Abstract
Introduction: This study aimed to explore the impact of comorbid hyperuricemia on disease severity in Japanese patients with coronavirus disease 2019 (COVID-19). This retrospective cohort study included patients with COVID-19 between July 2020 and February 2021.
Methods: We divided patients into mild, moderate, and severe groups according to the degree of disease severity. Clinical and biochemical parameters on admission and comorbidities were compared between the mild and severe groups.
Results: We enrolled 146 patients in this study: 36 patients were allocated to the mild group, 96 to the moderate group, and 14 to the severe group. The male sex, age, body mass index (BMI), systolic blood pressure, pulse rate, white blood cell counts, levels of serum urea nitrogen and uric acid were significantly higher in the severe group than in the mild group (p<0.05), while lymphocyte counts and estimated glomerular filtration rate were significantly lower (p<0.05). As for comorbidities, malignant tumor, diabetes mellitus, hypertension, chronic kidney disease (CKD), hyperlipidemia, and hyperuricemia were associated with COVID-19 severity. Logistic regression analysis indicated that hyperuricemia was significantly positively associated with the severity of COVID-19 independent of age, sex, BMI, comorbidities of diabetes mellitus, and malignant tumor. However, the association between hyperuricemia and COVID-19 severity was eliminated by correction with hypertension or CKD.
Conclusion: These data suggested that comorbidities of hyperuricemia may indicate an increased risk of COVID-19 progression. Furthermore, patients with hyperuricemia comorbidities may require careful and intensive multidisciplinary treatment for hyperuricemia and hypertension and/or CKD to prevent progression of COVID-19.
Keywords
Chronic kidney disease, COVID-19, Hyperuricemia, Hypertension
Introduction
Coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has rapidly spread worldwide, promoting the World Health Organization (WHO) to declare COVID-19 a pandemic and a public health emergency [1]. As of 29 May 2022, WHO reported over 526 million confirmed COVID-19 cases. Furthermore, over six million COVID-19-related deaths have been identified around the world [2]. The high contagiousness of SARS-CoV-2 and severity of COVID-19 are serious challenges of this infectious disease.
Several factors have previously been reported as severity risk factors of COVID-19, such as advanced age, obesity, hypertension, diabetes, smoking, malignancy, coronary heart disease, chronic liver disease, chronic obstructive pulmonary disease, and chronic kidney disease (CKD) [3-6]. Conflicting reports exists on the association between serum uric acid (UA) levels and severity of COVID-19. One study showed that hyperuricemia is an independent risk factor for COVID-19-related death [7], while another found that hypouricemia is positively associated with COVID-19 severity [8]. In addition, a U-shape phenomenon between serum UA levels and the COVID-19 severity has been reported [9]. The variability in the results of previous studies may be attributed to the fluctuation of serum UA levels at different times of measurement. Therefore, the aim of the current study was to investigate if and how comorbidity of hyperuricemia is associated with disease severity in Japanese patients with COVID-19.
Materials and Methods
Patients
This study was conducted at Tokyo Women’s Medical University Hospital, Tokyo, Japan. The institutional ethics committee approved the study protocol (approval #: 5612-R). In this retrospective cohort study, adult Japanese patients with COVID-19 admitted to Tokyo Women’s Medical University Hospital between July 2020 and February 2021 were included. The diagnosis of COVID-19 was confirmed when the result of a real-time reverse-transcriptase polymerase-chain-reaction assay for SARS-CoV-2 virus was positive. Information on clinical and biochemical parameters on admission and comorbidities were collected. The patients were divided into 3 groups based on the degree of COVID-19 severity: mild (negative computed tomography [CT] findings and saturation of percutaneous oxygen [SpO2] ≥ 94% on admission), moderate (positive CT findings or SpO2 <94% on admission), and severe (requirement of admission to the intensive care unit [ICU] or oxygen inhalation >10 L/h).
