DOI: 10.31038/CST.2024921
Abstract
Objective: Local recurrence and abdominal metastasis are the main reasons for reducing the survival. It is of great clinical value to identify patients with more malignant biological features at high recurrence and metastasis risk. We want to evaluate the efficacy and sensitivity of XPO1 as a biomarker to stratify gastric cancer patients at high biological aggressive risk.
Method: We retrospectively analyzed the pathological records of 100 enrolled patients with gastric cancer who underwent gastric cancer resection in the department of surgery of our hospital from January 2017 to December 2022; all enrolled patients had complete pathological data and follow up for survival. In this study, we analyzed the immunohistochemical staining patterns of gastrectomy tissue specimens with patients with follow-up survival information and evaluated the efficacy of a novel biomarker XPO1/CRM1, also called Exportin 1.
Results: The positive IHC of XPO1 was correlated with the following factors: primary tumor volume (P value=0.05), regional lymph node invasion (P value=0.008) and TNM staging (P value=0.069). We noticed a sequential upregulation of XPO1 IHC intensity in benign lesions, borderline tumors, invasive carcinomas biological changes. Kaplan-Meier survival analysis indicated that XPO1 positivity was associated with poor survival.
Conclusions: Our results revealed XPO1 as a sensitive and useful biomarker to stratify gastric cancer patients at high biological aggressive risk. We recommend supplementing XPO1 IHC to routine pathology test to stratify individual patients for intensive therapy and stringent follow-up plans.
Highlights
- High XPO1 can stratify tumors with more biology malignancy trend
- High XPO1 predicts poor prognosis in gastric cancer
- High XPO1 patients need stringent treatment and follow-up
Keywords
Biomarker, Gastric cancer, Immunohistochemistry, Prognosis, XPO1
Introduction
Gastric cancer has a high incidence and poor prognosis, particularly in China. Each year, most new cases of gastric cancer are diagnosed among Asians and Eastern Europeans [1]. In 2020, approximately 27,000 new cases will be diagnosed [2]. The survival rates for patients with gastric disease are 31% in the United States and 25% globally [3]. In addition to its high incidence, gastric cancer has a poor prognosis and survival rate. Local recurrence and abdominal metastasis substantially impaired long-term survival. Common causes of poor prognosis [3,4] include late-stage diagnosis with regional or distant metastases, intratumor heterogeneity, and chemotherapeutic resistance. The identification of novel and specific biomarkers with prognostic significance and novel targets in gastric cancer is urgently required. At present, gastric cancer remains a fatal disease with limited treatment options. In clinical practice, clinicians execute TNM staging for patients primarily based on imaging; we believe it would be more beneficial if biomarkers that can predict the intrinsic metabolic characteristics of tumor cells could be identified for clinical applications. In addition to TNM staging, for instance, more effective prognostic assessment methods for gastric cancer can be identified, and patients who are more likely to experience recurrence can be identified. Recent reports have linked elevated XPO1 expression to a poor prognosis in a variety of tumors. XPO1, also known as CRM1, is a nuclear pheherin that belongs to the importin-superfamily [5-7] and can export at least 221 NES containing proteins and several nuclear Rnas to the cytoplasm [8,9]. The presence of conserved hydrophobic NES on carrier molecules was identified by XPO [9-11]. XPO1 participates in the localization and passive transport of diverse regulatory proteins between the nucleus and cytoplasm. Presently, it is known that XPO1 regulates a number of tumor suppressor genes that play a significant role in the pathogenesis and progression of cancer. Among the cargo proteins detected to be transported by XPO1 are the tumor suppressor p53, CDK1, adenomatous colonic polyposis (APC), BRCA1 and BRCA2, survivin, etc. [12,13]. Therefore, targeting XPO1 has promising potential as a cancer treatment. Intriguingly, XPO1 inhibitors effectively discriminate between tumor and normal tissue. XPO1 inhibitors are more likely to selectively and preferentially target tumor cells. The mechanism may be that, compared to non-malignant tumors, tumor cells express more XPO1 and cancer cells have an increased rate of cell proliferation and metabolism, making them more susceptible to nuclear trafficking inhibition [14,15]. First, we selected 100 gastric cancer patients with comprehensive clinical data from the pathology center of our hospital; all of these patients underwent surgical resection of gastric cancer in our hospital. Immunohistochemical staining was used to determine the XPO1 protein expression level in paraffin-embedded specimens of gastric carcinoma. We analyzed XPO1 IHC results in various TNM stages, as well as the correlation between XPO1 positivity and patient clinical data. Second, we analyzed XPO1 positivity variations in benign lesions, ambiguous tumors, and invasive carcinomas. We observed a pathological upregulation of XPO1 in malignant transformation of tumors, indicating its role in tumorigenesis. We performed a Kaplan-Meier analysis of survival to determine the impact of XPO1 on the clinical prognosis and survival of patients with gastric cancer. High XPO1 was able to stratify high-risk patients and predict a poorer prognosis, according to the findings. We advise these patients to adhere to rigorous treatment regimens and frequent follow-up appointments. Finally, we extended our findings to additional cancer categories. By comparing pan-cancer XPO1 expression and conducting survival analyses, we identified XPO1 as a biomarker for a poor prognosis in a variety of cancer types.
