DOI: 10.31038/AFS.2024612
Abstract
The biofloc system uses the presence of microorganisms in the culture system to generate flocs from nitrogen waste, thus permitting continued water use. Factors like carbon source, carbon-to-nitrogen ratio, and stocking density affect the quality and density of microorganisms and the productivity of the system. This study aims to determine the growth, feed conversion ratio (FCR), and condition indices of catfish reared in a biofloc system using rice bran (RBB), fermented rice bran (FRB), and hydrolyzed fermented rice bran (HFRB) as carbon sources. Fingerling catfish with an initial mean weight of 11.15 ± 1.60 g were stocked in outdoor 200-liter plastic tanks in a randomized design with the three treatments in two replications. A biomass (g) to volume (l) ratio of 1:2 was maintained throughout the experiment. The carbon-nitrogen content was adjusted to 5:1 C-N in the system. The results showed that the water quality parameters of all the treatments were within the range recommended for aquaculture. The HFRB treatment showed significantly higher floc (P<0.05) compared with RBB and FRB. The weight of the catfish at the end of the 8-week rearing trials showed the catfish culture using RBB (80.26 ± 3.20 g), FRB (81.70 ± 2.5 g), and HFRB (85.50 ± 2.55 g) were significantly different (P<0.05). A similar trend was observed in the feed conversion ratio. The condition indices of catfish were also higher in FRB treatment. The FCR value and protein efficiency ratio were not significantly different (P>0.05) between RBB and HFRB treatments. However, the percentage survival was significantly lower in the HFRB treatment (P < 0.05) compared with the FRB and RBB treatments. While fermentation of rice bran has gained much consideration, this study demonstrated that acid-hydrolyzed fermentation of rice bran could boost its performance as a biofloc carbon source.
Keywords
Fermented rice bran, Acid-hydrolyzed rice bran, Biofloc, Carbon sources
Introduction
The African catfish, Clarias gariepinus, is valued as the most economically important fish cultured in Africa. This species is reputed for its desired aquaculture traits, including high fecundity, fast growth, hardiness, and market attractiveness. It is one of the most researched culturable fish in Nigeria. Modern culture techniques such as aquaponics and biofloc systems have also adopted catfish as one of the experimental species for the viability of the systems. Biofloc aquculture systems transform fish waste into microorganism biomass through the addition of carbon sources. The type of carbon source significantly influenced the water quality of the system. The floc serves as additional food for the fish, leading to faster growth and higher production of fish compared to traditional methods. Microorganism- enriched floc confers immunological enhancement to fish, thereby improving fish health. Many ingredients, such as grains, sucrose, sugarcane byproducts, tapioca, rice, wheat bran, etc., have been used as carbon supplements in biofloc systems. Ligno-cellulose materials like bran showed limited success in biofloc systems due to the to the slow release of carbon, but tends to have higher microbial diversity and immune-boosting potential for cultured organisms. Researcher efforts to improve carbon release and utilization of lignified and cellulose materials in biofloc systems remain pertinent. This work compared the growth performance and condition indices of catfish in biofloc systems where untreated, fermented, and hydrolyzed fermented rice bran were used as carbon sources. In ethanol production, acid hydrolysis of rice bran resulted in the conversion of its starch and cellulose component into reducing sugar, and fermentation has been used to increase the nutrient value of rice bran. While fermented rice bran has been well experimented with in biofloc, we hypothesize that acid hydrolyzed once could also serve in the system [1-12].
Materials and Methods
Experimental Setup
The experiment was conducted at the biological garden, Umaru Musa Yar’adua University. Fingerlings of African catfish, Clarias gariepinus, of 11.15 ± 1.50 g initial weight were obtained from hatchery-reared stock and kept in a 200-liter plastic tank for a 7-day acclimation period. The fish were fed a diet containing 40% crude protein at 5% body weight during 8:00–18.00 hours daily. Experimental tanks were seeded with 1 liter of water from a pre-fertilized earthen fish pond containing abundant phytoplankton. The fish were randomly distributed to the three experimental treatments of untreated, fermented, and hydrolyzed fermented rice bran biofloc in a triplicate of 20 fish per tank at a 2:1 biomass (g) to volume (l) ratio. Each experimental setup was seeded with 2 liters of water from a pre-fertilized earthen fish pond containing abundant phytoplankton.