Data Collection
Data on patient characteristics, such as sex, smoking, alcohol use, and comorbidities were retrospectively collected from medical records. Clinical data were obtained on admission and laboratory data were obtained within the first 24 hours of admission. Additionally, clinical data such as body mass index (BMI), SpO2, blood pressure, and pulse rate were collected. BMI was calculated as a person’s weight in kilograms divided by the square of the height in meters. Blood cell counts, urea nitrogen (UN), alanine aminotransferase, UA, c-reactive protein (CRP), d-dimer, and creatinine were measured using standard laboratory methods at our clinical laboratory. The estimated glomerular filtration rate (eGFR) was calculated using the following equation: eGFR (mL/min/1.73 m2) = 194 × creatinine−1.094 × age−0.287 (×0.739, if female) [10]. The amount of oxygen required and admission to the ICU were retrospectively followed up during the hospitalized period from medical records.
Statistical Analyses
Data are expressed as the median with interquartile range (IQR). Baseline characteristics between the mild and severe groups were compared using the chi-squared or Fisher’s test. The correlation between UA level and the odds ratio (OR) for COVID-19 severity was investigated using Spearman’s rank correlation by calculating the OR using the following formula: OR = (true positive rate/false negative rate)/(false positive rate/true negative rate). Comorbidities were compared by logistic regression analysis between mild and severe groups and odds ratios for severity were calculated. Logistic regression analyses were subsequently applied to compare the strength of the relationship with the risk of COVID-19 severity between hyperuricemia and age, male sex, BMI, malignant tumor, diabetes mellitus, CKD, or hypertension. Patient characteristics, antiviral treatment, and outcome were compared between the patients with and without comorbidity of hyperuricemia using the Fisher’s test. Significance was defined as p<0.05. All statistical analyses were carried out using JMP pro version 15 (SAS Institute Inc., Cary, NC, USA).
Results
Patient Characteristics and Severity of COVID-19
Table 1 shows the characteristics of our COVID-19 patients upon admission who were classified into the mild (n=36), moderate (n=96), or severe group (n=14). Compared to those in the mild group, patients in the severe group were older, more likely to be men, had a higher BMI, systolic blood pressure, pulse rate, white blood cell counts, neutrophil counts, levels of serum UN, UA, and CRP, plasma d-dimer levels, and had lower lymphocyte counts and eGFR. Figure 1 reveals the scattergram showing the relationship between the serum UA level on admission and the OR for severe COVID-19. There was a positive linear correlation (R2=0.5285, p<0.0001).
Table 1: Characteristics of COVID-19 patients on admission
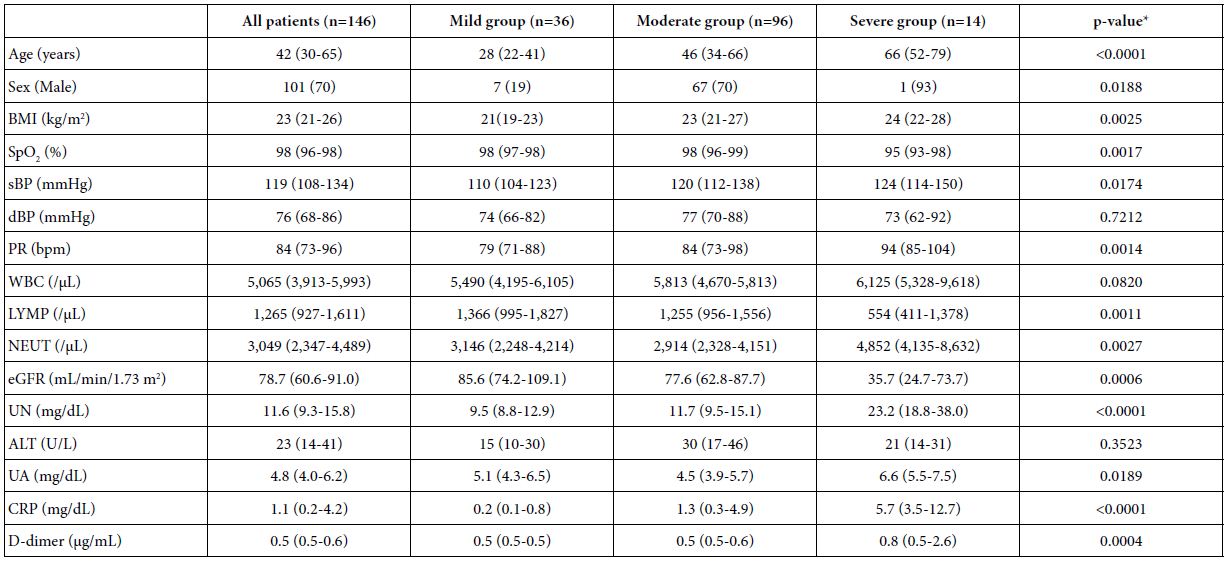
Data are expressed as median (interquartile range), or n (%).
