumayee M, Jayaprakash S, Umavathi S, Basheer SK (2014) Studies of ammonia toxicity on haematological parameters to freshwater fish Cyprinus carpio (Common carp) Int J Curr Microbiol Appl Sci 3: 535-542.
- Shaw JB, Genan AB, Richard DH (2012) Effects of waterborne copper nanoparticles and copper sulphate on rainbow trout, Oncorhynchus mykiss: Physiology and accumulation. Aqu Toxicol 117: 90-101. [crossref]
- Mayilathal K, Thamizhselvi N (2014) Impact of lead nitrate on haematological parameters of Cyprinus Carpio (Common Carp) Int JAdv Phar Bio Chem 3: 919-925.
- John R, Ahmad K, Gadgil, Sharma S (2007) Antioxidative response of Lemna polyrhiza L. to cadmium NPs stress. J Enviro Biol 28: 583-589. [crossref]
- Lavanya R, Veerappan N (2011) Antibacterial potential of six seaweeds collected from Gulf of Mannar of Southeast Coast of India. Adv Biol Res 5: 38-44.
- Kori-Siakpere O, Oghoghene UE (2008) Sublethal haematological effects of zinc on the freshwater fish, Heteroclarias sp. Osteichthyes: Clariidae. Afr J Biotech 7: 2068-2073.
- Keerthika V, Ramesh R, Rajan MR (2017) Toxicity assessment of iron oxide nanoparticles in Labeo rohita. Int J Fish Aqua Stu 5: 01-06.
- Sevcikova M, Modra H, Blahova J, Dobsikova R, Plhalova L, et al. (2016) Biochemical, haematological and oxidative stress responses of common carp (Cyprinus carpio ) after sub-chronic exposure to copper. Veterinarni Medicina 1: 35-50.
- Vutukuru SS, Sharada R, Kalpana Ba (2013)Acute exposure to nano Titanium dioxide causes biochemical and physiological alterations in the Zebrafish (Danio rerio)-A Case Study. Int J Chem Tech Res 5: 646-653.
- Mehibeen J, Nazura U (2015) Stress response of biomolecules (carbohydrate, protein, and lipid profiles) in fishes Channa punctatus inhabiting river polluted by thermal power plant effluent. Saudi J Biol Sci 22: 237-242.
- Abdel-Tawwab M, Mohamed NM, Mousaad, Khaled M, Sharafeldin, et al. (2013) Changes in growth and biochemical status of common carp, Cyprinus carpio exposed to water-born zinc toxicity for different periods. Int Aqu Res 5: 1-9.
- Rajeshwari S, Sevarkodiyone SP (2017) Biochemical parameters of Common Carp Cyprinus carpio exposed to Cadmium nanoparticles change to the leaf extract of Abutilon indicum. J Clin Micro Biochem Tech 3: 54-57.