Methods
Patients’ Enrollment
We selected 100 patients who underwent surgical resection for gastric cancer at Suqian Hospital Affiliated with Xuzhou Medical University between January 1, 2017 and December 31, 2022. All patients enrolled in this study were informed of the study’s purpose and procedures, and all provided written consent to participate. The included patients must have comprehensive basic and clinic pathological information. Their paraffin-embedded tissue specimens were retrieved from the pathology department archives. Bormann grade of gross morphology and WHO grade of histopathology were used as the pathological diagnostic criteria [16]. The TNM classification of the 5th edition of the International Union against Cancer (UICC) was utilized for cancer staging [17]. Patients’ clinical information was gathered, recorded, and analyzed in detail. Indicators analyzed included the patient’s gender, age, tumor size, gastric wall invasion depth, histopathological grade, regional lymph nodes, and distant metastasis. Each patient was individually contacted via telephone to inquire about their survival status and to obtain a death date from their family. None of the patients included in the study received radiotherapy, chemotherapy, or immunotherapy prior to surgery. The study protocol was approved by the Ethics Committee Board of Suqian Hospital Affiliated to Xuzhou Medical University, and all experiments were carried out in accordance with Xuzhou Medical University’s guidelines.
Immunohistochemistry Staining
Tissues embedded in paraffin were sliced into 5-mm-thick sections. The portions were deparaffinized with xylene three times for five minutes each and rehydrated with 90, 75, and 50 percent ethanol in each container for two minutes. To recover antigenicity, the sections were submerged in a 10 mmol/L citrate buffer solution (pH 6.0) and microwaved for 12 minutes. To inhibit the activation of endogenous peroxidase, the samples were treated for 12 minutes with 3% hydrogen peroxide–methanol and then rinsed with distilled water. Anti-XPO1 rabbit polyclonal antibody (sc5595; Santa Cruz Biotechnology, Santa Cruz, California; 1: 100 dilution) was applied and incubated for one hour. Following washing, sections were rinsed with TBS and incubated with horseradish peroxidase-conjugated anti-rabbit antibody (Dako Cytomation, Carpinteria, CA). Phosphate-buffered saline (PBS) was substituted for the primary antibody to create negative controls.
Interpretation and analysis of immunohistochemistry results: Two pathologists independently examined the radiographs without knowledge of the patient’s clinical history. Each slide was investigated individually using a light microscope. When the results of two pathologists’ reviews are incongruent, the conclusion of the review is reached through mutual consultation between the two pathologists. The following criteria were used to interpret the XPO1 staining results: The intensity and proportion of positive cells were used to evaluate the immunostaining for XPO1. The staining intensity scores were as follows: 0 (negative), 1 (mild positive), 2 (medium positive), and 3 (strong positive). The following four kinds of scores were calculated based on the proportion of XPO1-positive cells: 0% to 10% was 1, 11 to 50% was 2, 51 to 80% was 3, and 81 to 100% was 4. As indicated previously, the final XPO1 staining score was calculated by multiplying the intensity score by the percentage score [18]. Positive results were defined as > 10% of cells with dark brown nuclei staining, and negative results were defined as < 10% of cells with staining. We determined the cutoff point for XPO1 IHC scores using the X-tile software (Rimm Lab at Yale University, http: //www.tissuearray.org/rimmlab).
Kaplan-Meier Survival Analysis in TCGA
The Kaplan-Meier curves for overall survival (OS) have been calculated for the high/low XPO1 expression group dichotomized by the 75% quantile of XPO1 expression. The log-rank test was utilized to investigate the difference in survival between those with high and low XPO1 expression.
Statistical Analysis
We used the chi-square test to assess the relationship between XPO1 expression and various clinicopathological features of gastric cancer. Cox’s proportional hazards regression models were used to determine univariate and multivariate analyses in order to identify independent factors associated with disease-free survival and overall survival. The Kaplan-Meier method was utilized to assess the relationships between XPO1 expression and patient outcomes. *, P < 0.05, **, P < 0.01, ***, P < 0.005, and exact P values are stated in the source data for each figure panel.
Results
Gastric Cancer Exhibits Higher XPO1 with Immunohistochemistry
The clinical characteristics of the patients were summarized and exhibited (Table 1). The ages of the patients ranged from 30 to 85 years. High XPO1 expression was specifically correlated with TNM stage (p=0.003), tumor stage (p=0.05), and lymph node metastasis positivity (p=0.007). In contrast, no significant correlation was found between XPO1 expression and other clinical factors, including gender, tumor diameter, age, and status of distant metastasis. To determine if there are any differences in XPO1 expression between gastric patient samples and normal gastric tissues, we compared XPO1 expression between gastric cancer tumors and normal gastric epithelial tissues. Each clinic sample contains comprehensive information regarding the pathology cell type and tumor stage. Two pathologists independently evaluated the results of XPO1 immunohistochemistry staining, with no knowledge of the patient’s clinical history. When there was disagreement, a conclusion was reached via consensus. Evaluation of the immunostaining was based on the intensity and percentage of XPO1-positive cells. The stain’s intensity was measured as follows: 0 (negative), 1 (weakly positive), 2 (moderately positive), and 3 (strongly positive). In addition, the percentage of XPO1-positive cells was scored based on four categories: 1 for 0 to 10%, 2 for 11 to 50%, 3 for 51 to 80%, and 4 for 81 to 100%. Multiplying the intensity and percentage scores produced the final XPO1 staining score. The emblematic images of IHC were displayed. We observed a substantial difference in XPO1 expression between tumor and normal tissue samples. Strong XPO1 positivity was observed in gastric tumors, and XPO1 intensity increased with TNM stages II, III, and IV (Figure 1).