BMI: Body Mass Index; SpO2: Percutaneous Oxygen Saturation; sBP: Systolic Blood Pressure; dBP: Diastolic Blood Pressure; PR: Pulse Rate; bpm: Beats per Minute; WBC: White Blood Cell; LYMP: Lymphocyte; NEUT: Neutrophil; eGFR: Estimated Glomerular Filtration Rate; UN: Urea Nitrogen; ALT: Alanine Aminotransferase; UA: Uric Acid; CRP: c-Reactive Protein.
*p-value: comparing mild versus severe groups.
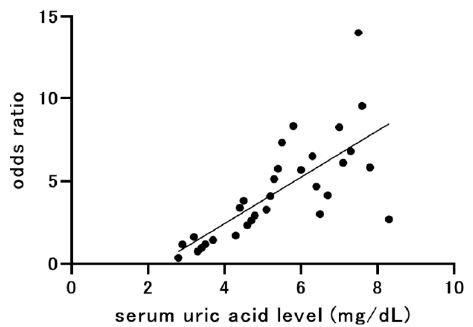
Figure 1: Scattergram showing the relationship between serum uric acid level on admission and odds ratio for severe COVID-19.
R2=0.5285, p<0.0001.
COVID-19: Coronavirus Disease 2019.
Relationships between Comorbidities and Severity of COVID-19
Table 2 shows the comorbidities of the COVID-19 patients included in this study. Patients in the severe group presented a higher complication rate of diabetes mellitus, CKD, hypertension, and hyperuricemia than the mild group. There were no significant differences in the complication rate of liver disorder, heart failure, smoking, or alcohol use between the mild and severe groups.
Table 2: Comorbidities of COVID-19 patients
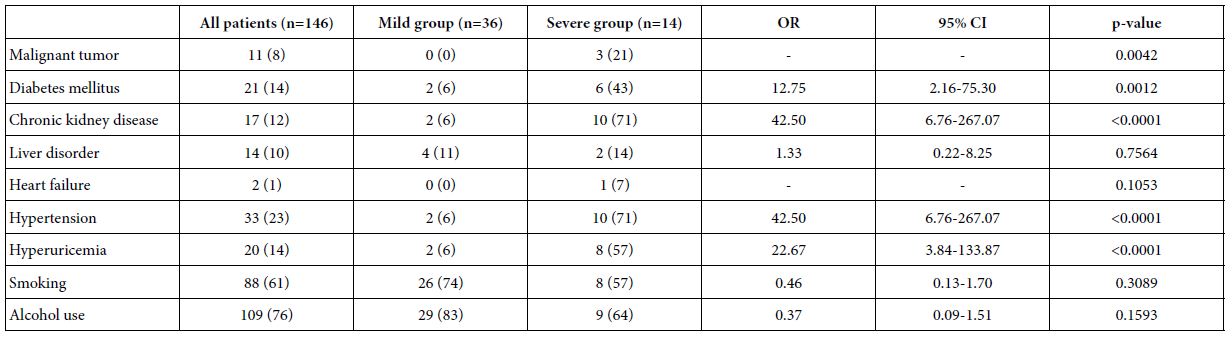
Data are expressed n (%).
OR: Odds Ratio; CI: Confidence Interval.
