Abstract
Background: Galectin-3 has been reported to have substantial accuracy in detection or excluding malignant nodules with prior indeterminate FNAC and per operative findings. Keeping this fact in mind, Thus, Galectin-3 can have a pivotal role in separating benign from the malignant thyroid neoplasms.
We aim to determine the frequency and intensity of Galectin-3 immunohistochemical expression studied in the benign and malignant thyroid neoplasms confirmed on histopathology.
Materials and Methods: This descriptive, observational, cross-sectional study was conducted from 5th November 2017 to 4th May 2018 in the Department of Histopathology, Foundation University Medical College, Islamabad Campus & Department of Surgery, Fauji Foundation Hospital, Rawalpindi.
We studied 78 thyroid specimens diagnosed with thyroid neoplasms on histopathology. Out of these 39 were benign cases (follicular adenoma and hurthle cell adenoma) and 39 were malignant cases (papillary thyroid carcinomas, follicular carcinoma, medullary carcinoma and poorly differentiated carcinoma). Each specimen was examined grossly and microscopically and checked for immunohistochemical staining pattern of Galectin-3 under the microscope.
Results: Age range in this study was from 15 to 65 years with mean age of 44.97 ± 10.78 years. Out of these 78 patients, 17 (21.79%) were male and 61 (78.21%) were female with male to female ratio of 1:3.6. Frequency of positive Galectin-3 immuno histochemical expression among thyroid neoplasms was found in 32 (41.03%) cases with Galectin-3 showing positive staining in 21 (53.85%) of all malignant and 11 (28.21%) of all benign cases. Among the malignant neoplasms, positivity was seen most frequently in papillary thyroid carcinomas as compared to the other malignancies.
Conclusion: This study concluded that positive Galectin-3 immunohistochemical expression is seen both in benign and malignant thyroid neoplasm, but its expression is more in malignant thyroid neoplasms (53.85%) as compare to the benign lesions (28.21%). Therefore, we recommend that Galectin-3 immunohistochemical marker cannot be used alone for the routine diagnosis of malignant thyroid lesions as it has shown less sensitivity and specificity. Moreover it also has shown no significant role in differentiating between the benign and the malignant thyroid neoplasms.
Keywords
neoplasm, marker, malignant, benign, expression
Introduction
Thyroid gland is an important part of the endocrine system located at the base of the neck. It is chiefly composed of two types of cells, follicular and parafollicular cells. The follicular cells make thyroxine, which has important functional impacts on various systems and general metabolism. The parafollicular cells, also known as C cells arise from the neural crest and are involved in the calcitonin production, which has vital role in maintaining calcium homeostasis [1].
Thyroid neoplasms including both benign and malignant lesions are common entities encountered in daily clinical practice. Most of the lesions (95%) arise from the follicular epithelial cells of the thyroid gland [2].
Thyroid cancer is the most common among the endocrine tumors and its incidence has been increasing in the last three decades [3]. An estimated mortality rate of thyroid cancer is 0.5 to 10 cases per 100,000. The annual male and female percentage is 6.3% and 7.1% for white population, 4.3% and 8.4% for blacks and for Asian population patients it is 3.4% and 6.4% respectively.3These tumors can clinically present as a solitary nodule along with the normal thyroid gland or as a dominant nodule in the background of a multinodular goiter. 5% of the solitary thyroid nodules are found to be neoplastic [4].
In Pakistan, thyroid neoplasms are common especially in the northern areas, which are mainly attributable to the iodine deficiency or excess. Thyroid cancer accounts for 1.2% of all the malignancies diagnosed in our country with the papillary thyroid carcinoma being most common. The female to male ratio in Pakistan is reported as 2.2:1 [5].
