Abstract
Purpose: We aimed to assess the fracture rate in patients who were placed on a drug holiday (DH) after minimum adequate therapy versus those who continued therapy (CT) in a real-life setting.
Methods: This is a retrospective cohort study conducted in a tertiary academic center. Inclusion criteria involved osteoporotic adults who received minimum adequate bisphosphonate therapy (≥ 3 years), otherwise, patients were excluded.
Results: Of 1,814 charts randomly selected and reviewed, 272 patients met the inclusion criteria. In our cohort, females were 90.9%, White 50.0%, and African American 40.5%. A DH was initiated in 119 patients (43.8%). In the CT versus DH cohorts, the mean duration of therapy was 6.0 ± 2.6 versus 5.7 ± 2.3 years, total duration of follow-up 6.9 ± 2.9 versus 7.8 ± 2.7 years, and fractures occurred in 11.7% versus 9.2% respectively, not statistically different. The mean duration of follow-up after starting DH was 2.5 ± 1.9 years. Upon risk stratification using FRAX scoring, in the high-risk cohort, fragility fractures occurred in 16.5% (n=22/133) of the CT group versus 13.5% (n=7/52) of the DH cohort (P=0.66). In the lower risk cohort based on FRAX scoring, fragility fractures occurred in 7.1% (n=10/131) of the CT group versus 6.0% (n=4/63) of the DH cohort (P=1.0).
Conclusion: In our cohort, continued drug therapy did not provide additional fracture protective benefits beyond the minimum adequate duration of therapy. A drug holiday after three to five years of treatment may be considered after review of risk factors for future fracture.
Keywords
Osteoporosis, Fracture, Fragility fracture, Drug holiday, Continuous therapy
Introduction
Osteoporosis (OP) is a silent disease that may initially present as a fragility fracture with subsequent high morbidity, mortality, and healthcare financial burden [1]. Screening patients using fracture risk assessment modalities is suggested for case detection and institution of appropriate preventative therapeutics to prevent fragility fractures [2]. Fracture risk assessment modalities include DXA scanning (Dual-Energy X-ray Absorptiometry), online risk assessment tools such as the FRAX algorithm, and determining the presence of prevalent or incident fragility fractures [3]. High-risk patients are candidates for treatment while intermediate-risk patients may be monitored more frequently versus initiating a moderate intensity therapy like zoledronate 5 mg every other year [4].
Several classes of effective OP medications are available that significantly decrease the risk of initial and subsequent fragility fractures [5]. The pharmacological therapies for osteoporosis at the time of this analysis were broadly classified as antiresorptive therapies (bisphosphonates, denosumab, hormonal therapy, selective estrogen receptor modulators [SERM]) and osteoanabolic therapies (teriparatide, abaloparatide). Bisphosphonates (BP) include alendronate, risedronate, ibandronate, and zoledronate (FDA approved 1995, 2000, 2005, and 2007, respectively).
The concept of a drug holiday (DH) was introduced in 2008 after several reports of rare severe side effects including osteonecrosis of the jaw and atypical femur fracture [6]. Accurate fracture risk assessment is critical for appropriate risk stratification in a variety of clinical settings inclusive of whether a patient should be initially started on medical therapy, when to consider a DH, and continued surveillance every 1-2 years while on a DH to determine when reinstitution of pharmacological therapy will be necessary [7]. It is important to note that a DH is presently considered for only bisphosphonate therapy, it should not apply to other classes of therapy due to the rapid loss of bone mineral density (BMD) and increased fracture risk associated with their withdrawal [8].
In this study, we aimed to retrospectively assess incidence rates of fractures between patients on continuous osteoporosis treatment versus patients placed on a DH after minimum adequate therapy in a tertiary academic center.
Methods
This is a retrospective cohort study. Data were collected by chart review of patients who were followed at a tertiary academic center from October 2007 to September 2016 for treatment of osteoporosis.
Definitions
- Osteoporotic fracture: a fracture caused by an injury that would be insufficient to fracture a normal bone as a result of reduced compressive and/or torsional strength of bone [9]. Typical fractures in patients with osteoporosis include vertebral (spine), proximal femur (hip), distal forearm (wrist), and proximal humerus [9,10]. Osteoporotic fractures may involve any bone except the hand (distal to carpal bones), foot (distal to ankle), face, and skull [11]. Osteoporotic fractures are also termed fragility fractures in this study.
- DXA scan: Dual-energy X-ray Absorptiometry
- Drug holiday: A period when treatment is stopped after a patient has been on continuous treatment. However, the term ‘holiday’ implies the temporary withdrawal of treatment that may be restarted in the future [12]
- FRAX score: An online validated tool for fracture risk assessment (https://www.shef.ac.uk/FRAX/tool.jsp), FRAX web version 4.0 was utilized in this study.