Single correlation analyses showed that the serum UA level and comorbidity of hyperuricemia were significantly correlated with COVID-19 severity (Tables 1 and 2). Table 3 shows prevalences of comorbidities in patients with or without hyperuricemia. Prevalences of diabetes mellitus, CKD, heart failure and hypertension were significantly higher in patients with hyperuricemia than those without hyperuricemia. To determine whether the correlation between comorbidity of hyperuricemia and COVID-19 severity was independent of other factors, multiple regression analyses were conducted (Table 4). Comorbidity of hyperuricemia was significantly associated with COVID-19 severity independent of age (Model 1), male sex (Model 2), BMI (Model 3), comorbidity of malignant tumor (Model 4), and diabetes mellitus (Model 5), while the association between hyperuricemia and severity of COVID-19 was eliminated by correction with hypertension (Model 6) or CKD (Model 7). Table 5 shows relationships between COVID-19 severity and comorbidity of hyperuricemia in all patients, patients without hypertension or CKD. In all patients, the prevalence of comorbidity of hyperuricemia was significantly higher in the severe COVID-19 group than the mild COVID-19 group. However, the prevalence of comorbidity of hyperuricemia were not significantly different between the two groups when the patients were limited to those without hypertension or CKD.
Table 3: Comorbidities of COVID-19 patients with or without hyperuricemia
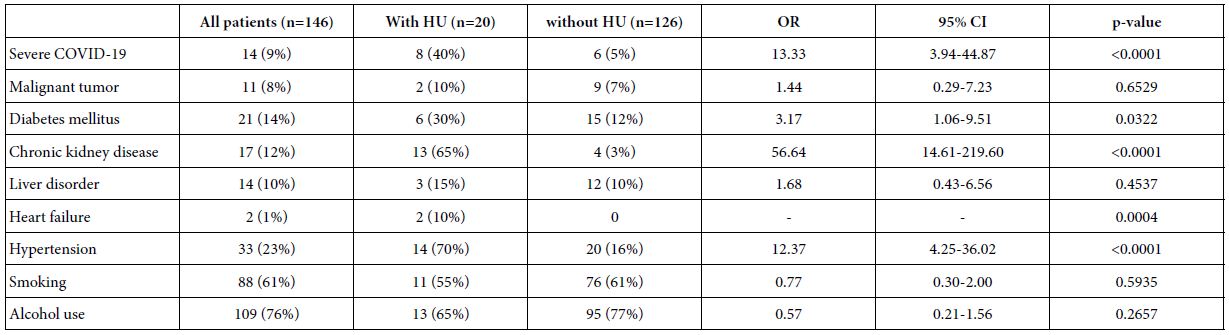
Data are expressed n (%).
HU: Hiperuricemia; OR: Odds Ratio; CI: Confidence Interval.
Table 4: Comparison of the relationship between COVID-19 severity and comorbidity of hyperuricemia and other parameters.
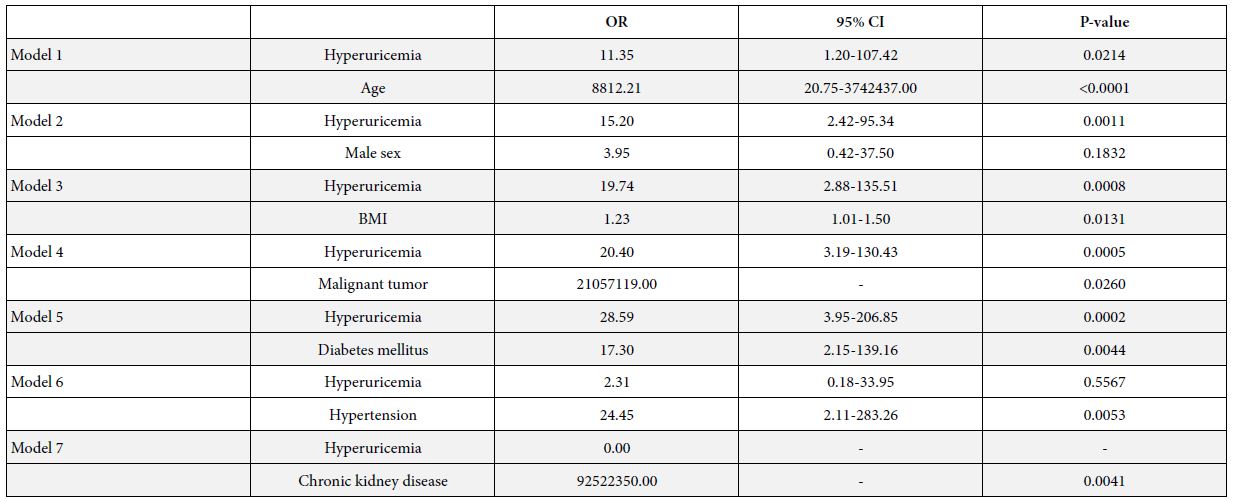
OR: Odds ratio; CI: Confidence interval; BMI: Body mass index
Table 5: COVID-19 severity and comorbidity of hyperuricemia in all patients, patients without hypertension or chronic kidney disease.