Fermentation and Acid Hydrolysis of Rice Bran
Milled rice bran was sieved through a 0.50 mm sieve and sterilized in an autoclave. The solid-phase fermentation procedure described by [13] was followed with slight modifications. 50 g of rice bran was added to 45 ml of distilled water, while 5 g of baker’s yeast dissolved in 5 ml of water was added to make up a 1:1 weight-to-volume ratio of rice bran to water. The mixture was incubated in a beaker at a temperature of 27°C for 24 hours. Acid hydrolysis of rice bran was carried out using 50 ml of 2% sulfuric acid mixed with 50 g of rice bran, and the mixture was incubated at 90°C for 3.5 h. This was modified from. The hydrolyzed product was subjected to fermentation as described before. The fermented and hydrolyzed fermented products were oven dried at 45°C for 6 h, powdered, and sieved through 100 µm mesh. The fermented rice bran (FRB) and hydrolyzed fermented rice bran (HFRB) were used as carbon sources in biofloc production catfish [11].
Water Quality and Floc Monitoring
Water parameters were measured every two weeks. The temperature (°C) was determined using a mercury in glass thermometer, while the pH was measured using a Metrohm Herisau E520 pH meter. Dissolved oxygen concentration was determined through the Winkler-Azide method [14]. Chemical oxygen demand (COD) was determined titrimetrically, while biological oxygen demand (BOD) was determined using the incubation method at 20°C for five days [15]. The total ammonia nitrogen concentration was determined using the phenate method [16], while nitrate was determined using spectrophotometry [14]. By assuming 16% of protein is nitrogen and 46% carbon in rice bran, the amount of carbon to be added was calculated following. The total feed consumed per day was estimated, and the C:N was adjusted daily to 15:1 by adding treatments C of untreated, fermented, or hydrolyzed rice bran. The treatment carbon was mixed with 1 liter of water from the treatment before being added to the experimental setup. Biofloc volume (ml/L) was measured every 14 days for each experimental treatment using an Imhoff cone. The floc solution was allowed to settle down for one hour before the reading was taken [17-19].
Data Collection
The total length (TL) of fish in centimeters from each replicate was measured from the tip of the snout to the end of the caudal fin using a meter rule. Body weight was measured using an electronic digital balance. At the end of the experiment, all fish in the tank were counted, and the survival rate was determined. Growth performance in each treatment was estimated by weighing 10 randomly selected fish from each treatment and replicates on a biweekly basis, and the following growth and condition indices parameters were estimated:
Absolute growth (ΔG, g) = (W2 – W1), g.
Absolute growth rate (AGR, g/day)= (W2−W1)/t Relative growth (RG %) = (W2 – W1/ W1) × 100
Specific growth rate weight (%∕day) = (ln mean final weight − ln mean initial weight)/ no. of culture days ×100,
Where W1 is the initial mean weight (g), W2 is the final mean weight ( g), and t is the experimental duration.
Survival (%) = (Number of harvested fish/ number of stocked fish) × 100,
FCR = Total Feed fed (g)/Total wet weight gain (g).
Protein efficiency ratio PER = (Body weight gain g)/protein intake g)
Condition factor (CF) = [Body weight, g)/ Body length, cm3)] ×100 [20]
Hepatosomatic index (HIS) = (Liver weight, g)/(Whole body weight, g) ×100
Viscerosomatic index (VSI) = (Viscera weight, g)/ (Whole body weight, g) ×100
Statistical Analysis
Results were presented as mean ± SD. A one-way ANOVA was used to analyze the data, and the means werecompared using Duncan’s multiple range test. All the analyses were performed using SPSS 21.
Results
Water Quality Parameters
The average temperature recorded in this experiment did not differ significantly among all treatments (Table 1). A significant lower (p < 0.05) dissolved oxygen (DO) level was observed in FRB (5.95 ± 0.40 mg/L) compared to RBB (6.55 ± 0.50 mg/L) and HFRB (6.15 ± 0.10 mg/L). The highest DO and COD levels were recorded in the RBB treatment (Table 1). The average values for biological oxygen demand (BOD), total dissolved solids (TDS), and pH were highest in RBB treatment, while TDS and BOD had the highest average values in FRB treatment. HFRB treatment recorded the highest average value for nitrite. A significantly lower value of pH (6.75) was recorded in HFRB treatment (p < 0.05).
