Abstract
Background: Molecular tools for detection of low-density asymptomatic Plasmodium infections are needed in malaria elimination efforts. This study reports results from the hitherto largest implementation of loop-mediated isothermal amplification (LAMP) for centralized mass screening of asymptomatic malaria in Zanzibar.
Methods: Healthy individuals present and willing to participate in randomly selected households in 60 villages throughout Zanzibar were screened for malaria by rapid diagnostic tests (RDT). In 50 % of the study households, participants were asked to provide 60 μL of finger-prick blood for additional LAMP screening. LAMP was conducted in two centralized laboratories in Zanzibar, by trained technicians with limited or no previous experience of molecular methods. The LAMP assay was performed with LoopampTM MALARIA Pan/Pf Detection Kit (Eiken Chemical Company, Japan). Samples positive for Plasmodium genus (Pan)-LAMP were re-tested using Plasmodium falciparum-specific LAMP kits.
Results: Paired RDT and LAMP samples were available from 3983 individuals. The prevalence of asymptomatic malaria was 0.5 % (CI 95 % 0.1-0.8) and 1.6 % (CI 95 % 1.1-2.2) by RDT and Pan-LAMP, respectively. LAMP detected 3.4 (CI 95 % 2.2-5.2) times more Plasmodium positive samples than RDT. DNA contamination was experienced, but solved by repetitive decontamination of all equipment and reagents.
Conclusions: LAMP is a simple and sensitive molecular tool, and has potential in active surveillance and mass-screening programmes for detection of low-density asymptomatic malaria in pre-elimination settings. However, in order to deploy LAMP more effectively in field settings, protocols may need to be adapted for processing larger numbers of samples. A higher throughput, affordable closed system would be ideal to avoid contamination.
Keywords
Plasmodium, Malaria, Low-density, Asymptomatic, Loop-mediated isothermal amplification, Mass screening, DNA contamination
Background
Asymptomatic Plasmodium infections are an important reservoir for continued malaria transmission that needs to be addressed in the context of malaria elimination [1]. Detection of asymptomatic infections, which are often sub-patent, i.e., fall beneath the threshold of detection of both microscopy and rapid diagnostic tests (RDT), requires highly sensitive molecular tools. The use of polymerase chain reaction (PCR)-based assays in field settings is, however, limited due to the need for a cold chain, specialized equipment and know-how [2]. Loopmediated
isothermal amplification (LAMP) offers several advantages over PCR in field settings. LAMP requires minimal equipment, has short time-to-result (30 min-1 h), with an analytical sensitivity similar to PCR, and results that can be read by eye using UV fluorescence [3–5].
The Loopamp™ MALARIA Pan/Pf Detection Kit (Eiken Chemical Company, Japan) has been developed as a
field-friendly kit, comprising strips of reaction tubes containing vacuum-dried and temperature-stable reaction
components for either Plasmodium genus (Pan)-specific or Plasmodium falciparum-specific malaria detection. The
kit has been evaluated both in laboratory and field settings [6–8], and was piloted on a small scale in Zanzibar as a health facility-based, point-of-care, diagnostic tool for mass screening and treatment in 2013 [9].
This study reports results from the hitherto largest implementation of LAMP in the field, for scaled-up,
centralized mass screening of asymptomatic malaria in Zanzibar, a pre-elimination setting.
Methods
Study sites and study design
Zanzibar, located 35 km off the coast of mainland Tanzania, consists of two main islands, Unguja and Pemba, with respective populations of approximately 900,000 and 400,000. This study was performed as part of a larger knowledge, attitude, practice, and behavior (KAPB) malaria survey, conducted in Zanzibar April-May 2014. Household visits were carried out in 60 villages in ten districts (six in Unguja and four in Pemba) covering
the whole of Zanzibar. A proportional number of households were sampled from each village to reach a sample
size of 2162 households, powered for the KAPB study. Healthy individuals present and willing to participate in
the randomly selected households were screened for malaria by RDT. In 50 % of study households (in even
house numbers), participants were asked to provide 60 μL of finger-prick blood for additional LAMP screening.
Nexus seven tablet computers were used to conduct questionnaires as part of the KAPB survey. All participants or
guardians provided written informed consent prior to blood sampling. Ethical approvals were obtained from the ethical committees in Zanzibar (ZAMREC/0002/FEBRUARY/014) and the Regional Ethics Committee in Stockholm (2009/387-31).