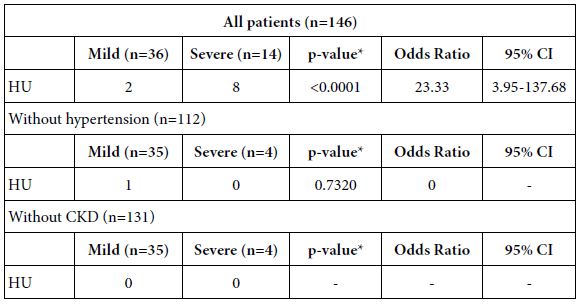
HU: Hyperuricemia; CKD: Chronic Kidney Disease.
*p-value: comparing mild versus severe groups.
Relationships between the Comorbidity of Hyperuricemia and Treatment or Outcome of COVID-19
The treatment and outcome of COVID-19 was compared between patients with and without comorbidity of hyperuricemia (Table 6). Regarding treatment with antiviral agents such as favipiravir, ciclesonide, and remdesivir, treatment with favipiravir and remdesivir was significantly higher in patients with comorbidity of hyperuricemia than those without hyperuricemia. In addition, the rate of patients who had severe COVID-19 or died of COVID-19 was significantly greater in patients with comorbidity of hyperuricemia than those without hyperuricemia.
Table 6: Treatment and outcome comparisons between patients with and without comorbidity of hyperuricemia.
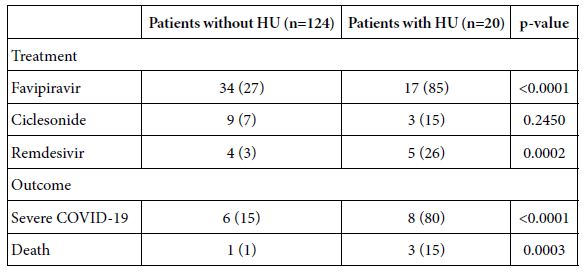
Data are expressed as n (%).
HU: Hyperuricemia; COVID-19: Coronavirus Disease 2019.
Discussion
This study showed that patients with severe COVID-19 had a higher serum UA level (Table 1) and higher incidence of comorbidity of hyperuricemia (Table 2) than patients with mild COVID-19. Comorbidity of hyperuricemia was associated with the risk for severe COVID-19 independent of age, sex, BMI, comorbidity of malignant tumor, and diabetes mellitus (Table 4). However, the association between hyperuricemia and COVID-19 severity disappeared by correction with hypertension or CKD (Table 4), suggesting that as a risk factor of COVID-19 progression, comorbidity of hyperuricemia may be confounded by hypertension and/or CKD.
Hyperuricemia is more frequently observed in men and is caused by aging, obesity, alcohol and purine body intake, hypertension and reduced excretion due to kidney dysfunction [11]. These causes may overlap with known risk factors of COVID-19 severity such as male sex, advanced age, obesity, hypertension and CKD (5,6), while some reports showed that hypertension is not a risk factor for severe COVID-19 [12-14].
Previous studies have shown that hyperuricemia, hypertension, and CKD are inseparable, and that the relationship between hyperuricemia and kidney dysfunction is bidirectional [15]. The kidney eliminates 70% of the body’s daily UA production. CKD and hyperuricemia coexist because UA is excreted by the kidney and serum UA levels are negatively associated with GFR [15], while hyperuricemia is an early detection marker for renal dysfunction [16]. One possible mechanism by which hyperuricemia causes renal dysfunction may be via the formation of UA crystals in the renal tubules [17]. Hyperuricemia may also be involved in hypertension [18-21]. These strong associations among hyperuricemia, hypertension, and CKD may account for the phenomenon observed in our study where the relationship between hyperuricemia and COVID-19 severity was eliminated by correction with hypertension or CKD.