Risk Stratification
Risk Assessment was based on the recommendations of the National Osteoporosis Foundation (NOF) (USA) [13-16]:
- High risk:
- FRAX Score: Major Osteoporotic Fracture [MOF] risk ≥20% and/or Hip Fracture [HF] risk ≥3%
- DXA scan findings: T-score ≤ -2.5 SD at the lumbar spine, femur neck, or total hip
- Presence of a fragility fracture (sites as previously mentioned in the protocol)
- Only one positive high risk categorical finding is enough to be classified as “high-risk”.
- Intermediate risk:
- FRAX Score: MOF risk 10-19% and/or HF risk 1.5-2.9%
- Low risk:
- FRAX Score: MOF risk <10% and/or HF risk <1.5%
- Lower risk:
- Patients in the low or intermediate-risk categories to facilitate their combined risk as compared to high-risk patients.
Medications for the treatment of osteoporosis [16]:
Bisphosphonates: Alendronate, ibandronate, risedronate, and zoledronate.
Inclusion and Exclusion Criteria
Inclusion criteria consisted of adults aged ≥18-year-old with a history of receiving continuous bisphosphonate therapy (alendronate, ibandronate, risedronate, zoledronate) for treatment of osteoporosis or patients at high risk of fracture. Continuous therapy was defined as a minimum of three years of continuous bisphosphonates therapy.
Exclusion criteria included adult patients who received a shorter duration of bisphosphonates therapy for treatment of osteoporosis or receiving other osteoporosis treatment medications solely; receiving treatment to manage hypercalcemia or osteolytic lesions related to malignancy or other medical conditions. Institutional Review Board (IRB) approval was obtained.
Aim, Data, and Analysis
We aimed to assess fracture incidence rates between patients on continuous treatment (CT) for osteoporosis versus patients placed on a drug holiday (DH) after minimum adequate therapy.
Descriptive analysis was performed to assess baseline patient demographics, clinical characteristics, duration of therapy, and fracture rates. Categorical variables were presented as frequencies, proportions, and percentages. Continuous variables were presented as means ± standard deviations. The χ2 test was used for the analysis of categorical variables and the t-test was used for continuous variables. Fracture-free survival analysis was performed using the Kaplan-Meier method, comparison and assessment of statistical difference were performed using Mantel-Cox analysis. SPSS (Statistical Package for the Social Sciences) ≥23 was used for all the statistical analyses.
To compare data between different proportions or means, a fixed-effects statistical model for meta-analysis was used [17]. The means, SD, and proportions were weighted based on the sample sizes of the different cohorts in each study. The confidence intervals for the variables were calculated for the p values of 0.05, 0.01, and 0.001. These were compared with corresponding means and proportions in the other cohort to determine the statistical significance [17].
Results
A total of 12,885 patients were identified based on the presence of at least one prescription for an osteoporosis medication in the electronic medical records from 2007 to 2016. The research group reviewed 1,814 randomly selected charts and 272 patients met the inclusion criteria as shown in Figure 1. The mean age of the cohort ( ± standard deviation) was 68.8 ± 10.7 years, females accounted for 90.9%. Most of the patients were Caucasian (50.0%) and African American (40.5%). A Drug holiday was initiated in 119 (43.8) patients. Table 1 summarizes the baseline clinical characteristics of the cohort and the medical specialty of the treating providers. Table 1 discloses the prevalence of comorbidities and risk factors for osteoporosis and fragility fractures in our cohort.