Training of field enumerators and sample collection
Household visits were conducted by 40 field enumerators in teams of two, together with four field supervisors with prior experience of similar studies. All enumerators attended five days of training for RDT performance, blood sample collection for LAMP, and use of tablet computers. There were 14 teams in Unguja and six teams in Pemba, and each team visited six or seven households per day. RDT screening was conducted with either SD-Bioline Malaria Ag P.f/PanW (Standard Diagnostic, Inc, USA) (used for >90 % of screening) or First ResponseW Malaria Ag Combo (pLDH/HRP2) (Premier Medical Corporation Limited India). Results were recorded on the tablet computer during household visits, and RDT positive indi-viduals were referred to the closest health facility for treat-ment and registration in the Zanzibar malaria surveillance system. In 50 % of study households, 60 μL of finger-prick blood was collected using a plastic capillary tube (Dropstir, Medical Precision Plastics, USA), dispensed into a 1.5-ml pre-labelled sample collection tube containing 60 μL of pre-aliquoted DNA extraction buffer (400 nM NaCl, 40 mM Tris pH 6.5, 0.45 SDS), and mixed by flicking. Blood samples were collected in microtube storage racks with lids and transported at the end of each day to two centralized laboratories, one on each island, where they were stored at 4 °C overnight.
Training of laboratory technicians
Four technicians, two for each laboratory, with limited or no experience of LAMP were trained over three-and-a-half days. Training included a theoretical introduction to LAMP and the LAMP protocol, hands-on practical sessions with malaria positive blood samples diluted to different known concentrations, how to record results on tablet computers, and a half-day field trial with sam-ples collected the same day by the field enumerators.
Screening by LAMP in centralized laboratories
LAMP procedures were similar to the pilot study [9], with some modifications for scale-up of sample sizes. One cen-trifuge, three heat-blocks (1.5-ml block at 95 °C, 0.2-ml block at 65 °C and a 0.2-ml block at 95 °C) and a UV lamp were required in each laboratory. All samples collected in Pemba and half of the samples collected in Unguja (see below) were processed within 24 h of sampling. To reduce the risk of mix-up of samples and contamination, sets of pre-labelled sample collection tubes (containing 60 μL of aliquoted DNA extraction buffer) and pre-labelled DNA dilution tubes (containing 300 μL of aliquoted sterile water) were prepared prior to the start of the study. DNA extraction and the LAMP assays were performed in separ-ate areas to avoid contamination. DNA was extracted by the boil and spin method [10] and 26 μL of the super-natant was transferred to the DNA dilution tubes. The LAMP assay was performed with Loopamp™ MALARIA Pan/Pf Detection Kit (Eiken Chemical Company) as per protocol [10]. Samples positive for Pan-LAMP were retested using P. falciparum-LAMP specific kits. LAMP positive individuals (who were not positive by RDT) were visited by malaria surveillance officers and provided treat-ment within 48 h of sampling where possible.
Freezing of samples
Due to a delay in the shipment of LAMP kits, half of the LAMP samples collected in Unguja (N = 1414) were stored at −20 °C after DNA extraction and dilution, until the remaining reaction tubes arrived five weeks later. Dilution tubes from LAMP-positive samples in Unguja were also stored at −20 °C, for quality control of frozen DNA.
Statistical
Results are reported from individuals for which both RDT and LAMP were conducted (i.e., where paired data are available). Statistical analyses were conducted using Stata/SE 12.1 (StataCorp LP, Texas, USA). The survey de-sign was taken into consideration when calculating 95 % confidence intervals (CI 95 %) for prevalence estimations, using the survey [svy] command in Stata accounting for household and village sampling/stratification. The sensi-tivity and specificity of RDT was calculated using LAMP as the gold standard. McNemar’s test was used to compare the methods. Statistical significance was determined as p < 0.05.