Furthermore, inflammation plays a key role in the relationship between UA and COVID-19 pneumonia. COVID-19 progresses to severe acute respiratory syndrome (ARDS) via hyperinflammation responses [22]. During ARDS, the abnormal generation of reactive oxygen species (ROS) occurs and causes organ damage [23] which may be counteracted by UA’s strong antioxidant capabilities [24]. A recent study reported a higher incidence of severe COVID-19 symptoms in individuals with low serum UA levels [8], suggesting that low serum UA levels may progress organ damage due to the reduction in ROS scavenging. High UA levels were also associated with a high incidence of severe COVID-19 [7]. Hyperuricemia is associated with systemic inflammatory markers and also higher CRP levels [25], both of which are observed in severe COVID-19 patients. Altogether, these findings suggest that both low and high serum UA levels are associated with severe COVID-19 [9]. In our study, serum UA levels were higher in the severe group upon admission compared to the mild group (Table 1); however, no U-shape association with UA and odds ratio for COVID-19 severity was observed (Figure 1).
UA levels upon admission may also be modified by dehydration and other factors. Favipiravir, an antiviral agent undergoing clinical trials for the treatment COVID-19 in Japan, is known to elevate UA levels [26]. We initially speculated that attending physicians may have hesitated to prescribe favipiravir in patients with hyperuricemia to avoid worsening both the condition and progression of COVID-19. However, this was not the case because the rate of patients treated with this agent was significantly higher in patients with comorbidity of hyperuricemia than those without comorbidity of hyperuricemia.
This study has some limitations. First, the study was conducted as a single center study and the sample size was relatively small. Second, owing to the retrospective cohort design of the study, we could not determine the causal relationship between hyperuricemia or comorbidity of hyperuricemia and COVID-19 severity; therefore, larger-scale prospective studies are needed to investigate this further.
Conclusion
Overall, we demonstrated that serum UA levels upon admission and comorbidity of hyperuricemia were associated with COVID-19 severity in Japanese patients, although the hyperuricemia comorbidity association was confounded by hypertension or CKD. These data suggest that comorbidity of hyperuricemia may indicate a risk of COVID-19 progression. Furthermore, patients with hyperuricemia comorbidity may require careful and intensive multidisciplinary treatment for hyperuricemia and hypertension and/or CKD to prevent progression of COVID-19.
Statements
Statement of Ethics
Study approval statement: This study protocol was reviewed and approved by Tokyo women’s medical university ethics committee, approval number 5612-R.
Consent to Participate Statement
Written informed consent was obtained by all the participants.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Authorship
All authors (1) made substantial contributions to the study concept or the data analysis or interpretation; (2) drafted the manuscript or revised it critically for important intellectual content; (3) approved the final version of the manuscript to be published; and (4) agreed to be accountable for all aspects of the work.
Acknowledgment
We would like to thank the ward staff and doctors who cared for the patients enrolled in this study. We thank Editage (www.editage.com) for English language editing.
References
- World Health Organization. Coronavirus disease (COVID-19) outbreak. https://www.who.int/health-topics/coronavirus [Accessed 7 June 2022].
- WHO (2022) COVID-19 weekly epidemiological update. World Heal Organ 58: 1-23.
- Lippi G, Henry BM (2020) Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19). Respir Med 167: 105941. [crossref]
- Berlin I, Thomas D, Le Faou A-L, Cornuz J (2020) COVID-19 and Smoking. Nicotine Tob Res 22: 1650-1652.
- Rodacki M (2020) Severity of COVID-19 and diabetes mellitus: there is still a lot to be learned. Arch Endocrinol Metab 64: 195-196.