Patients can present with both the features of hyper and hypothyroidism in both benign and malignant lesion. This makes it clinically difficult to diagnose the exact underlying cause. Here comes the role of histopathology, which can correctly diagnose the lesion, but there are some neoplasms that have very confusing morphological details and these cannot be exactly categorized into benign or malignant, only on the basis of histopathology. This scenario is mostly seen in the follicular and the Hurthle cell neoplasms. The gross appearance and the microscopic details are perplexing for a pathologist. Moreover, the cytological details are also much overlapping in various benign versus malignant lesions [2].
The final diagnosis of the lesion being benign and malignant has profound effects on the clinical outcome and prognosis of the patient. Several articles have reported the significance of immnohistochemical markers to solve this problem. Galectin-3, p63 and Ki67 have been reported quite accurate to detect or exclude malignancy in nodules with prior indeterminate FNAC and per operative findings [6].
In this study, role of Galectin-3 will be quantified to differentiate and classify the thyroid lesions into benign and malignant categories. Galectin-3 belongs to the family of lectins. Galectin-3 is synthesized in both the nucleus and cytoplasm, and also expressed at the cell surface. It is also found extracellularly in the general circulation. Galectin-3 specifically binds to the beta galactoside containing intracellular, extracellular and cell surface associated glycol conjugates so it is over expressed in oncogenic pathology of thyroid [7-8].
In Pakistan, limited data is available regarding the role of galectin-3 as a diagnostic tool to differentiate malignant thyroid neoplasms from benign lesions. So, this study can have beneficial effects in the diagnostics and further treatment of such lesions.
Materials and Methods
There was a total of 78 thyroid specimens included in this study (39 benign and 39 malignant neoplasms). All of these patients were operated at the Department of Surgery, Fauji Foundation Hospital Rawalpindi during a period of six months from 5th November 2017 to 4th May 2018. The specimens were processed in the department of Histopathology, Foundation University Medical College Islamabad. The benign conditions included Follicular adenoma and Hurthle cell adenoma. The malignant conditions included Papillary thyroid carcinoma (both classic type and follicular variant), Follicular thyroid carcinoma, Medullary thyroid carcinoma and poorly differentiated carcinoma.
The hospital ethical committee granted the approval for data collection. The data included patient’s demographic details, clinical presentation, previous laboratory test record and clinical suspicion. The specimens were examined both grossly and microscopically in the laboratory. The thyroid specimens were fixed in 10% formalin and were sliced properly. The representative sections were processed in the tissue processor (SAKURA TISSUE TEK-R TEC5 MODEL 220-240) for the paraffin sectioning. After this step, 4-5µm thick sections were cut using rotatory microtome (SAKURA ACCU-CUT MODEL SRM 200 CW). Hematoxylin and eosin stain (H&E) was used for staining the slides and get them ready to see under the microscope.
For the immunohistochemistry, representative histological sections of the thyroid neoplasm were used. The sections were deparaffinised by xylene and then were rehydrated by ethanol. Tri-sodium citrate buffer (pH 6.0 to 6.2) was used for the antigen retrieval. When the slides came back to room temperature, endogenous peroxidase activity was blocked by 0.6% H2O2. After this step lyophilized mouse monoclonal Galectin-3 antibody in the dilution of 1:100 was applied for an hour. Washing was done with tris- buffered saline (TBS). Then for 20 minutes super enhancer was added. Polymer horseradish peroxidase (HRP) was applied for 30 minutes as a secondary antibody and washing was done again with TBS. Subsequently Diamine Benzidine (DAB) chromogen was applied for 5 minutes. Mayer’s Haematoxylin was used for counter staining followed by clearing and mounting. Positive and negative controls were also applied.
Two consultant histopathologists examined the H&E stain and immunohistochemical marker (Galectin-3) under the Olympus light microscope. The sections with the best staining were selected for examination and reported likewise. Morphology and staining was noted and grading of Galectin-3 was done by Weber KB et al and Hermann ME et al guidelines. The intensity and distribution of Galectin-3 staining (cytoplasmic) on a scale of 0 to 3 was done as follows:
0 No staining
1+ Weak/slight staining
2+ Moderate staining
3+ Intense staining
The proportion of stained cells was interpreted as;
1+ < 5% of cells
2+ 5% to 50% of cells
3+ >50% of cells
The lesions with the particular cytoplasmic staining of more than 5% ofthe tumor cells was taken as positive for Galectin-3 regardless of its intensity.