Table 1: Demographic characteristics of the 100 gastric cancer patients. High XPO1 expression was associated with TNM stage (p=0.003), tumor stage (p=0.05) and positive lymph node metastasis (p=0.007). No significant correlation was discovered between XPO1 expression and other clinical parameters, such as gender, age, tumor diameter, and distant metastasis status.
|
n |
Negative (%) (n = 55) | Positive (%) (n = 45) |
P-value |
||
| Gender (M: F)
Age (years) Longest diameter (cm) T stage T1 T2 T3 T4 Nodal stage N0 N1 N2 N3 Distant metastasis M0 M1 TNM stage I II III IV |
33 24 38 5 48 34 14 4 97 3 44 17 33 6 |
38: 17(69%: 31%) 56.38 3.65 27(82%) 11(46%) 16(42%) 1(20%) 37(39%) 20(59%) 4(29%) 1(25%) 62(64%) 0(0%) 34(77%) 11(65%) 15(45%) 2(33%) |
26: 19(58%: 42%) 57.65 4.76 6(18%) 13(54%) 22(58%) 4(80%) 11(61%) 14(51%) 10(71%) 3(75%) 35(36%) 3(100%) 10(23%) 6(35%) 18(55%) 4(67%) |
0.531 0.723 0.125 0.05 0.007 0.072 0.03
|
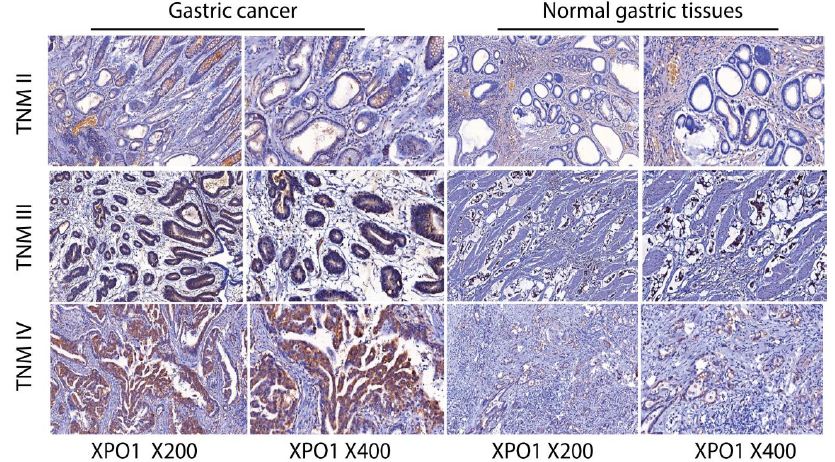
Figure 1: Gastric tumors in TNM II, III, IV stages exhibit increased XPO1 intensity with immunohistochemistry staining. Representative immunohistochemistry results for anti-human XPO1 staining were presented. Gastric tumors were strongly positive for XPO1, and XPO1 intensity increased with TNM II, III, IV stages. Compared with gastric cancer samples, the expression of XPO1 in normal tissues was limited or absent.
Compared with gastric cancer samples, the expression of XPO1 in normal tissues was limited or absent. There was a statistically significant difference between adjacent non-tumor tissues and tumor-infiltrated areas in XPO1 expression, P = 0.001. The rate of positivity in normal tissue was 6%, whereas the rate of positivity in tumor areas was significantly higher (45%). High XPO1 expression was detected in 45 of 100 (45%) gastric cancer tissues, while only 6 of 100 (6%) normal gastric tissues displayed XPO1 expression (Table 2). These results indicated that XPO1 signaling was strongly activated in gastric cancer.
Table 2: Overall XPO1 expression in tumor and surrounding normal tissues. IHC was employed to investigate the expression of XPO1 in gastric cancer. There was a statistical difference in the XPO1 expression between tissues adjacent non-tumor tissues and tumor-infiltrated areas (p=0.0001).
|
XPO1 |
Normal tissue |
Cancer |
| negative
1–10% 11–50% 51–100% P-value |
94 (94%) 4(4%) 2(2%) 0(0%) 0.0001 |
25(55%) 13(23%) 18(18%) 44(4%) |
XPO1/CRM1, also called Exportin 1, Cancer-related genes, FDA approved drug targets.
XPO1 Plays a Role in Tumor Initiation and Progression
Previous research indicates that XPO1 exports tumor suppressor genes from the nucleus and promotes tumorigenesis. We hypothesized that XPO1 facilitated tumor initiation, i.e., that XPO1 levels would increase during the carcinogenesis process. We compared the variance in XPO1 IHC intensity among benign lesions, ambiguous tumors, and invasive carcinoma groups. Although there was no XPO1 positivity in benign lesions, there was an increase in borderline tumors. Strong XPO1 positivity was observed in invasive carcinomas (Figure 2).