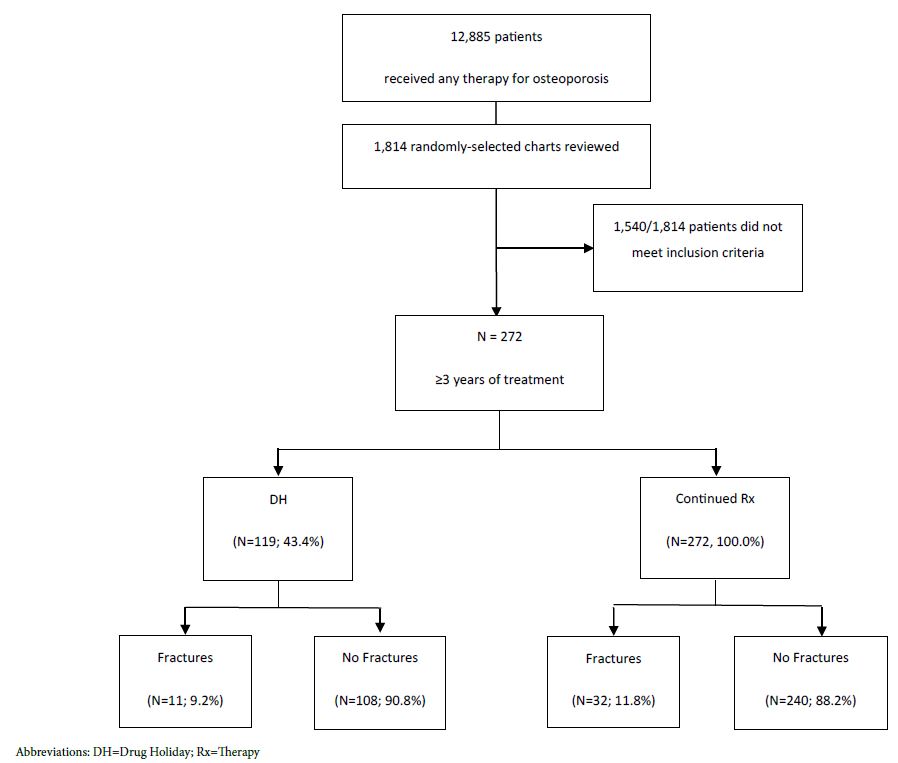
Figure 1: Consort Table
Table 1: Clinical characteristics, and comorbidities and risk factors for osteoporosis and fractures
| Age (years, mean ± SD) |
68.8 ± 10.7 |
| Female gender [n (%)] |
248 (91.2%) |
| Race [n (%)] | |
| Caucasian |
135 (49.6%) |
| African American |
111 (40.8%) |
| Hispanic |
22 (8.1%) |
| Asian |
3 (1.1%) |
| Unknown |
1 (0.4%) |
| Treating Provider | |
| PCP (IM) |
132 (48.5%) |
| Rheumatologist |
62 (22.6%) |
| PCP (FM) |
34 (12.5%) |
| Endocrinology |
19 (6.9%) |
| PCP (Geriatrics) |
12 (4.4%) |
| Others |
8 (2.9%) |
| Oncology |
5 (1.8%) |
| Comorbidities and risk factors for osteoporosis and fractures | |
| Falls |
146 (53.7%) |
| Smoking |
90 (33.1%) |
| Glucocorticoids |
59 (21.7%) |
| Prednisone (or equivalent) ≥7.5mg |
32/59 (54.2%) |
| Diabetes mellitus |
53 (19.5%) |
| Rheumatoid arthritis |
28 (10.3%) |
| Parent fractured hip |
10 (3.7%) |
| Premature menopause |
8 (2.9%) |
| Liver disease |
7 (2.6%) |
| Hyperthyroidism |
6 (2.2%) |
| Hypogonadism |
4 (1.5%) |
| Alcohol abuse |
1 (0.4%) |
| Osteogenesis imperfect |
0 (0%) |
Abbreviations: SD=Standard Deviation; DH=Drug Holiday; PCP=Primary Care Physician; IM=Internal Medicine; FM=Family Medicine.
The entire cohort received continued therapy beyond the minimum of three years and therefore, all the patients (n=272) were included in the analysis for the continued therapy (CT) group while they were receiving uninterrupted treatment. The mean duration of therapy in the CT group was 6.0 ± 2.6 years. A total of 119 patients were placed on a DH after a mean duration of prior bisphosphonate therapy of 5.7 ± 2.3 years. Any fragility fractures that occurred while receiving therapy and before initiating their DH were analyzed as being in the CT group. Fragility fractures that were present before starting anti-osteoporosis therapy were documented in 82 (29.9%) patients but were not included as occurring during therapy in the CT group. In the CT group, fragility fractures occurring during the initial three years of therapy were noted in 30/272 (11.0%) patients, as observed in Table 2 (fragility fractures within the first 3 years of therapy). These fractures were included in the calculation of each patient’s FRAX fracture risk assessment at the time of institution of their DH but were not considered a failure of therapy since these patients had not completed the predefined minimum adequate therapy of three years. A total of 159 patients received 5 or more years of continuous therapy.