Table 1: Physicochemical parameters of water in a catfish biofloc system where rice bran, fermented rice bran, and hydrolyzed fermented rice bran (HFRB) were used as carbon sources.
| Carbon sources | ||||
|
Parameters |
Rice bran (RBB) | Fermented rice bran (FRB) | Hydrolyzed fermented rice bran (HFRB) |
Significant level |
| Temp °C |
27.80 ± 0.30a |
27.50 ± 0.50a | 27.60 ± 0.40a |
P > 0.05 |
| pH |
7.70 ± 0.10a |
7.20 ± 0.35b | 6.75 ± 0.50c |
P < 0.05 |
| TDS (mg/l) |
195.00 ± 10.00a |
243.00 ± 15.50b | 205.00 ± 18.50c |
P < 0.05 |
| COD (mg/l) |
102.50 ± 5.50a |
115.20 ± 8.70b | 98.00 ± 5.50b |
P < 0.05 |
| BOD (mg/l) |
55.50 ± 6.50a |
60.45 ± 10.50a | 41.50 ± 8.00b |
P < 0.05 |
| DO (mg/l) |
6.55 ± 0.5a |
5.95 ± 0.4ab | 6.15 ± 0.10b |
P < 0.05 |
| TAN (mg/l) |
3.85 ± 0.55a |
2.52 ± .0.45b | 2.60 ± 0.50b |
P < 0.05 |
| Nitrite (mg/l) |
0.35± 0.10a |
0.30± 0.10b | 0.33 ± 0.10c |
P < 0.05 |
Means with a different superscript in the same row are significantly different (P < 0.05).
Floc Production
The biofloc volume was significantly higher in HFRB starting from week 2 of the experiment compared to all other treatments (Figure 1). The floc volume reached maximum in week 7 with values of 135.1 ml/l, 166.6 ml/l, and 111.0 ml/l for HFRB, FRB, and RBB treatments, respectively.
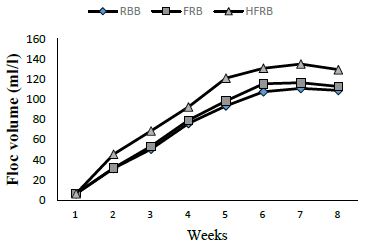
Figure 1: Floc production in the catfish biofloc system where rice bran, fermented rice bran, and hydrolyzed fermented rice bran were used as carbon sources.
Growth Parameters and Feed Efficiency
The growth rates, survival, and feed utilization of catfish in the biofloc system for the 8 weeks of rearing (Table 2) showed that treatment of rice bran through solid phase fermentation and acid hydrolysis enhanced its utilization as carbon sources in biofloc as the final weight and specific growth rates were higher compared with untreated rice bran. The highest final weight (85.5 g) was recorded in HFRB treatment, while the lowest value of weight gain (80.26) was in RBB. The feed conversion ratio was also highest in HFRB treatment. The survival was however lowest in HFRB, and this was significant (P < 0.05). The growth of catfish in biofloc utilizing hydrolyzed fermented rice bran as a carbon source in this experiment was better than that in untreated bran, as the growth rates were higher. The best FCR obtained for catfish (1.52 ± 0.05) was recorded in HFRB treatment, and this was significantly higher (P < 0.05). The percentage survival was significantly lower in HFRB treatment (P < 0.05).
Table 2: Growth parameters of catfish reared in a biofloc system where rice bran, fermented rice bran, and hydrolyzed fermented rice bran were used as carbon sources.