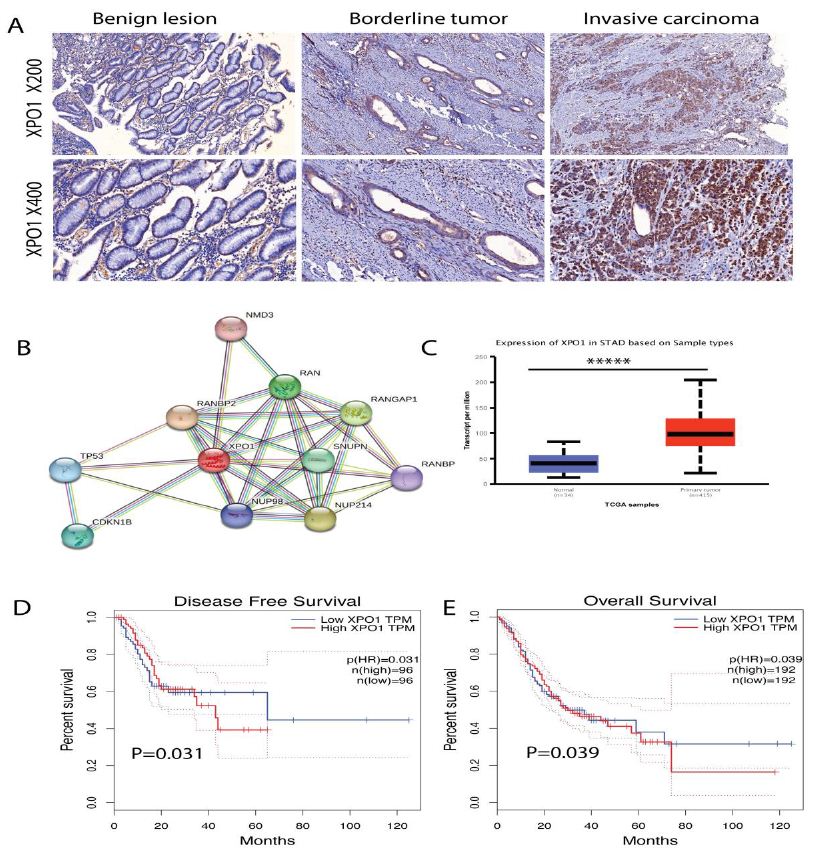
Figure 2: Gastric tumors exhibit higher XPO1 expression, which predicts shorter disease-free survival and overall survival. (A) Presentative XPO1 IHC staining in benign lesions, borderline tumors, and invasive carcinoma groups. There was no XPO1 positive staining in benign lesions, however, invasive carcinoma showed a very strong XPO1 positive staining. (B) STRING analysis showed the top genes interacting with XPO1 in gastric cancer, of which the top correlated genes were TP53, CDKN1B, RANBP2, NUP98, NUP214. (C) Gastric cancer had higher XPO1 expression than the normal tissues, results calculated from TCGA gastric cancer cohort. (D) Higher XPO1 expression predicts shorter disease-free survival in gastric cancer, p=0.031. (E) Higher XPO1 expression predicts shorter overall survival in gastric cancer, p=0.039.
To gain a more detailed understanding of how XPO1 may interact with other genes. We analyzed the top genes in TCGA gastric cancer cohorts that correlate with XPO1. According to gene STRING analysis, the XPO1 gene is closely related to a number of genes that promote malignancy. STRING analysis showed the top interacting genes with XPO1 in gastric cancer, of which the top genes were TP53 (responds to diverse cellular stresses, induce cell cycle arrest, apoptosis, senescence, DNA repair, or changes in metabolism), CDKN1B (cyclin-dependent kinase inhibitor, which shares a limited similarity with CDK inhibitor CDKN1A/p21), RANBP2 (RAN binding protein 2, enables SUMO ligase activity), NUP98 (the 96 kDa nucleoporin is a scaffold component of the nuclear pore complexes), NUP214 (the protein encoded by this gene is localized to the cytoplasmic face of the nuclear pore complex). Gastric cancer had higher XPO1 expression than the normal tissues. Higher XPO1 expression was related with shorter disease-free survival, p=0.031 and overall survival, p=0.039 in gastric cancer. To gain a more detailed understanding of how XPO1 may interact with other genes. We analyzed the top genes in TCGA gastric cancer cohorts that correlate with XPO1. According to gene STRING analysis, the XPO1 gene is closely related to a number of genes that promote malignancy. Results revealed a considerable increase in XPO1 during the progression of gastric cancer from benign lesions to borderline tumors and then to the terminal invasive carcinoma (Table 3).
Table 3: Increased expression of exportin 1/XPO1 located both in nuclear and cytoplasm. The specific number of XPO1 IHC stain location in benign lesions, borderline tumors, and invasive carcinoma groups were summarized and shown.
|
Number. of patients (%) |
|||||
|
XPO1 |
Invasive carcinomas, N=70 | Borderline tumors, n=20 | Benign lesions, n=10 |
P* |
|
| Nuclear and Cytoplasmic | Negative 20 (28.5)
Positive 50 (71.4) |
16(80) 4 (20) |
10 (100)
0 (0) |
0.001
|
|
| Nuclear | Negative 41 (58.6)
Positive 29 (41.4) |
18 (90) 2 (10) |
10 (100)
0 (0) |
0.002
|
|
| Cytoplasmic | Negative 49 (70)
Positive 21 (30) |
18 (90) 2 (10) |
10 (100)
0 (0) |
0.000
|
|
| XPO1/CRM1, also called Exportin 1, Cancer-related genes, FDA approved drug targets.
Nuc and Cyt, Nuclear and cytoplasmic. *Chi-square test. |
|||||
Since XPO1 is present in both the nucleus and cytoplasm, both patterns were evaluated separately. Immunohistochemistry for XPO1 was negative in benign lesions. In borderline tumors, XPO1 positivity was more prominent than in benign lesions. Nuclear (2 of 20) and cytoplasmic (2 of 20) expression was moderate in 4 of 20 borderline tumors. XPO1 nuclear expression was detected in 29 of 70 invasive carcinomas (41.4%), whereas XPO1 cytoplasmic expression was detected in 21 of 74 tumors (30%). In the majority of tumors, both expression patterns were found concurrently, albeit with differing intensities. XPO1 facilitates the transport of tumor suppressor genes outside of the nucleus and may facilitate and accelerate tumorigenesis, as suggested by these findings.
TP53 Mutant Gastric Cancer had Higher XPO1 Expression
To confirm the localization of XPO1, we examined the human protein atlas and cell atlas. We discovered that XPO1 was predominantly localized in the nucleus and cytoplasm of cancer cells, which is consistent with our results. The specific intracellular XPO1 localization were examined and analyzed (Figure 3).