Results
Age range in this study was from 15 to 65 years with mean age of 44.97 ± 10.78 years as shown in Table- I. Out of these 78 patients, 61 (78.21%) were female and 17 (21.79%) were male with female to male ratio of 3.6:1 (Figure I). On the basis of histopathological diagnosis half (39) cases belonged to benign neoplasms and other half (39) were diagnosed as malignant neoplasms as shown in Figure II.
Table 1: Age distribution of patients (n=78), having Mean ± SD = 44.97 ± 10.78 years.
|
Age (in years) |
No. of Patients | %age |
| 15-40 | 27 |
34.62 |
|
41-65 |
51 | 65.38 |
| Total | 78 |
100.0 |
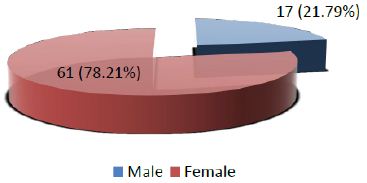
Figure 1: Distribution of patients according to Gender (n=78)
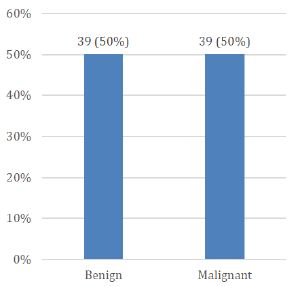
Figure 2: Distribution of patients according to histopathological features (n=78)
Frequency of positive Galectin-3 immunohistochemical expression among thyroid neoplasms was found in 32 out of 78 (41.03%) cases while 46 out of total 78 (58.97%) were showing negative galectin-3 staining (Figure III).
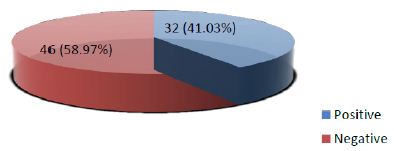
Figure 3: Frequency of Galectin-3 immunohistochemical expression among thyroid neoplasms confirmed on histopathology (n=78)
A detailed look at the further breakdown of galectin-3 staining among benign neoplasms reveal that 11(28.1%) among 39 benign cases were positive for the stain. For the malignant neoplasms, total 21(53.85%) among 39 cases were positive. On the other side 28(71.79%) benign cases and 18(46.15%) malignant cases showed negative galectin-3 staining. The p-value calculated was 0.021 which is not significant (Table- II)
Table 2: Stratification of Galectin-3 immunohistochemical expression among benign and malignant thyroid neoplasms
|
|
Galectin-3 immunohistochemical expression |
p-value |
|
| Positive |
Negative |
||
|
Benign |
11 (28.21%) | 28 (71.79%) |
0.021 |
| Malignant | 21 (53.85%) |
18 (46.15%) |
|
The Stratification of Galectin-3 immunohistochemical expression with respect to age groups showed total 27 cases within the age range of 115- 40 years out of which 10 cases were positive. Total 51 cases belonged to the age range of 41-65 years out of which 22 showed positive galectin-3 staining. The p-value calculated was 0.602 which is again insignificant (Table- III)
Table 3: Stratification of Galectin-3 immunohistochemical expression with respect to age groups
|
|
Galectin-3 immunohistochemical expression |
p-value |
|
| Positive |
Negative |
||
|
15-40 years |
10 | 17 |
0.602 |
| 41-65 years | 22 |
29 |
|
Similarly Table IV shows the breakdown of the cases according to gender. Total 8 out of 17 cases among male patients were positive for Galectin-3 and 24 out of 61 cases of female patients were showing the positive staining. The p-value calculated was 0.567 which is again insignificant.