|
Parameters |
Rice bran (RBB) | Fermented rice bran (FRB) |
Hydrolyzed rice bran (HFRB) |
| Initial weight (g) |
11.15 ± 1.60a |
11.15 ± 1.60a |
11.55 ± 1.60a |
| Final weight (g) |
80.26 ± 3.20a |
81.70 ± 2.50b |
85.50 ± 2.55b |
| Absolute growth (g) |
70.15 ± 2.80a |
70.65 ± 1.80c |
74.53 ± 1.50b |
| Absolute growth rates(g/day) |
0.992 ± 0.03a |
1.000 ± 0.03c |
1.031 ± 0.02b |
| Relative growth (%) |
8.643 ± 0.21a |
8.695 ± 0.11a |
8.964 ± 0.10b |
| Specific growth rate(%/day) |
1.211 ± 0.16a |
1.23 ± 0.14a |
1.28 ± 0.13b |
| Survival% |
92 ± 1.55a |
95 ± 1.50a |
86 ± 1.52b |
| Feed conversion ratio |
1.65 ± 0.04a |
1.68 ± 0.03a |
1.52 ± 0.05b |
| Protein efficiency ratio |
0.403 ± 0.03a |
0.405 ± 0.04a |
0.421 ± 0.05b |
Means (n=10) followed by different letters in each rows are significantly different (P < 0.05).
Condition Indices
Condition factor (Fulton factor) calculated showed that catfish in the FRB treatment had higher values (0.54) followed by those in RBB (0.47) and HFRB (0.44) treatment fishes (Figure 2). Similar trends were observed with hepatosomatic index and viscerosomatic index in this experiment. The HIS showed a significantly higher value of 5.9% in the FRB treatment (P < 0.05).
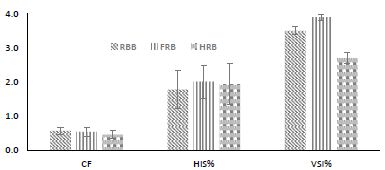
Figure 2: HIS (hepatosomatic index), CF (condition factor), VSI (viscerosomatic index) African catfish, C. gariepinus fingerlings reared in a biofloc system where rice bran, fermented rice bran, and hydrolyzed fermented rice bran were used as carbon sources.
Discussion
This experiment demonstrated that differential treatment of rice bran influenced its performance as a carbon source in biofloc production of catfish. Even though fermented bran has been well researched in this system, our findings suggest that acid-hydrolyzed fermented rice bran also has potential for consideration as well. Acid hydrolysis of rice bran resulted in the conversion of its starch and cellulose components into reducing sugar [11]. This may improve its performance in carbon release in the biofloc system. Slow carbon release by rice bran has been attributed to its low performance as a biofloc carbon source [5,21]. Rice bran is a cheap carbon source; efforts towards boosting its carbon release have been the focus of research. All the water quality parameters recorded in the system were within the range recommended for aquaculture and catfish production. Temperature is an important ecological factor with a profound effect on fitness, growth, and metabolism performance in aquatic organisms [22-24]. Variations in the temperature in our research were not significant, indicating no external influence on the treatment used. DO is an important abiotic factor influencing the growth and survival of fish. A significant reduction in the level of dissolved oxygen (DO) observed in FRB compared to the rest of the treatment could be due to higher microorganisms in this system, as reported in previous findings [25-27]. The pH range of 7-8.5 was said to be suitable for biofloc system performance, while recommends an average pH of 6.7, as biofloc systems tend to lose their buffering capacity at lower pH. The stability of the bioflocs was found to be dictated by the pH. All the treatments used in the current research maintained the pH of catfish biofloc within the recommended range [28-31], even though the pH level in the HFRB treatment was significantly lower (P < 0.05). Recovery of the acid after the hydrolysis process is a major bottleneck, and this informed our modified method for reducing acid utilization in the hydrolysis process. Future research towards optimizing acid hydrolysis of bran for biofloc carbon usage may be important. The survival rates of RBB and FRB treatments were significantly higher (P < 0.05) than HFRB treatments. This may be connected to lower pH in HFRB treatment. In this study, a higher concentration of total ammonium nitrate was recorded in HFRB treatment even though not significant (P > 0.05). This is in line with the findings of [27,32], who reported the use of complex carbon such as bran to decrease ammonia concentration in biofloc when compared to other carbon sources with simple sugar. The growth parameters of the catfish in the biofloc system after 8 weeks of rearing in this experiment showed that treatment of rice bran through solid phase fermentation and fermentation after acid hydrolysis enhanced its utilization as carbon sources in biofloc as the final weight and specific growth rates were higher compared with untreated rice bran. Organosomatic indices of catfish in this research showed a direct link between the effects of change in carbon source and environment on the fish; the response in such indices in response to nutrition has been well reported [33,34]. The use of acid-hydrolyzed fermented bran in this research proved efficacious in the system, as the growth parameters of this fish therefrom were well above the untreated rice bran treatment. The floc level was also higher in the hydrolyzed fermented bran treatment in this research. The observed drawback in this use of hydrolyzed bran is the low pH produced in the biofloc system. This might account for the reduced survival rates of the fish in this system. In conclusion, the results of this study suggest that hydrolyzed fermented rice bran can be used in catfish biofloc systems as a carbon source without negative consequences on water quality, growth, and feed utilization. However, adjusting the pH may be required for better performance and is recommended. Further research is needed to investigate the optimal hydrolization condition of bran for their usage as carbon sources in biofloc systems.