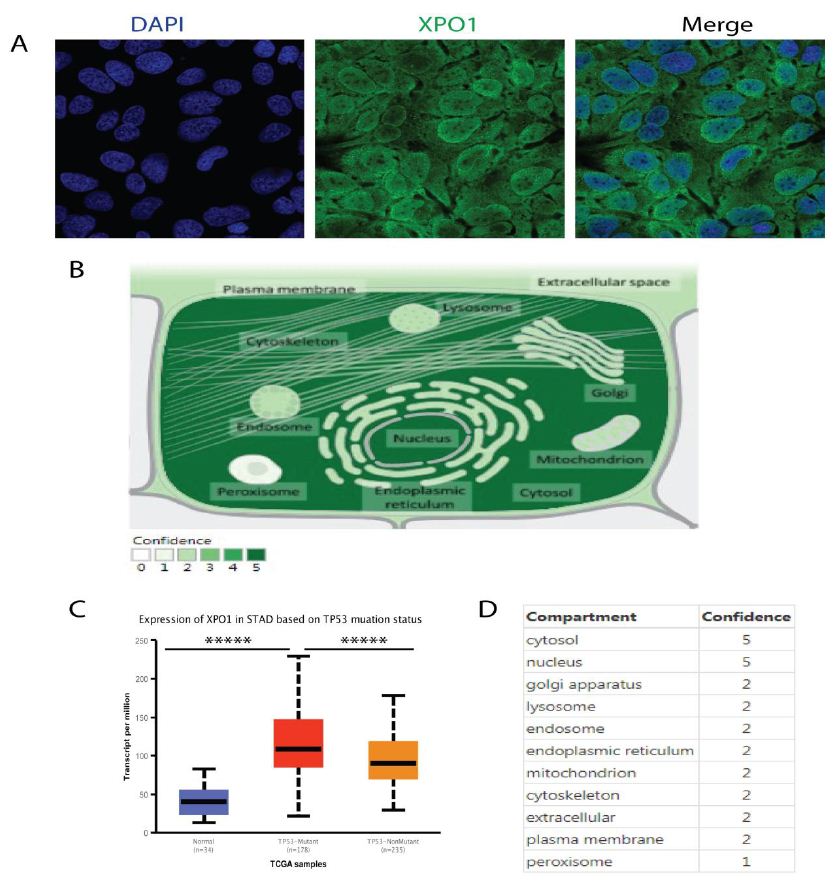
Figure 3: XPO1 has many subcellular locations, with cytosol and nucleus as the two most frequent sites. (A) Representative confocal images stained with anti-XPO1 (CAB010184) antibody. In addition to localized at the cytosol & vesicles, XPO1 mainly localize to the nucleoplasm & nuclear membrane. XPO1, also called exportin 1, was cancer-related genes, a transporter which localized to the nucleoplasm (enhanced), and nuclear membrane (enhanced). (B) The specific subcellular XPO1 location were examined and analyzed from COMPARTMENTS. (C) TP53 mutant gastric cancer exhibited higher XPO1 expression. (D) Subcellular locations of XPO1 from the Human Protein Atlas (HPA) COMPARTMENTS, cytosol (5), nuclear membrane (5), nucleoplasm (2), vesicles (2).
The XPO1 localization intensity was calculated. We observed that XPO1 can be found in numerous locations within the cell, with the cytosol and nucleus being the most common. XPO1 facilitates tumorigenesis and confers drug resistance by transporting the tumor suppressor TP53. Given that p53 was a cargo protein for XPO1, it was hypothesized that inhibiting XPO1 could activate TP53. We observed that TP53 mutant gastric cancer has increased XPO1 expression. The significance of TP53 mutational and functional status on XPO1 inhibitor sensitivity in gastric cancer cell lines and the functional role of apoptosis signaling mediated by TP53 were correlated with nuclear accumulation of TP53.
Pan-Cancer XPO1 Expression and Survival Analysis
Finally, we wanted to extend our discovery to other cancer types. We investigated The Cancer Genome Atlas (TCGA) database for pan-cancer XPO1 expression analysis in cancer and normal tissues. Box graphs were used to illustrate the differential gene expressions (Figure 4).
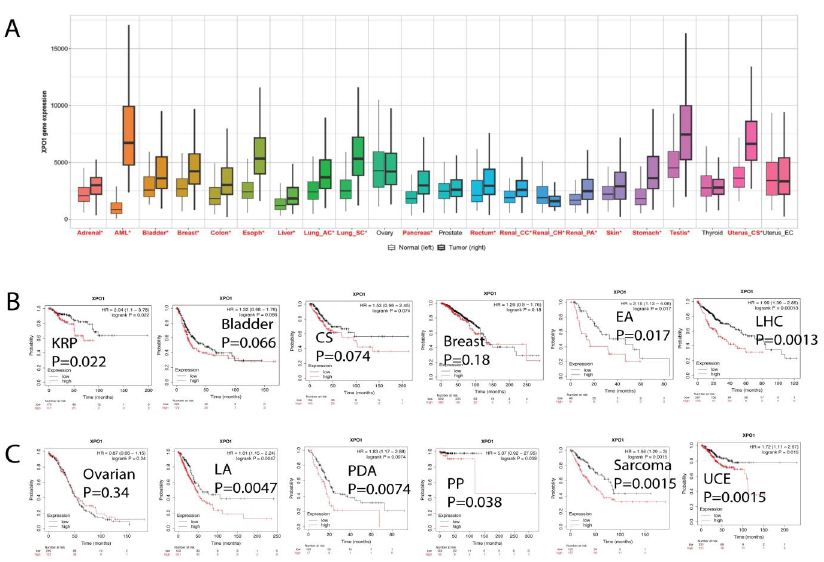
Figure 4: Pan cancer XPO1 expression and Kaplan-Meier survival analysis. (A) We studied the differential expression between tumor and adjacent normal tissues for XPO1 in all TCGA tumors. Distributions of gene expression levels were displayed using box plots. The statistical significance computed by the Wilcoxon test was annotated by the number of stars (*, p-value < 0.05). (B) Kaplan-Meier survival analysis between XPO1 expression and clinical outcome in multiple cancer types. In the kidney renal papillary (KRP) carcinoma, bladder tumor, cervical squamous (CS), liver hepatocellular cancer (LHC), and esophageal adenocarcinoma (EA) cohort, high XPO1 predicts shorter overall survival. The p value was labeled on each graph. (C) In lung adenocarcinoma (LA), pancreatic ductal adenocarcinoma (PDA), pheochromocytoma and paraganglioma (PP), sarcoma, and uterine corpus endometrial carcinoma (UCE) cohort, high XPO1 predicts shorter overall survival. The p value was labeled on each graph.