Table 2: Continued therapy and drug holiday
|
Continued Therapy |
Drug Holiday |
P-Value |
|
| Number of patients |
272 |
119 |
|
| Duration of therapy (y; mean ± SD) |
6.0 ± 2.6 |
5.7 ± 2.3 |
P>0.05 |
| Follow-up duration (y; mean ± SD) |
6.9 ± 2.9 |
7.8 ± 2.7 |
P=0.05 |
| Fragility fractures within the first 3 years of therapy (%; n) | |||
| Total |
11.0% (30/272) |
13.4% (16/119) |
P>0.05 |
| During 1st year of Rx |
3.3% (9/272) |
3.4% (4/119) |
P>0.05 |
| During 2nd year of Rx |
2.2% (6/272) |
4.2% (5/119) |
P>0.05 |
| During 3rd year of Rx |
5.5% (15/272) |
5.9% (7/119) |
P>0.05 |
| Fragility fractures beyond the first 3 years of therapy (%; n) | |||
| Total |
11.7% (32) |
9.2% (11) |
P>0.05 |
| 3-4.9y of therapy |
6.3% (17/272) |
14.0% (8/57) |
P>0.05 |
| ≥5y of therapy |
9.4% (15/159) |
4.8% (3/62) |
P>0.05 |
Fragility Fractures in the Continued Therapy versus Drug Holiday Cohorts
In the CT versus DH cohorts, mean duration of therapy was 6.0 ± 2.6 versus 5.7 ± 2.3 years (p>0.05) and total duration of follow-up was 6.9 ± 2.9 in CT group versus 7.8 ± 2.7 years in DH group (P=0.05). The mean duration of follow-up after starting DH was 2.5 ± 1.9 years. The mean duration of follow-up until the occurrence of first fracture (after a minimum of three years of therapy) or last follow-up if there were no fractures was 2.6 ± 2.3 years for the CT group and 2.3 ± 1.8 years for the DH group. As observed in Table 2, the total number of fragility fractures during the entire study period were 32/272 (11.8%) of the CT group versus 11/119 (9.2%) of the DH cohort (P=0.60). A total of 272 patients continued to receive therapy beyond three years and 159 patients received therapy for ≥5 years. Fragility fractures occurred in 17/272 (6.3%) patients on CT for 3-4.9 years and in 15/159 (9.4%) patients on CT for 5 or more years (p>0.05). Fragility fractures after initiation of a DH occurred in 8/57 (14.0%) patients who completed 3-4.9 years of prior bisphosphonate therapy and 3/62 (4.8%) patients who received ≥5 prior treatment, P>0.05. The mean duration for the occurrence of the first fragility fracture was 2.3 ± 2.7 years in the CT cohort versus 1.5 ± 1.2 years in the DH cohort (P<0.01). The fracture-free survival analysis for the whole cohort using Kaplan Meier analysis revealed no significant difference in fracture rates between the CT and DH groups (P = 0.74) as shown in Figure 2.
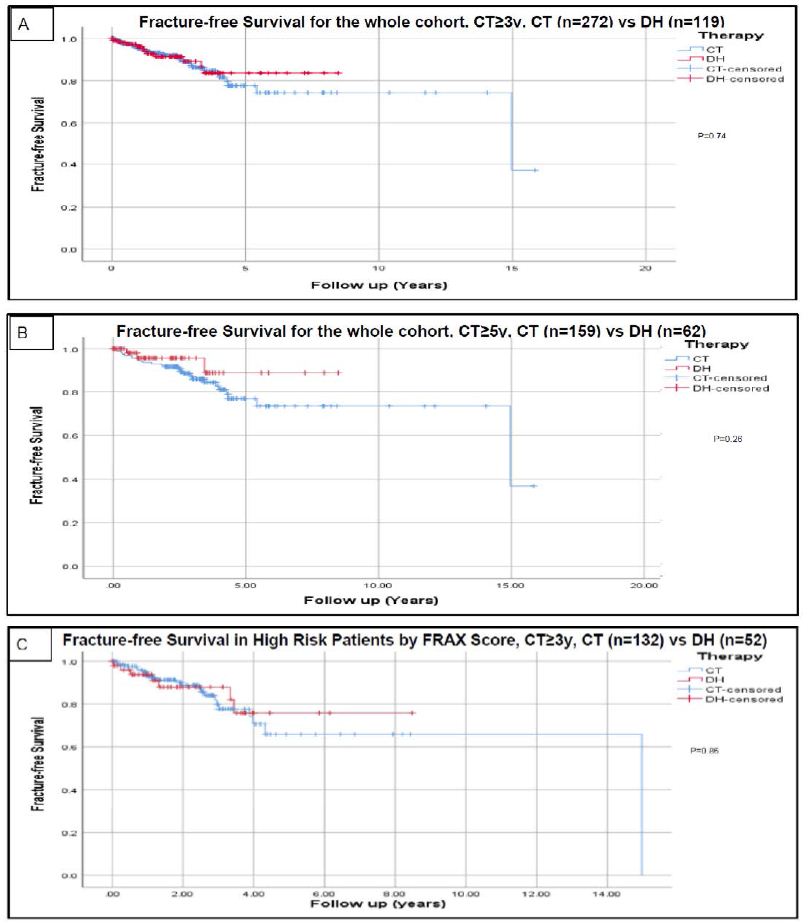
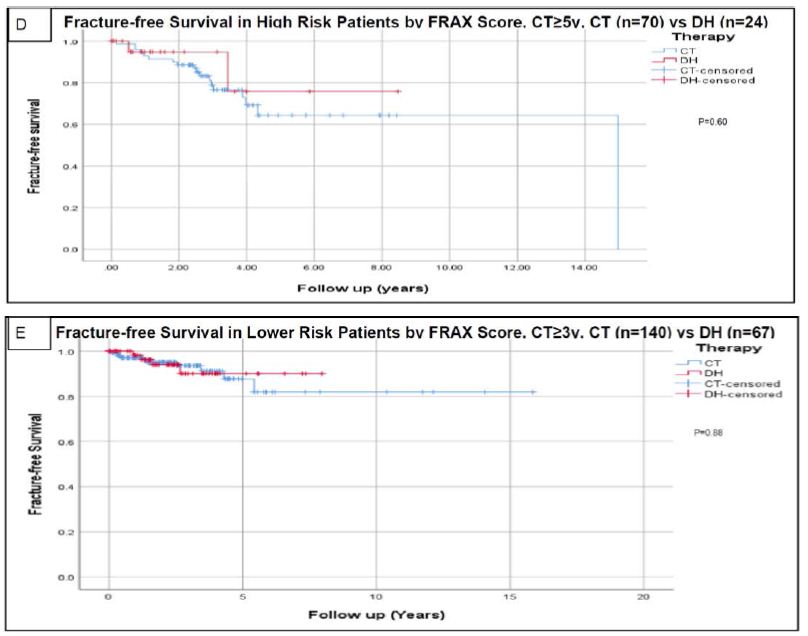
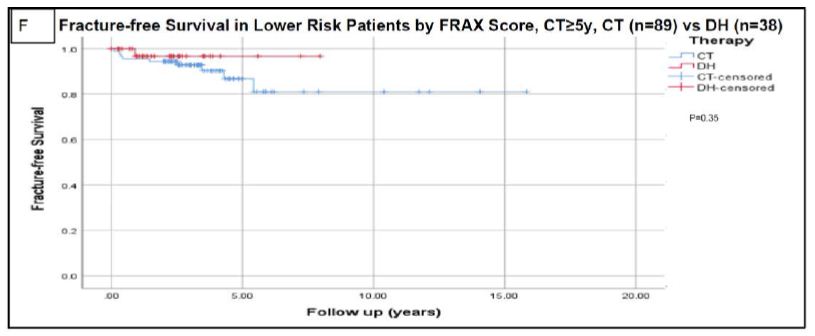
Figure 2: Fracture-free survival using Kaplan-Meier analysis.