Acknowledgements
We would like to thank, Umaru Musa Yar’Adua University for their technical assistance. This work was supported by the Tertiary Education Trust Fund, Nigeria.
References
- Okomoda VT, Koh ICC, Shahreza SM (2018) A simple technique for accurate estimation of fertilization rate with specific application to Clarias gariepinus (Burchell 1822). Aquaculture Research 49(2).
- Tesfahun A (2018) Feeding biology of the African catfish Clarias gariepinus (Burchell) in some of Ethiopian lakes: a International Journal of Fauna and Biological Studies 5(1) 19-23.
- Babatunde TA, Ibrahim K, Abdulkarim B, Wagini NH (2019) Co-production and biomass yield of amaranthus (Amaranthus hybridus) and tilapia (Oreochromis niloticus) in gravel-based substrate filter aquaponics. International Journal of Recycling of Organic Waste in Agriculture V(8): 1-7.
- Chauhan RS, Mishra A (2022) New innovative technologies for sustainable aqua In Biodiversity 97-111 CRC Press.
- Dauda AB, Romano N, Ebrahimi M, Karim M, Natrah I, et al. (2017) Different carbon sources affects biofloc volume water quality and the survival and physiology of African catfish Clarias gariepinus fingerlings reared in an intensive biofloc technology system. Fisheries Science 83(6).
- Minabi K, Sourinejad I, Alizadeh M, Ghatrami ER, Khanjani MH (2020) Effects of different carbon to nitrogen ratios in the biofloc system on water quality growth and body composition of common carp Cyprinus carpio L fingerlings. Aquaculture International 28(5).
- Hargreaves JA (2013) Biofloc production systems for aquaculture 4503 1-11 Stoneville MS USA: Southern Regional Aquaculture Center.
- Bakhshi F, Najdegerami EH, Manaffar R, Tukmechi A, Farah KR (2018) Use of different carbon sources for the biofloc system during the grow-out culture of common carp Cyprinus carpio L fingerlings. Aquaculture 484: 259-267.
- Sasikumar R, Saranya S, Lincy LL, Sathyan A, Chellapandi P (2024) Fermented rice extract as a carbon source for biomass production of aquaculture Biomass Conversion and Biorefinery 1-9.
- Arreola LR, Quiroz-Guzmán E, Caballero JL, García EIP, Murueta JHC, et (2023) Shrimp Hepatopancreatic Crude Enzymes as Aids in Rice Bran Hydrolysis: Potential Contributors to Sustainable Aquaculture Turkish Journal of Fisheries and Aquatic Sciences 23(9).
- Sutanto S, Go AW, Chen KH, Nguyen PLT, Ismadji S, et (2017) Release of sugar by acid hydrolysis from rice bran for single cell oil production and subsequent in-situ transesterification for biodiesel preparation. Fuel Processing Technology 16: 7281-291.
- Christ-Ribeiro A, Chiattoni LM, Mafaldo CRF, Badiale-Furlong E, de Souza-Soares LA (2021) Fermented rice-bran by Saccharomyces cerevisiae: Nutritious ingredient in the formulation of gluten-free cookies. Food Bioscience V(40).
- Chinma C, Ilowefah M, Muhammad K (2013) Optimization of Rice Bran Fermentation Conditions Enhanced by Baker’s Yeast for Extraction of Protein Nigerian Food Journal 321: 126-132.
- APHA (1998) Standard Methods for the Examination of Water and Wastewater (20th Ed) American Public Health Association American Water Works Association Water Environmental Federation Washington DC.
- APHA (2012) Standard methods for the examination of water and waste water 19th edition American Public Health Association Inc New York 1193.