The Wilcoxon test’s statistical significance was indicated by the number of stars (*: p-value 0.05). We were able to determine whether XPO1 was up- or down-regulated in tumors relative to their normal counterparts for each cancer type. The 33 malignancies analyzed by the TCGA/Pan Cancer Initiative were represented schematically according to their tissue of origin. XPO1 was expressed substantially more (red) in cancerous tissues than in normal tissues, with the exception of ovarian, prostate, thyroid, and uterine cancers (black). We then performed a Kaplan-Meier analysis of survival between XPO1 expression and clinical prognosis in multiple types of cancer. In the cohort of patients with kidney renal papillary (KRP) carcinoma, bladder tumor, cervical squamous (CS), liver hepatocellular cancer (LHC), and esophageal adenocarcinoma (EA), a high XPO1 level predicts a shortened overall survival. In lung adenocarcinoma (LA), pancreatic ductal adenocarcinoma (PDA), pheochromocytoma and paraganglioma (PP), sarcoma, and uterine corpus endometrial carcinoma (UCE) cohorts, XPO1 expression was associated with shorter overall survival at a 75% quantile threshold. Collectively, these findings demonstrated that XPO1 is a potential broad-spectrum biomarker for cancer prognosis and could be a therapeutic target for treatment.
Discussion
In this retrospective study, we evaluated the immunohistochemical staining of XPO1 in gastric tumor samples and investigated the correlation between XPO1 level and multiple clinicopathology factors in predicting its clinical significance in patients with gastric cancer. XPO1 levels were substantially elevated in cancer samples compared to normal counterparts. Statistically, the degree of XPO1 positivity did not correlate with tumor size; however, higher expressions were found in patients with higher T values, more regional lymph node invasion, and advanced TNM staging, which could predict a substantially lower survival rate. We examined the differential expression of XPO1 in various phases of gastric cancer and the correlation between XPO1 immunohistochemical staining and patient clinical characteristics. Our results demonstrated that XPO1 is a valuable biomarker for stratifying gastric cancer patients based on their biologically malignant nature. Chemotherapy and surgery for gastric cancer have improved over the past few decades [19,20]. Nonetheless, patients with gastric cancer continue to have a poor prognosis due to therapeutic failure and disease progression [21]. Identification of novel and validated prognostic biomarkers in practice has clinically significance for gastric cancer. In this study, we discovered that XPO1 was a useful marker in gastric cancer that had the potential to be used as a candidate for targeted therapy. The regulation of material transport across the nuclear membrane was essential for maintaining homeostasis, which required the correct nuclear-cytoplasm positioning of large molecules; nevertheless, this process was typically dysregulated in cancer cells [22]. XPO1, an export receptor responsible for the nuclear-cytoplasm transport of multiple proteins and RNA species, was frequently overexpressed or mutated in human malignancies and served as a potential oncogenic driver [23]. Unlike small molecules, which can passively diffuse through the nuclear pore complex (NPC), larger cargo molecules (>40 kDa) require active transport via transport receptors [24-26], which belong to the karyopherin beta family and are classified as importins (nuclear import), exportins (nuclear export), and transportins (for both import and export) [25]. Studies show that exportins are potential targets in tumorigenesis [27,28], of which XPO1 was the most important and well-studied target. XPO1 was initially identified as a chromosomal mutation in the yeast Schizosaccharomyces pombe [29]. XPO1, also known as CRM1, transported over 200 proteins, the majority of which were tumor suppressors and oncoproteins [29-31]. CRM1-mediated cargos include p27, p53, FOXOs, nucleophosmin, PI3K/AKT, Wnt/-catenin, BCR-ABL, p21, NF-kB, APC, and Rb; these cargos all play important roles in tumorigenesis [28,32]. For the first time, we investigated the clinical and prognosis value of XPO1 in gastric cancer. IHC analysis revealed a higher XPO1 concentration in gastric cancer tissues compared to normal gastric tissues. Consistent with previous research, our findings indicated that a variety of malignancies exhibited a higher level of XPO1 expression than their normal counterparts [33,34]. In addition, elevated XPO1 levels in gastric cancer were associated with certain clinical-pathologic factors, including AJCC stage, positive lymph node metastasis, and tumor grade. The Kaplan-Meier analysis demonstrated that the disease-free survival and overall survival of patients with increased XPO1 expression were shorter than those of patients with negative or decreased expression. A univariate analysis revealed that XPO1 expression, AJCC stage, and lymph node metastasis were correlated with gastric cancer patients’ survival (both disease-free survival and overall survival). High levels of XPO1 and advanced AJCC staging independently predicted unfavorable disease-free survival and overall survival outcomes for patients with gastric cancer, as determined by multivariate analysis. XPO1 overexpression was identified in solid tumors and hematologic malignancies and was reported as an indicator of poor prognosis and potential drug resistance in