Results
Study population
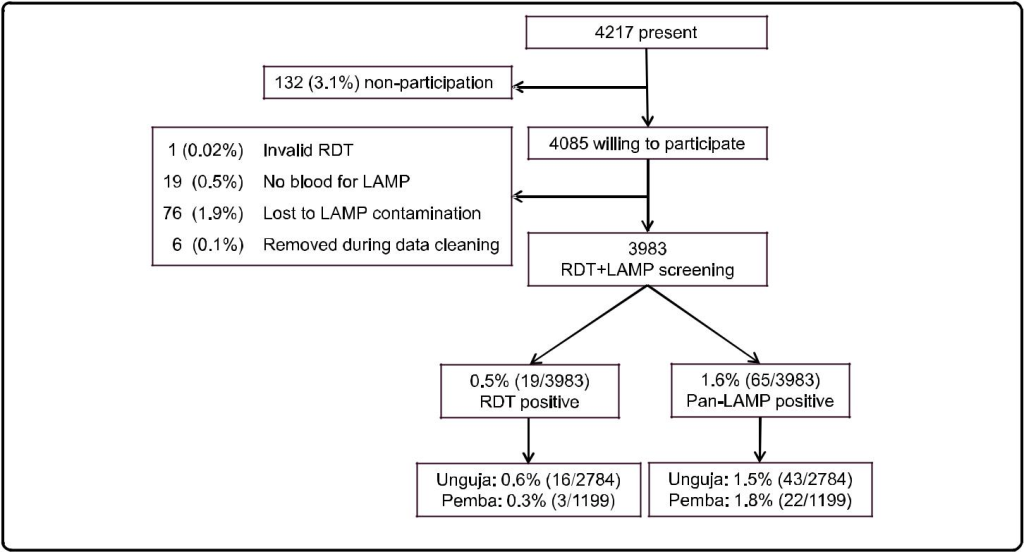
Participation was high; informed consent was given by 96.9 % of those present at the time of the survey (Fig. 1). Both RDT and LAMP results were available for 3983/4085 (97.5 %) of the individuals willing to participate. The remaining 102 (2.5 %) were excluded from further ana-lysis. The study population consisted of all ages (median: 18 years, range 0–98), with a higher proportion of females (59.0 %). Sample collection was conducted during a total of 19 days with an average of 220 samples processed per day in the two laboratories combined.
Prevalence of malaria by RDT and LAMP
The prevalence of asymptomatic malaria was 0.5 % (CI 95 % 0.1-0.8) and 1.6 % (CI 95 % 1.1-2.2) by RDT and Pan-LAMP, respectively (Table 1). Pan-LAMP detected 3.4 (CI 95 % 2.2-5.2) times more Plasmodium positive samples than RDT. Out of the Pan-LAMP positive sam-ples 64.6 % (42/65) were also positive by P. falciparum-LAMP. RDT had a sensitivity of 24.6 % (14.7-36.9) and specificity of 99.9 % (99.7-100.0) when compared to Pan-LAMP. Comparison by McNemar’s test showed a signifi-cant difference between the two methods (p <0.001).
Table 1. Prevalence of malaria detected by RDT and LAMP
| RDT | LAMP | |
| Overall prevalence (%; CI 95 %a) | 0.5; 0.1-0.8 19/3983 |
1.6; 1.1-2.2 65/3983 |
| Relative proportion positive in: | ||
| Only Panb (%; CI 95 %) | 5.3; 0.0-16.4 1/19 |
35.4; 23.4-47.4 23/65 |
| Pan + P. falciparumc (%; CI 95 %) | 31.6; 8.5-54.6 6/19 |
64.6; 52.6-76.6 42/65 |
| Only P. falciparumd (%; CI 95 %) | 63.2; 39.2-87.1 12/19 |
NDe |
Both RDT brands used for malaria screening are two-band RDTs detecting P. falciparum HRP2 and Pan-Plasmodium LDH simultaneously, although with different detection limits (50–100 parasites/μL for P. falciparum HRP2 and 200–500 parasites/μL for Pan-Plasmodium LDH). In contrast, only the Pan-LAMP positive samples were assessed for P. falciparum during the LAMP screening, with a detection limit of 2–5 parasites/μL for both Pan-Plasmodium
and P. falciparum
aConfidence intervals for prevalences were calculated using the survey [svy] command in Stata, accounting for household and village sampling/stratification
bPositive for Plasmodium genus only
cPositive for Plasmodium and P. falciparum
dPositive for P. falciparum only
eND = not determined
Discrepancies in LAMP after freezing of samples
DNA extracted from half of the samples (N = 1414) in Unguja was stored at −20 °C prior to LAMP testing due to a delay in the shipment of LAMP kits. Among these samples, 32 (2.3 %) were positive by Pan-LAMP, out of which 12 was also positive by RDT. However, amongst the frozen samples there were also three RDT positive samples that were found negative by Pan-LAMP. These three samples were positive for P. falciparum HRP2 only, Pan-Plasmodium LDH only, and both P. falcip-arum HRP2 and Pan-Plasmodium LDH, respectively. Among the samples from Unguja that were screened be-fore freezing (N = 1370), 11 (0.8 %) were positive by Pan-LAMP, out of which one was also positive by RDT. The 11 Pan-LAMP positive samples were stored at −20 °C, as a quality control of freezing DNA, however only 7/11 (63.6 %) were positive when re-tested after thawing.