- Li X, Zhong X, Wang Y, Zeng X, Luo T, et al. (2021) Clinical determinants of the severity of COVID-19: A systematic review and meta-analysis. PLoS One 16: e0253894. [crossref]
- Ishii M, Terai H, Kabata H, Masaki K, Chubachi S, et al. (2020) Clinical characteristics of 345 patients with coronavirus disease 2019 in Japan: A multicenter retrospective study. J Infect 81: e3-5. [crossref]
- Hu F, Guo Y, Lin J, Zeng Y, Wang J, et al. (2021) Association of serum uric acid levels with COVID-19 severity. BMC Endocr Disord 21: 97. [crossref]
- Chen B, Lu C, Gu HQ, Li Y, Zhang G, et al. (2021) Serum Uric Acid Concentrations and Risk of Adverse Outcomes in Patients With COVID-19. Front Endocrinol (Lausanne) 12: 633-767. [crossref]
- Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, et al. (2009) Revised Equations for Estimated GFR from Serum Creatinine in Japan. Am J Kidney Dis 53: 982-992. [crossref]
- BT E (1996) The management of gout. N Engl J Med 334: 445-451. [crossref]
- Mcfarlane E, Linschoten M, W. Asselbergs F, Lacy PS, et al. (2022) The impact of pre-existing hypertension and its treatment on outcomes in patients admitted to hospital with COVID-19. Hypertens Res 45: 834-845.
- Zhang S, Zhong Y, Wang L, Yin X, et al. (2022) Anxiety, home blood pressure monitoring, and cardiovascular events among older hypertension patients during the COVID-19 pandemic On behalf of the STEP Study Group. Hypertens Res 45: 856-865.
- Kow CS, Ramachandram SD, Hasan SS (2022) Clinical outcomes of hypertensive patients with COVID-19 receiving calcium channel blockers: a systematic review and meta-analysis. Hypertens Res 45: 360-363. [crossref]
- Russo E, Viazzi F, Pontremoli R, Barbagallo CM, Bombelli M, et al. (2021) Serum Uric Acid and Kidney Disease Measures Independently Predict Cardiovascular and Total Mortality: The Uric Acid Right for Heart Health (URRAH) Project. Front Cardiovasc Med 8: 713652. [crossref]
- Iseki K, Oshiro S, Tozawa M, Iseki C, Ikemiya Y, et al. (2001) Significance of hyperuricemia on the early detection of renal failure in a cohort of screened subjects. Hypertens Res 24: 691-697. [crossref]
- Umekawa T, Chegini N, Khan SR (2003) Increased expression of monocyte chemoattractant protein-1 (MCP-1) by renal epithelial cells in culture on exposure to calcium oxalate, phosphate and uric acid crystals. Nephrol Dial Transpl 18: 664-669. [crossref]
- Sato Y, Fujimoto S, Iseki K, Konta T, Moriyama T, et al. (2020) Higher baseline uric acid concentration is associated with non-attainment of optimal blood pressure. PLoS One 15: e0236602. [crossref]
- Kuwabara M, Hisatome I, Niwa K, Hara S, Roncal-Jimenez CA, et al. (2018) Uric Acid Is a Strong Risk Marker for Developing Hypertension from Prehypertension: A 5-Year Japanese Cohort Study. Hypertension 71: 78-86. [crossref]
- Kawasoe S, Kubozono T, Ojima S, Kawabata T, Miyahara H, et al. (2021) J-shaped curve for the association between serum uric acid levels and the prevalence of blood pressure abnormalities. Hypertens Res 44: 1186-1193. [crossref]
- Mori K, Furuhashi M, Tanaka M, Higashiura Y, Koyama M, et al. (2022) Serum uric acid level is associated with an increase in systolic blood pressure over time in female subjects: Linear mixed-effects model analyses. Hypertens Res 45: 344-353. [crossref]
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, et al. (2020) COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395: 1033-1034. [crossref]
- Kellner M, Noonepalle S, Lu Q, Srivastava A, Zemskov E, et al. (2017) ROS signaling in the pathogenesis of Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS). Adv Exp Med Biol 967: 105-137. [crossref]
- Bowman GL, Shannon J, Frei B, Kaye JA, Zemskov E et al. (2010) Uric acid as a CNS antioxidant. J Alzheimer’s Dis 19: 1331-1336. [crossref]
- Chen PH, Chen YW, Liu WJ, Hsu SW, Lee CL (2019) Approximate mortality risks between hyperuricemia and diabetes in the United States. J Clin Med 8: 1-13. [crossref]
- Mishima E, Anzai N, Miyazaki M, Abe T (2020) Uric acid elevation by favipiravir, an antiviral drug. Tohoku J Exp Med 251: 87-90. [crossref]