cancers [35]. One potential mechanism for XPO1 overexpression was associated with altered transport, which promoted cancer-promoting outcomes [36]. XPO1 facilitated the import of growth regulatory proteins, such as c-myc or BCR-ABL, into the cytoplasm and consequently activated downstream signaling, resulting in sustained cell proliferation. Similarly, tumor suppressor proteins (TSPs), such as p53, p21, Rb, and p27, were rendered inactive by exportin and lost their ability to inhibit uncontrolled cell proliferation. Collectively, these findings support the notion that XPO1 inhibition is an attractive therapeutic target for its ability to target a variety of hallmarks of oncogenesis signaling. In addition, the combination of SINE compounds with existing standard regimens in multiple cancer types was feasible and well tolerated in clinical trials. Common inhibitors of nuclear export (SINE) XPO1 antagonists included KPT-185, KPT-276, KPT-251, and KPT-330, which were reported to inhibit the proliferation of triple-negative breast cancer (TNBC) cell lines and also demonstrated efficacy in human breast cancer xenograft models. Mechanically, SINE compounds inhibit XPO1 and suppress STAT3 trans-activation, thereby inhibiting the oncogenic potential of TNBC and their clinical application [36]. Priming cancer cells with XPO1 inhibitors followed by doxorubicin, melphalan, bortezomib, or carfiltiamob may sensitize de novo and adaptive cancer cell lines to drug resistance [37]. Inhibiting the activation of the XPO1 pathway would accelerate the apoptosis of tumor cells and induce cell cycle arrest [38,39]. In summary, XPO1 expression or upregulation may replicate the natural process of gastric cancer bio-evolution, and XPO1 may therefore predict and stratify patients with a poor prognosis. In another sense, we may consider the XPO1 level as a molecular staging biomarker for oncologists employing intensive surgical intervention or chemotherapy. High XPO1 expression in gastric cancer was a reliable molecular biomarker for staging and prognostic prediction during both the diagnostic and treatment phases. High XPO1 expression in gastric cancer is indicative of an aggressive phenotype requiring intensive treatment and careful monitoring. Our findings supported XPO1 as a novel prognostic biomarker for patients with gastric cancer, and targeting XPO1 may provide a beneficial strategy for gastric cancer patients with positive XPO1 expression, which is typically accompanied by TP53 mutation. As mentioned previously, inhibiting XPO1 signaling with SINE may restore the functions of common tumor suppressors. Thus, targeting XPO1 in gastric cancer may provide new treatment options for gastric cancer patients, particularly those with advanced disease and a high recurrence risk. In addition, our pan-cancer analysis of the TCGA dataset revealed that XPO1 was commonly elevated in all cancer types. Consequently, our findings illuminated the potential universal application of XPO1 inhibitors in multiple types of cancer. Future clinical studies are required to evaluate the therapeutic effects of KPT-SINE compounds (small molecules for XPO1) alone and in combination with XPO1-targeted therapy. Our research had several limitations. We detected XPO1 positivity solely through immunohistochemistry, so there is a possibility for diagnostic error. Several other techniques, such as immune blotting and qRT-PCR for mRNA expression, have been considered in an effort to achieve more precise diagnostic results. Second, all clinical data, including recurrence and survival rates, were retrospectively collected. Thirdly, the relatively small number of patients enrolled in our study may result in a lack of statistical power; therefore, a larger prospective study is needed in the future.
Availability of Data and Materials
The data generated in the present study may be requested from the corresponding author through 1822991734@qq.com
Authors’ Contributions
Conception and design were performed by Rui Wang and Yanli Cheng. Data analysis and interpretation were performed by Ruimin Wang. Manuscript writing was performed by Rui Wang and revised by Yanli Cheng. Final approval of manuscript was performed by all authors who read and approved the final manuscript.
Grant Support
Rui Wang is founded by China Scholarship Council (202206920039). This research was supported by funds from Natural Science Foundation of Suqian Science and Technology Bureau (K201903, Z2018076, Z2018213 and Z2022065). Jiangsu Association for Science and Technology (JSTJ-2022-004).
Ethics Approval and Consent to Participate
The patient reported in this study was informed for the purpose and process of this study and had written informed consent according to the guidelines of the hospital’s human associated research.
Patient Consent for Publication
Not applicable.
Competing Interests
The authors indicated no potential conflicts of interest.
Declaration of Interest
The authors declare that there is no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Financial Support
Rui Wang is founded by China Scholarship Council (202206920039). This research was supported by funds from Natural Science Foundation of Suqian Science and Technology Bureau (K201903, Z2018076, Z2018213 and Z2022065). Jiangsu Association for Science and Technology (JSTJ-2022-004).
Acknowledgements
The authors would like to thank Dr. Xiaohong Shi for comments and discussion on the manuscript. We also would like to thank Dr. Quanquan Guo for data analysis.