Analysis for the whole cohort using continued therapy after a minimum of 3 years is presented in figure A. Analysis for the whole cohort using continued therapy after a minimum of 5 years is presented in figure B. Analysis based on risk assessment using FRAX scoring are presented in figures C (high risk with continued therapy ≥3 y), D (high risk with continued therapy ≥5 y), E (lower risk with continued therapy ≥3 y), and F (lower risk with continued therapy ≥5 y). High risk patients based on FRAX score were defined as having major osteoporotic fracture risk ≥20% and/or hip fracture risk ≥3. Censored data refers to incomplete data for patients like those who lost follow up or deceased before experiencing the primary outcome (fractures) and who could have otherwise experienced it if continued followed up [2]. Abbreviations: CT=Continued Therapy; DH=Drug Holiday; FRAX=An Online Fracture Risk Assessment Tool.
Fragility Fractures Using Different Risk Assessment Tools
Fragility fractures in the high risk versus lower-risk patients in the CT cohort based on FRAX high risk (HR) were 16.5% versus 7.1% (P=0.01). In the combined FRAX HR plus DXA HR groups, fragility fractures occurred in 13.2% of the high-risk group versus 9.0% in the lower-risk groups (P=0.20). Based on fragility fractures that occurred during the first three years of therapy, 0.0% in the higher risk group versus 13.2% in the lower risk group (P=0.02) respectively. Fragility fractures in the high-risk versus lower-risk patients in the DH cohort based on FRAX HR were 13.5% versus 6.0% (P=0.14). In the combined FRAX HR plus DXA HR group, fragility fractures occurred in 11.3% versus 6.3% in the lower risk group (P=0.28). Based on the presence of fragility fractures during the first three years of therapy, 14.3% were in the high risk versus 8.2% in the lower risk group (P=0.30).
Fragility Fractures in FRAX High Risk versus Lower Risk Patients and Drug Holiday
In the FRAX high-risk group of 133 patients, 87/133 (65.4%) continued therapy whereas 46/133 (34.6%) were placed on a drug holiday. For the high-risk cohort, the mean duration of follow-up until the time of the first fracture or last follow-up if no fractures occurred ( ± standard deviation) was 2.4 ± 2.0 years for the CT group (after 3 years of minimum therapy), and 2.1 ± 1.8 years for the DH group. Among the high-risk patients at initial FRAX risk stratification, fragility fractures occurred in 22/133 (16.5%) of the CT group versus 7/52 (13.5%) of the DH cohort (P=0.66). The mean duration for the occurrence of the first fragility fracture was 2.5 ± 3.1 years in the CT cohort after minimum adequate therapy versus 1.4 ± 1.4 years in the DH cohort.