LAMP-amplified DNA contamination
During the study DNA contamination of LAMP arose in the central laboratory in Pemba [see Additional file 1 for flow chart of events]. The contamination resulted from using a heat block with a heated pressurized lid during the 95 °C enzyme inactivation stage, and not allowing the samples to cool to room temperature before removing the strips for recording of results. ‘Fizzing’ was observed around the lid of the LAMP strips resulting in leakage of LAMP-amplified DNA. All equipment and reagents were subjected to repetitive decontamination with 5 % sodium hypochlorite over three days, and moved away from the epicentre of the contamination to a laboratory space avail-able in another building. The final enzyme inactivation step of the protocol [10] was removed as this was thought to be the source of contamination; instead results were read and recorded immediately after the amplification re-action. A negative control was included in each strip of eight reaction tubes, and any Pan-LAMP positive samples were repeated and only recorded as positive if positive in both runs. During the first few days following the contam-ination there were some samples that were considered false positive, but the numbers declined and reached zero within one week after the contamination.
Discussion
This is the hitherto largest reported implementation of LAMP for detection of asymptomatic malaria in a field setting. In order to scale-up the breadth of sampling, LAMP testing was centralized in two laboratories, meaning samples could be collected from all over the islands with fewer resources. The time-to-result was ap-proximately 24 h, compared with three hours in the pilot study where LAMP was used as a health facility-based, point-of-care, diagnostic tool for mass screening and treatment [9].
The results confirm the improved sensitivity of LAMP over RDT, as has been shown previously [3, 9]. The MALARIA Pan/Pf Detection Kit has a detection limit of 2–5 parasites/μL [3, 6], for both Pan-Plasmodium and P. falciparum. This is comparable to PCR, and substantially lower than the detection limits of P. falciparum-specific HRP2 (50–100 parasites/μL) and Pan-Plasmodium LDH (200–500 parasites/μL) in combo RDTs. The proportion of samples detected only by Pan-LAMP (35.4 %) sug-gests the presence of species other than P. falciparum. Similarly, other studies in Zanzibar have shown that up to 40 % of PCR-detectable malaria infections contained non-falciparum species [11, 12]. Non-falciparum infec-tions tend to be of lower parasite densities than P. falcip-arum infections [13], emphasizing the need for more sensitive species-specific methods for non-falciparum Plasmodium detection. The sensitivity (83.8 %) and speci-ficity (99.7 %) of Pan-LAMP, calculated using PCR as the reference standard, was high in the pilot study conducted in Zanzibar [9]. This is similar to previously reported sen-sitivities and specificities [6–8, 14, 15] and, together with the results of this study, suggests malaria LAMP is a use-ful molecular tool sensitive enough for detection of low-density asymptomatic malaria infections in field settings.
Importantly, some discrepancies were shown amongst samples screened following freezing of diluted DNA. RDT false positivity due to recently cleared infections has been well documented when detecting P. falciparum HRP2 [16], although none of the three study participants who were RDT positive/LAMP negative reported receiv-ing malaria treatment within the previous two weeks, and two of the RDTs were positive for Pan-Plasmodium LDH suggesting ongoing infections. The lack of repro-ducibility of results following freezing of samples sug-gests that DNA extracted by simple methods such as boil and spin may not be suitable for long-term storage and should be amplified by LAMP within a short period of time [17]. Alternatively, low reproducibility of PCR for detection of low-density infections has been reported [18] and parasite densities close to the LAMP detection limit could also explain the lack of reproducibility.