Table 4: Stratification of Galectin-3 immunohistochemical expression with respect to gender
|
Galectin-3 immunohistochemical expression |
p-value |
||
| Positive |
Negative |
||
|
Male |
08 | 09 | 0.567 |
| Female | 24 |
37 |
|
The breakdown of Galectin-3 positivity in the various histological types of malignant and benign thyroid neoplasms is also shown in figure IV & V respectively.
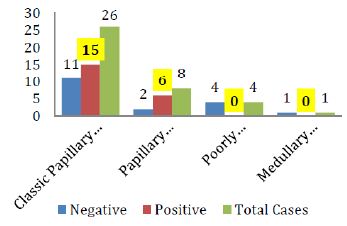
Figure 4: Galectin-3 staining in various histological types of Thyroid carcinomas
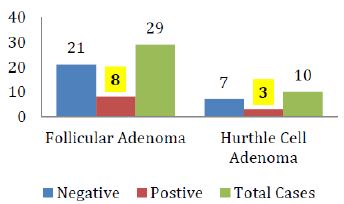
Figure 5: Galectin-3 staining in various histological types of Thyroid adenomas
Discussion
Immunohistochemical markers have been extensively investigated for their potential diagnostic and prognostic utility in different thyroid tumors. Among these, they have deduced Galectin-3 to be a promising marker. Galectin-3 belongs to lectin family [9]. And a constellation of other normal tissues and tumors masses express Galectin-3 [10]. An intense nuclear localization of galectin-3 in tumors is seen in malignant transformation of thyroid tissue [11]. Research studies suggest Galectin-3 expression may serve as a potential diagnostic and prognostic marker of some cancers [12]. However, galectin-3 has not been established as a universal and specific marker of thyroid neoplasia. Yet it can serve as a parameter in diagnostic approach for these tumors and for possible potential therapeutic target [13-16].
In 2015 a study was conducted in India regarding the staining pattern of Galectin-3 in thyroid neoplasms. The results showed 86% sensitivity and 85% specificity, with Galectin-3 showing positive staining in 87% of all malignant and 15% of all benign cases [2] In 2016, another study was conducted in Italy to check the diagnostic accuracy of the various immunohistochemical stains and they found galectin-3 to be 84.2% sensitive and 94.5% specific in detecting the thyroid neoplasms [7].
In contrast, our study shows less frequency of positive Galectin-3 immunohistochemical expression among thyroid neoplasms i.e. total 32 (41.03%) cases with Galectin-3 showing positive staining in 53.85% of all malignant and 28.21% of all benign cases. In 2002 a study was conducted regarding the staining pattern of Galectin-3 in thyroid neoplasms. The results showed high frequency of staining in papillary thyroid carcinomas only and no significant staining in the other type of carcinomas. Moreover, it also showed positive results in follicular adenomas which made them conclude that galectin-3 is not a sensitive marker if used alone [8] Our study also shows the same results.
A systematic review and meta-analysis on galectin-3 as a biomarker found that it may be a potentially useful immuno-marker to distinguish between patients with papillary thyroid carcinoma (PTC) and patients without PTC. In addition, lymph node metastasis is more frequently seen in PTC patients with positive expression of galectin-3 [17]. Our study also gives us the result that although galectin-3 is sensitive for detecting papillary thyroid carcinomas but it is not much sensitive in detecting other carcinomas from benign lesions (adenomas) as shown in figures IV & V.
Galectin-3, HBME-1, and cytokeratin-19 may be helpful in diagnosis of malignant thyroid tumors as evidenced by a study, although the expression of these markers may be seen in benign lesions as well. However, cytokeratin-19 is investigated for its diagnostic prowess. It was found that the marker and its combinations with other markers have higher sensitivities in accurate diagnosis of papillary carcinoma than the other combinations. But these immunohistochemical markers have limited role in differentiation between benign and malignant lesions [18].