- APHA (1985) Standard methods for the examination of water and wastewater, 15th American Public Health Association (APHA), New York.
- Choi HJ (2020) Agricultural biowaste rice bran as carbon source to enhance biomass and lipid production: analysis with various growth rate models. Water Science and Technology 82(6).
- Avnimelech Y (1999) Carbon/nitrogen ratio as a control element in aquaculture Aquaculture 176: 227–235.
- Saeedi KH, Chapara M (2024) Isolation, Identification, and Biofloc Production: Potential of Floc-Forming Bacteria Using a Novel Monoculture Approach and Aquaculture Studies 24(4).
- Fulton T (1902) Rate of Growth of Sea Fish 20th Annual Report of the Fishery Board for Scotland London.
- Mahadik PU, Wasave SS, Chavan BR, Meshram SJ, Ghode GS, et (2024) Effect of fermented rice bran as a carbon source for rearing genetically improved farmed Tilapia Oreochromis niloticus Linnaeus 1758 fry in biofloc system. Aquaculture V(592).
- Pekkoh J, Chaichana C, Thurakit T, Phinyo K, Lomakool S, et al. (2022) Dual- bioaugmentation strategy to enhance the formation of algal-bacteria symbiosis biofloc in aquaculture wastewater supplemented with agricultural wastes as an alternative nutrient sources and biomass support materials. Bioresource Technology V (359).
- Abakari G, Luo G, Kombat EO, Alhassan EH (2021) Supplemental carbon sources applied in biofloc technology aquaculture systems: Types effects and future Reviews in Aquaculture 13: 1193-1222.
- Green BS, Fisher R (2004) Temperature influences swimming speed growth and larval duration in coral reef fish Journal of Experimental Marine Biology and Ecology 299(1).
- Taylor JC, Miller JM (2001) Physiological performance of juvenile southern flounder Paralichthys lethostigma Jordan and Gilbert 1884 in chronic and episodic hypoxia. Journal of Experimental Marine Biology and Ecology 25(82).
- Adineh H, Naderi M, Jafaryan H, Khademi Hamidi M, Yousefi M, et (2022) Effect of stocking density and dietary protein level in biofloc system on the growth digestive and antioxidant enzyme activities health and resistance to acute crowding stress in juvenile common carp (Cyprinus carpio). Aquaculture Nutrition 1-12.
- Ajamhasani E, Akrami R, Najdegerami EH, Chitsaz H, Shamloofar M (2023) Different carbon sources and probiotics in biofloc based common carp (Cyprinus carpio) culture: Effects on water quality growth performance fish welfare and liver Journal of the World Aquaculture Society 54(6).
- Pérez-Rostro CI, Pérez-Fuentes JA, Hernández-Vergara MP (2014) Biofloc a technical alternative for culturing Malaysian prawn Macrobrachium rosenbergii. Sustainable Aquaculture Techniques 87-104.
- Azim ME, Little DC (2008) The biofloc technology (BFT) in indoor tanks: water quality biofloc composition and growth and welfare of Nile tilapia (Oreochromis niloticus). Aquaculture 283: 29-35.
- De Schryver P, Crab R, Defoirdt T, Boon N, Verstraete W (2008) The basics of bio- flocs technology: the added value for aquaculture. Aquaculture 277(3-4).
- Yustiati A, Ayuningtyas AP, Andriani Y (2023) The Effectiveness of the Red Water System RWS Technique in African Catfish Culture Clarias gariepinus: A review. Asian Journal of Fisheries and Aquatic Research 23(3).
- Abiri SA, Chitsaz H, Najdegerami EH, Akrami R, Jalali AS (2022) Influence of wheat and rice bran fermentation on water quality growth performance and health status of Common carp (Cyprinus carpio L) juveniles in a biofloc-based Aquaculture V(555).
- Akani NP, Daka ER (2015) Evaluation of weight changes condition factor and organosomatic indices of Clarias gariepinus exposed to sub-lethal concentrations of an oilfield wastewater. Current Studies in Comparative Education Science and Technology 2(2).
- Eroldoğan OT, Kumlu METİN, Aktaş M (2004) Optimum feeding rates for European sea bass Dicentrarchus labrax L reared in seawater and freshwater. Aquaculture 231(1-4).