References
- Rawla P, A Barsouk (2019) Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol 14(1): 26-38. [crossref]
- Gu E (2020) . SCDb: an integrated database of stomach cancer. BMC Cancer 20(1): 490. [crossref]
- Rawla P, A Barsouk (2019) Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol 14(1): 26-38. [crossref]
- Stahl P (2015) Heterogeneity of amplification of HER2, EGFR, CCND1 and MYC in gastric cancer. BMC Gastroenterol 15(1): 70-78. [crossref]
- Fornerod M (1997) CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 90(6): 1051-60. [crossref]
- Fukuda M (1997) CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature 390(6657): 308-11. [crossref]
- Ossareh-Nazari B F. Bachelerie, C. Dargemont (1997) Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science 278(5335): 141-4. [crossref]
- Fu SC (2013) ValidNESs: a database of validated leucine-rich nuclear export signals. Nucleic Acids Res 41(Database issue): D338-43. [crossref]
- Okamura M H. Inose and S. Masuda (2015) RNA Export through the NPC in Eukaryotes. Genes (Basel) 6(1): 124-49. [crossref]
- Monecke T A. Dickmanns, R. Ficner (2014) Allosteric control of the exportin CRM1 unraveled by crystal structure analysis. FEBS J 281(18): 4179-94. [crossref]
- Cautain B (2015) Components and regulation of nuclear transport processes. FEBS J 282(3): 445-62. [crossref]
- Wang A Y, H Liu (2019) The past, present, and future of CRM1/XPO1 inhibitors. Stem Cell Investig 6(3): 69-78. [crossref]
- Schmidt J (2013) Genome-wide studies in multiple myeloma identify XPO1/CRM1 as a critical target validated using the selective nuclear export inhibitor KPT-276. Leukemia 27(12): 2357-65. [crossref]
- Angus L, PJ. van der Watt, VD Leaner (2014) Inhibition of the nuclear transporter, Kpnbeta1, results in prolonged mitotic arrest and activation of the intrinsic apoptotic pathway in cervical cancer cells. Carcinogenesis 35(5): 1121-31. [crossref]
- Kuusisto HV, DA Jans (2015) Hyper-dependence of breast cancer cell types on the nuclear transporter Importin beta1. Biochim Biophys Acta 1853(8): 1870-8. [crossref]
- Schlemper RJ ( 2000) The Vienna classification of gastrointestinal epithelial neoplasia. Gut 47(2): 251-5. [crossref]
- Hermanek P (1987) [TNM classification of malignant tumors: the new 1987 edition]. Rontgenblatter. 40(6): 200. [crossref]
- Gong W (2005) Expression of activated signal transducer and activator of transcription 3 predicts expression of vascular endothelial growth factor in and angiogenic phenotype of human gastric cancer. Clin Cancer Res 11(4): 1386-93. [crossref]
- Barchi LC (2016) MINIMALLY INVASIVE SURGERY FOR GASTRIC CANCER: TIME TO CHANGE THE PARADIGM. Arq Bras Cir Dig 29(2): 117-20. [crossref]
- Kinoshita J (2021) Current status of conversion surgery for stage IV gastric cancer. Surg Today 11(4): 1386-93. [crossref]
- Digklia A, AD Wagner (2016) Advanced gastric cancer: Current treatment landscape and future perspectives. World J Gastroenterol 22(8): 2403-14. [crossref]
- Gravina GL (2014) Nucleo-cytoplasmic transport as a therapeutic target of cancer. J Hematol Oncol (7): 85-96. [crossref]
- Azizian NG, Y Li (2020) XPO1-dependent nuclear export as a target for cancer therapy. J Hematol Oncol 13(1): 61. [crossref]
- Schmidt HB, D Gorlich (2016) Transport Selectivity of Nuclear Pores, Phase Separation, and Membraneless Organelles. Trends Biochem Sci 41(1): 46-61. [crossref]
- Ullman KS, MA Powers, DJ Forbes (1997) Nuclear export receptors: from importin to exportin. Cell 90(6): 967-70.
- Jamali T (2011) Nuclear pore complex: biochemistry and biophysics of nucleocytoplasmic transport in health and disease. Int Rev Cell Mol Biol 287(3): 233-86. [crossref]
- Sun Q et al. (2016) Inhibiting cancer cell hallmark features through nuclear export inhibition. Signal Transduct Target Ther (1): 160-175. [crossref]
- Das A et al. (2015) Selective inhibitors of nuclear export (SINE) in hematological malignancies. Exp Hematol Oncol (4): 79-86. [crossref]
- Stade K et al. (1997) Exportin 1 (Crm1p) is an essential nuclear export factor. Cell 90(6): 1041-50. [crossref]
- Senapedis WT, E Baloglu, Y Landesman (2014) Clinical translation of nuclear export inhibitors in cancer. Semin Cancer Biol (27): 74-86. [crossref]
- Xu D, NV Grishin, YM.Chook (2012) NESdb: a database of NES-containing CRM1 cargoes. Mol Biol Cell 23(18): 3673-6. [crossref]
- Senapedis WT, E Baloglu, Y Landesman (2014) Clinical translation of nuclear export inhibitors in cancer. Semin Cancer Biol (27): 74-86. [crossref]
- Zhang S (2012) ROR1 is expressed in human breast cancer and associated with enhanced tumor-cell growth. PLoS One 7(3): e31127. [crossref]
- Zhang S (2012) The onco-embryonic antigen ROR1 is expressed by a variety of human cancers. Am J Pathol 181(6): 1903-10. [crossref]
- Sun Q et al. (2016) Inhibiting cancer cell hallmark features through nuclear export inhibition. Signal Transduct Target Ther (1): 160-179. [crossref]
- [36]. Cheng Y et al. (2014) XPO1 (CRM1) inhibition represses STAT3 activation to drive a survivin-dependent oncogenic switch in triple-negative breast cancer. Mol Cancer Ther 13(3): 675-86. [crossref]
- Turner JG (2014) Inhibition of CRM1-dependent nuclear export sensitizes malignant cells to cytotoxic and targeted agents. Semin Cancer Biol (27): 62-73. [crossref]
- Huang WY (2009) Prognostic value of CRM1 in pancreas cancer. Clin Invest Med 32(6): E315. [crossref]
- Kim J (2016) XPO1-dependent nuclear export is a druggable vulnerability in KRAS-mutant lung cancer. Nature 538(7623): 114-117. [crossref]