Another important point which was noted in this study was that the carcinomas showing positive galectin-3 gave mostly focal positivity rather then the diffuse strong positivity in comparison to the results of the study done by Manivannan et al [18] . That study, which was done in 2012, demonstrated that galectin-3 staining pattern is significant in differentiating benign from malignant follicular neoplasms as well as follicular variant of papillary thyroid carcinoma. Diffuse positivity for galectin-3 was associated with malignant thyroid follicular neoplasms while focal weak positivity favours adenomas. On the other hand, a previous study have demonstrated that there was no marked difference in the staining intensity for intra cytoplasmatic or intranuclear expression of galectin-3 in benign and malignant thyroid neoplasms [19].
Thin-Prep fine needle aspiration cytology showing increased expression levels of galectin-3 were seen with cellular hyperproliferation, hypertrophy, and pathophysiological situations associated with adenomas and thyroid carcinomas [20-21]. A comparison of glypican-3 (a member of the glypican family of heparan-sulfate proteoglycans bound to the plasma membrane) with galectin-3 demonstrated that galectin-3 had higher sensitivity in diagnosing thyroid carcinoma; however, specificity is low for differentiating follicular-patterned neoplasm [22]. These markers have also been investigated preoperatively and postoperatively, the preoperative serum galectin-3 level showed potential diagnostic value, as it was significantly higher in the cancer patients than in the control subjects (p < 0.05). [23]
Galectin-3 is also used in combination with other biomarkers for a differential diagnosis of thyroid lesions. The most commonly combined biomarkers are Hector Battifora mesothelial epitope-1 (HBME-1) and cytokeratin-19 [24-27]. However galectin-3 may not be used as single discriminators between follicular thyroid adenoma and carcinoma [24-27]. Some studies show that galectin-3 and HBME-1 have an excellent sensitivity and specificity for malignant thyroid lesions (100 and 89.1%, respectively) [26]. Despite core needle biopsies leading to the diagnosis of the majority of thyroid nodules, the accuracy is increased by also observing the galectin-3, cytokeratin-19 and HBME-1 panels, indicating their additional diagnostic value when combined with routine histology and not when used alone [24-27]. It was also reported that galectin 3, cluster of differentiation (CD) and, to an extent, HBME-1, are useful immunocytochemical parameters with the potential to support the fine needle aspiration cytology diagnosis of PTC, particularly in situations where the differential diagnoses is complicated [28].
Studies have noted variable Galectin-3 expression in poorly differentiated thyroid cancers also [29]. However, in majority of cases (75% to 100% of reported cases) of anaplastic thyroid carcinoma, Galectin-3 positivity was identified suggesting that differentiated thyroid carcinoma can progress or undergo anaplastic transformation [26,29]. In the present study and study by Herrmann et al., small number of cases of MTC and poorly differentiated carcinoma are reported with inconsistent Galectin-3 expression, making diagnostic application of Galectin-3 in these rare histological subgroups unlikely [30].
Zhu et al. reported several markers expression like HBME-1, CK-19, Galectin-3, and RET in several papillary thyroid carcinoma. The expression was found to be higher in papillary thyroid carcinoma as compared to benign neoplasia. However, they did not report any of these as specific markers for papillary thyroid carcinoma [31].
Conclusion
Galectin-3 immunohistochemical expression is found in the cases of both benign thyroid tumors and malignant neoplasms although more commonly seen in malignant ones. So, it cannot be used alone for the routine diagnosis of malignant thyroid lesions as it shows less sensitivity and specificity. It also has expressed limited role in differentiating between the benign and the malignant thyroid neoplasms.
References
- Braverman LE, Cooper D. (2012) Werner & Ingbar’s The Thyroid: A Fundamental and Clinical Text. 10th Philadelphia: Lippincott Williams & Wilkins. p. 10.