In the 141 lower-risk patients, 68 (48.2%) continued therapy and 73 (51.8%) were placed on a DH. For the lower risk cohort, the mean duration of follow-up until the time of first fracture or last follow-up if no fractures occurred ( ± standard deviation) was 2.8 ± 2.6 years for the CT group (after 3 years of minimum therapy), and 2.4 ± 1.8 years for the DH group. Among the lower risk patients at initial FRAX risk stratification, fragility fractures occurred in 10/141 (7.1%) of the CT group versus 4/67 (6.0%) of the DH cohort (P=1.0). The mean duration for the occurrence of the first fragility fracture was 2.0 ± 1.9 years in the CT cohort after 3 years of therapy versus 1.6 ± 0.8 years in the DH cohort. The fracture-free survival analysis for the high-risk and low-risk cohorts using Kaplan Meier analysis revealed no significant difference in the fracture rates between the CT and DH groups (P = 0.87 and 0.88 respectively) as shown in Figure 2.
Five Years of Therapy
The rate of fractures was also assessed for patients who continued therapy for a minimum of five years. In FRAX high-risk patients on CT for 3-4.9 years versus ≥5 years of minimum therapy, fractures occurred in 11/63 (17.5%) versus 11/70 (15.7%) patients, respectively, P=0.88. In FRAX lower-risk patients on CT for 3-4.9 years versus ≥5 years of minimum therapy, fractures occurred in 6/52 (11.5%) versus 4/89 (4.5%) patients, respectively, P=0.34. Among the entire cohort, 159/272 patients continued therapy ≥5 years (mean 7.1 ± 2.5y) while the mean duration of therapy for the DH cohort (n=119) was 5.7 ± 2.3 years. Fracture rates were comparable between both groups as shown in Figure 2, P=0.61.
Discussion
The duration of osteoporosis therapy and when institution of a drug holiday should be considered is an under-researched area. There are differences in guidance regarding a DH among the osteoporosis-related societies [7,18-23]. At present, there are few prospective clinical trials or retrospective studies available to assess the risk of fracture while on continuous osteoporosis pharmacological therapy versus a drug holiday. The present consensus states that high-risk patients should continue therapy for no less than 5 years. Our goal was to assess the pattern of osteoporosis pharmacological treatment and fracture rates in a real-life setting in patients on continued therapy (CT) and a drug holiday (DH). Patients in the CT and DH subgroups were further stratified by FRAX scoring into high risk versus lower risk categories.
The first clinical trial to prospectively assess the concept of DH was the FLEX trial comparing continuing alendronate for a total of 10 years versus a DH after 5 years of therapy [24]. DH did not increase the risk of non-vertebral fractures or x-ray-detected vertebral fractures over the 5 years of follow-up, but the risk of clinically diagnosed vertebral fractures was significantly lower among CT 2.4% (16/662) versus DH cohort 5.3% (n=23/437); relative risk 0.45; 95% confidence interval 0.24–0.85). However, post hoc analysis of this data disclosed increased risk of fractures in the DH group was associated with lower baseline BMD and increased number of fractures prior to starting therapy. Significant limitations of the FLEX trial included the lack of assessing shorter duration of therapy (namely three years of treatment) and inability to utilize fracture risk assessment tools such as FRAX scoring (2008) [24]. The second clinical trial to prospectively assess CT versus DH was the Zoledronate HORIZON-Pivotal Fracture Trial, 3 years of therapy (Z3) versus placebo (P3) [25]. Two subsequent extension trials assessed CT versus DH, 6 years of CT (Z6) versus 3 years of therapy followed by a DH (Z3P3), followed by 9 years of CT (Z9) versus 6 years of therapy followed by a DH (Z6P3) [26,27]. In the first extension trial (Z6 versus Z3P3), there was no significant difference in non-vertebral or hip fractures, although patients who continued therapy had a lower rate of new vertebral fractures: 3.0% versus 6.2% (Odds ratio 0.51, 95% confidence interval [0.26, 0.95], P 0.035), and >60% of the patients in each cohort were at high risk of fractures. In the second extension trial (Z9 versus Z6P3), there was no significant difference in fracture rates between CT versus DH. Limitations of the HORIZON extension trials included the lack of risk stratification at either baseline or at the time of starting a drug holiday using a well-validated fracture risk assessment tool. In the study assessing Risedronate in osteoporosis, there was no DH comparator group but rather a comparison of 7 years of CT versus 5 years of placebo followed by 2 years of therapy [28]. DH after the use of denosumab was found to be associated with a rebound rapid increase in bone remodeling rates and a high risk of vertebral fragility fractures [29]. DH after teriparatide therapy is associated with loss of accrued bone mass and loss of the fracture protective effect of the drug and as such the general recommendation has been to follow osteoanabolic therapy with an antiresorptive therapy [19]. It should be noted that all the recommendations regarding drug holidays are primarily based on the alendronate and zoledronate clinical trials with the noted limitations of the trials and reliance on expert opinion.
Based on the previously mentioned two prospective placebo-controlled trials and their extensions, the general recommendation has been to consider a DH after 5 years of oral bisphosphonate therapy (FLEX Trial for alendronate) and 3 years for intravenous zoledronate. In our study, the statistical analysis was performed after a minimum of 3 years of therapy that is comparable to all bisphosphonate registration trials with assessment of fracture rates in both CT and DH groups. We performed our preliminary analysis after collecting data for 272 patients with the aim of re-estimating the power analysis and the number of patients to be reviewed afterward. Unexpectedly, the rate of fractures was not statistically different in CT as compared to DH. The absolute rate of fractures was numerically higher in the CT group, and therefore the study was terminated at that point.
In our study, the rate of fractures was assessed for the entire cohort and FRAX high and lower-risk patients. Comparison of the rate of fractures between CT and DH cohorts in each of these groups was performed at treatment thresholds of ≥3 and ≥5 years. The cut-off of 3 years was suggested to assess the efficacy after the use of 3 years of oral bisphosphonate therapy. The mean duration of therapy in patients who were placed on a drug holiday was 5.7 ± 2.3 years, and as most of the initial therapy was oral bisphosphonates, followed the general recommended guidelines of 5 years of oral therapy. Therefore, analysis of CT ≥5 years was necessary as well to avoid the bias of under-treatment using the cut-off of 3 years and having a falsely higher number of fractures in the CT cohort. Among the 6 comparison studies as shown in Figure 2, there was no significant difference between the patients on CT versus DH, independent of the risk status (high or lower FRAX risk) or duration of therapy (≥3 or ≥5 years).
The position statements of the American Society of Bone and Mineral Research, International Osteoporosis Foundation (IOF), AACE/ACE, FDA commentary, and the Endocrine Society guidelines agree that initial therapy with oral bisphosphonates of 5 years or 3 years of intravenous zoledronate should be considered standard of care [7,18-23]. In high-risk patients, these guidelines suggest the continuation of therapy with some advocating at least 10 years of oral therapy and 6 years of intravenous zoledronate. In low to intermediate-risk patients, it was suggested that clinicians consider a DH with frequent risk assessment every 2-4 years [7,18]. Although it is a reasonable approach to consider continuation of therapy and avoidance of fragility fractures in high fracture risk patients started on a premature DH after less than 5 years of therapy, there is little objective evidence to confirm this position. In our study, the rate of fractures was comparable between the high-risk patients who continued therapy as compared to those who were placed on a DH.
Our study is the first retrospective cohort study to perform an in-depth fracture risk assessment based on calculating FRAX scores and assessing fracture rates in different risk strata. The pre-treatment fracture rate in our cohort was 29.9% consistent with the inclusion of a significant number of high-risk patients comparable to several prospective studies and as such avoiding under-powering of the study. The mean duration of therapy, as well as post-drug holiday follow-up, is reasonable considering the introduction of electronic health records in 2007. There are some limitations of this study including the retrospective nature of the study, small number of patients, and relatively short duration of follow-up. Patients who had their bone density scans or received treatment at outside facilities were not available in the EMR via notes/charts, per verbal discussion with the provider, or through use of cross-EMR observations (Care Everywhere®) reference. Most of our patients received alendronate without sufficient information available in the EMR regarding medication compliance. Bone remodeling markers were rarely checked. Lastly, no significant episodes of ONJ or AFF were observed although outside medical records were not always available, and assessment of these rare complications was limited.
Conclusion
In our cohort study, continued drug therapy beyond 3 years did not provide additional protective benefit as compared to a drug holiday in high-risk patients. Future studies with larger cohorts and a longer duration of follow-up are needed to validate these findings. Although not uniformly performed in all patients of our cohort, annual reassessment of the response to pharmacological therapy and fracture risk assessment should be performed. The present common use of the term “Drug Holiday” in osteoporosis management should be replaced and endorsed by all societies as “Bisphosphonate Drug Holiday”.
References
- Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17: 1726-1733. [crossref]
- Goel MK, Khanna P, Kishore J (2010) Understanding survival analysis: Kaplan-Meier estimate. Int J Ayurveda Res 1: 274-278. [crossref]
- Baim S (2011) Assessment of fracture risk. Rheumatic diseases clinics of North America 37: 453-470.