- Sumana BS, ShaShidhar S, Shivarudrappa AS, (2015) Galectin-3 Immunohistochemical Expression in Thyroid Neoplasms. Journal of Clinical and Diagnostic Research 9:EC07. [crossref]
- Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. (2013) Worldwide Increasing Incidence of Thyroid Cancer: Update on Epidemiology and Risk Factors. Journal of Cancer Epidemiology. [crossref]
- Dwivedi SS, Khandeparkar SG, Joshi AR, Kulkarni MM, Bhayekar P, Jadhav A, et al. (2016) Study of Immunohistochemical Markers (CK-19, CD-56, Ki-67, p53) in Differentiating Benign and Malignant Solitary Thyroid Nodules with Special Reference to Papillary Thyroid Carcinomas. Journal of Clinical and Diagnostic Research 10:EC14-9. [crossref]
- Asif F, Ahmad MR, Majid A. (2015) Risk Factors for Thyroid Cancer in Females Using a Logit Model in Lahore, Pakistan. Asian Pacific Journal of Cancer Prevention 16:6243-7. [crossref]
- Fortuna-Costa A, Gomes AM, Kozlowski EO, Stelling MP, Pavão MS. (2014) Extracellular Galectin-3 in Tumor Progression and Metastasis. FrontOncol 4:138. [crossref]
- Trimboli P, Guidobaldi L, Amendola S, Nasrollah N, Romanelli F, Attanasio D, et al. (2016) Galectin-3 and HBME-1 Improve the Accuracy of Core Biopsy in Indeterminate Thyroid Nodules. Endocrine 52:39-45. [crossref]
- Fukumori T, Kanayama H, Raz A. (2007) The Role of Galectin-3 in Cancer Drug Resistance. Drug Resistance Updates 10:101-8. [crossref]
- Hirabayashi J, Kasai K. (1993) The Family of Metazoan Metal-Independent Beta-Galactoside-Binding Lectins: Structure, Function and Molecular Evolution. Glycobiology 3:297-304. [crossref]
- Dumic J, Dabelic S, Flogel M. (2006) Galectin-3: An Open-Ended Story. Biochimica et Biophysica Acta 1760:616-35. [crossref]
- Paron I, Scaloni A, Pines A, Bachi A, Liu FT, Puppin C, Pandolfi M, Ledda L, Di Loreto C, Damante G et al. (2003) Nuclear Localization of Galectin-3 in Transformed Thyroid Cells: A Role in Transcriptional Regulation. Biochemical and Biophysical Research Communications 302:545-53.
- Danguy A, Camby I, Kiss R. (2002) Galectins and Cancer. Biochimica et Biophysica Acta 1572:285-93. [crossref]
- Van den Brule F, Califice S, Castronovo V. (2004) Expression of Galectins in Cancer: A Critical Review. Glycoconjugate Journal 19:537-42. [crossref]
- Johnson KD, Glinskii OV, Mossine VV, Turk JR, Mawhinney TP, Anthony DC, et al. (2007) Galectin-3 As a Potential Therapeutic Target in Tumors Arising from Malignant Endothelia. Neoplasia 9:662- 70. [crossref]
- Sawangareetrakul P, Srisomsap C, Chokchaichamnankit D, Svasti J. (2008) Galectin-3 Expression in Human Papillary Thyroid Carcinoma. Cancer Genomics & Proteomics 5:117- 22. [crossref]
- Nangia-Makker P, Nakahara S, Hogan V, Raz A. (2007) Galectin-3 in Apoptosis, a Novel Therapeutic Target. Journal of Bioenergetics and Biomembranes 1:79- 84.