- Reid IR, Horne AM, Mihov B, Stewart A, Garratt E, et al. (2018) Fracture Prevention with Zoledronate in Older Women with Osteopenia. N Engl J Med 379: 2407-2416. [crossref]
- Tu KN, Lie JD, Wan CKV, Cameron M, Austel AG, et al. (2018) Osteoporosis: A Review of Treatment Options. PT 43: 92-104. [crossref]
- Sebba A (2008) Osteoporosis: how long should we treat? Curr Opin Endocrinol Diabetes Obes 15: 502-507. [crossref]
- Adler RA, El‐Hajj Fuleihan G, Bauer DC, Camacho PM, Clarke BL, et al. (2016) Managing osteoporosis in patients on long‐term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. Journal of Bone and Mineral Research 31: 16-35. [crossref]
- Xu X-L, Gou W-L, Wang A-Y, Wang Y, Guo Q-Y, et al. (2013) Basic research and clinical applications of bisphosphonates in bone disease: what have we learned over the last 40 years? Journal of translational medicine 11: 303. [crossref]
- Bonjour JP, Ammann P, Rizzoli R (1999) Importance of preclinical studies in the development of drugs for treatment of osteoporosis: a review related to the 1998 WHO guidelines. Osteoporos Int 9: 379-393. [crossref]
- Brown JP, Josse RG, Scientific Advisory Council of the Osteoporosis Society of C (2002) 2002 Clinical practice guidelines for the diagnosis and management of osteoporosis in Canada. CMAJ 167: S1-34. [crossref]
- Seeley DG, Browner WS, Nevitt MC, Genant HK, Scott JC, et al. (1991) Which fractures are associated with low appendicular bone mass in elderly women? The Study of Osteoporotic Fractures Research Group. Ann Intern Med 115: 837-842. [crossref]
- Diab DL, Watts NB (2013) Bisphosphonate drug holiday: who, when and how long. In: SAGE Publications Sage UK: London, England. [crossref]
- Siris ES, Adler R, Bilezikian J, Bolognese M, Dawson-Hughes B, et al. (2014) The clinical diagnosis of osteoporosis: a position statement from the National Bone Health Alliance Working Group. Osteoporos Int 25: 1439-1443. [crossref]
- Watts NB, Lewiecki EM, Miller PD, Baim S (2008) National Osteoporosis Foundation 2008 Clinician’s Guide to Prevention and Treatment of Osteoporosis and the World Health Organization Fracture Risk Assessment Tool (FRAX): what they mean to the bone densitometrist and bone technologist. J Clin Densitom 11: 473-477. [crossref]
- Organization WH (2004) WHO scientific group on the assessment of osteoporosis at primary health care level. In: Summary meeting report 5-7.
- Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, et al. (2014) Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int 25: 2359-2381. [crossref]
- Brownlee KA (1965) Statistical theory and methodology in science and engineering. Wiley New York.
- Camacho PM, Petak SM, Binkley N, Clarke BL, Harris ST, et al. (2016) American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis. Endocrine Practice 22: 1-42. [crossref]
- Kaufman JM, Orwoll E, Goemaere S, San Martin J, Hossain A, et al. (2005) Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int 16: 510-516. [crossref]
- Silverman S, Adachi J, Dennison E (2016) Bisphosphonate drug holidays: we reap what we sow. In: Springer 27: 849-852. [crossref]
- Whitaker M, Guo J, Kehoe T, Benson G (2012) Bisphosphonates for osteoporosis-where do we go from here? New England Journal of Medicine 366: 2048-2051. [crossref]
- Eastell R, Rosen CJ, Black DM, Cheung AM, Murad M, et al. (2019) Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism 104: 1595-1622. [crossref]
- Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, et al. (2020) American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis. Endocrine Practice 26: 1-46. [crossref]
- Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, et al. (2006) Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. Jama 296: 2927-2938. [crossref]
- Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, et al. (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356: 1809-1822. [crossref]
- Black DM, Reid IR, Boonen S, Bucci-Rechtweg C, Cauley JA, et al. (2012) The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res 27: 243-254. [crossref]
- Black DM, Reid IR, Cauley JA, Cosman F, Leung PC, et al. (2015) The effect of 6 versus 9 years of zoledronic acid treatment in osteoporosis: a randomized second extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res 30: 934-944. [crossref]
- Mellstrom DD, Sorensen OH, Goemaere S, Roux C, Johnson TD, et al. (2004) Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif Tissue Int 75: 462-468. [crossref]
- Cummings SR, Ferrari S, Eastell R, Gilchrist N, Jensen JB, et al. (2018) Vertebral Fractures After Discontinuation of Denosumab: A Post Hoc Analysis of the Randomized Placebo-Controlled FREEDOM Trial and Its Extension. J Bone Miner Res 33: 190-198. [crossref]