- Tang W, Huang C, Tang C, Xu J, Wang H. (2016) Galectin-3 may serve as a Potential Marker for Diagnosis and Prognosis in Papillary Thyroid Carcinoma: A Meta-Analysis. OncoTargets and Therapy 9:455–60. [crossref]
- Manivannan P, Siddaraju N, Jatiya L, Verma SK. (2012) Role of Pro-Angiogenic Marker Galectin-3 in Follicular Neoplasms of Thyroid. Indian Journal of Biochemistry and Biophysics 49:392–4. [crossref]
- Sumana BS, Shashidhar S, Shivarudrappa AS. (2015) Galectin-3 Immunohistochemical Expression in Thyroid Neoplasms. Journal of Clinical and Diagnostic Research 9:EC07–11. [crossref]
- Papale F, Cafiero G, Grimaldi A, Marino G, Rosso F, Mian C, Barollo S, Pennelli G, Sorrenti S, De Antoni E, Barbarisi A. (2013) Galectin-3 Expression in Thyroid Fine Needle Cytology (t-FNAC) Uncertain Cases: Validation of Molecular Markers and Technology Innovation. Journal of Cellular Physiology 228:968–74. [crossref]
- Salajegheh A, Dolan-Evans E, Sullivan E, Irani S, Rahman MA, Vosgha H, Gopalan V, Smith RA, Lam AK. (2014) The Expression Profiles of the Galectin Gene Family in Primary and Metastatic Papillary Thyroid Carcinoma with Particular Emphasis on Galectin-1 and Galectin-3 Expression. Experimental and Molecular Pathology 96:212–8. [crossref]
- Al-Sharaky DR, Younes SF. (2016) Sensitivity and Specificity of Galectin-3 and Glypican-3 in Follicular-Patterned and other Thyroid Neoplasms. Journal of Clinical and Diagnostic Research 10:EC06–10. [crossref]
- Yilmaz E, Karsidag T, Tatar C, Tüzün S. (2015) Serum Galectin-3: Diagnostic Value for Papillary Thyroid Carcinoma. Ulus Cerrahi Derg 31:192–6. [crossref]
- Paunovic I, Isic T, Havelka M, Tatic S, Cvejic D, Savin S. (2012) Combined Immunohistochemistry for Thyroid Peroxidase, Galectin-3, CK19 and HBME-1 in Differential Diagnosis of Thyroid Tumors. APMIS 120:368–79. [crossref]
- Abd-El Raouf SM, Ibrahim TR. (2014) Immunohistochemical Expression of HBME-1 and Galectin-3 in the Differential Diagnosis of Follicular-Derived Thyroid Nodules. Pathology, Research and Practice 210:971–8. [crossref]
- Trimboli P, Guidobaldi L, Amendola S, Nasrollah N, Romanelli F, Attanasio D, et al. (2016) Galectin-3 and HBME-1 Improve the Accuracy of Core Biopsy in Indeterminate Thyroid Nodules. Endocrine 52:39–45.
- Durry MF, Miskad UA, Leiwakabessy WN, Cangara MH. (2016) Diagnostic Value of Galectin-3 and Hector Battifora Mesothelial Epitope (HBME)-1 as a Marker for Malignancy in the Diagnosis of Thyroid Lesions. Pathology International 48:S122–3.
- Das DK, Al-Waheeb SK, George SS, Haji BI, Mallik MK. (2014) Contribution of Immunocytochemical Stainings for galectin-3, CD44, and HBME1 to Fine-Needle Aspiration Cytology Diagnosis of Papillary Thyroid Carcinoma. Diagn Cytopathol 42:498–505.
- Bartolazzi A, Gasbarri A, Papotti M, Bussolati G, Lucante T, Khan A, et al. (2001) Application of an Immunodiagnostic Method for Improving Preoperative Diagnosis of Nodular Thyroid Lesions. Lancet 357:1644–50. [crossref]
- Herrmann ME, LiVolsi VA, Pasha TL, Roberts SA, Wojcik EM, Baloch ZW. (2002) Immunohistochemical Expression of Galectin-3 in Benign And Malignant Thyroid Lesions. Archives of Pathology & Laboratory Medicine 126:710–13. [crossref]
- Zhu X, Sun T, Lu H, Zhou X, Lu Y, Cai X, Zhu X. (2010) Diagnostic Significance of CK19, RET, galectin-3 and HBME-1 Expression for Papillary Thyroid Carcinoma. Journal of Clinical Pathology 63:786-9. [crossref]