The potential risk of contamination with LAMP is large, due to the high efficiency of the reaction, although the risk is reduced when using a closed system [3, 19]. The MAL-ARIA Pan/Pf Detection Kit is manufactured with tubes that cannot be re-opened once closed, in order to avoid contamination with amplified DNA. However, as demon-strated in this study, the exposure of such tubes to high temperatures, as during enzyme inactivation, results in softening of the plastic and leakage of the contents. While removing the inactivation step solved this problem in this case, contaminations have been experienced in other research settings [8, 20] and these issues are important to report. Although MALARIA Pan/Pf Detection Kit is a field-friendly option, three days’ training is not sufficient for dealing with such events. Successful decontamination requires a larger understanding of molecular techniques and rigorous repetitive methods to ensure that the area is free of contamination.
Standard malaria diagnostic tools including microscopy and RDT are not sensitive enough to detect low-density asymptomatic infections [12]. Nucleic acid amplification-based methods provide the, to date, most sensitive and ac-curate tools to detect and identify pathogens [21]. Recently published, highly sensitive quantitative PCR methods state detection limits as low as 0.02 and 0.03 parasites/μL blood [22, 23]. However, these methods lack the field applicability that LAMP offers. Furthermore, the cost of LAMP is estimated to be a tenth of that of conven-tional PCR [15], although the cost of the field friendly kit is still at 5.3 US$ per reaction i.e., considerably more expensive than RDTs [3].
The high cost and risk of contamination may yet limit the implementation LAMP at a point-of-care level, but LAMP will be valuable for research purposes and for evaluating malaria elimination efforts. LAMP may, for ex-ample, be useful in mass/focal screening and treatment (MSAT/FSAT) programmes, for which the deployment of RDTs, perhaps due to their low sensitivity, has had varying results [11, 24]. In any case it will be important to evaluate the impact and cost effectiveness of deploying LAMP, in comparison to the deployment of standard diagnostic tools as well as in comparison to alternative molecular methods.
Conclusions
LAMP is a simple and sensitive molecular tool, and has potential in active surveillance and mass-screening pro-grammes for detection of low-density asymptomatic mal-aria in pre-elimination settings. However, in order to deploy LAMP more effectively in field settings, protocols may need to be adapted for processing larger numbers of samples. A higher throughput, affordable closed system would be ideal to avoid contamination.
Competing interests
IG is an employee of the Foundation for Innovative New Diagnostics (FIND), a co-developer of the Loopamp™ MALARIA Pan/Pf Detection Kit. All other authors declare no competing interest.
Authors’ contributions
MIM, IJG, AM, ASA, AB, and JC conceived and designed the study. UM, MK, BAS, AKA, and JC carried out the training and were responsible for the fieldwork in Zanzibar. UM, BAS and MHN were responsible for the training and conducting of LAMP. UM and JC analysed the data, and drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgments
We would like to thank all participants, staff members, and ZAMEP employees involved in the KAPB survey for their dedicated participation. We would also like to acknowledge Colin Sutherland, Spencer Polly, Michelle Hsiang and Alanna Schwartz for their intellectual input regarding experience of dealing with DNA contamination. This work was supported by Global Fund [Grant number ZAN-809-G07–M, work plan GFRD 8 phase 2 2013]; President’s Malaria Initiative (PMI) [Implementation letter # 45, work plan for FY 2013]; the Swedish Medical Research Council (VR) [grant numbers 2009–3785 and 2013–6594]; the Foundation for Innovative New Diagnostics (FIND) with funds from the German Federal Ministry of Education and Research (BMBF) through the KfW Entwicklungsbank; and the Einhorn foundation. In memoriam of Ali K Abass, a much missed friend and colleague.
References
- Bousema T, Okell L, Felger I, Drakeley C. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol. 2014;12:833–40.
- Hänscheid T1, Grobusch MP (2002) How useful is PCR in the diagnosis of malaria? Trends Parasitol 18: 395-398. [crossref]
- Han ET1 (2013) Loop-mediated isothermal amplification test for the molecular diagnosis of malaria. Expert Rev Mol Diagn 13: 205-218. [crossref]
- Oriero EC, Jacobs J, Van Geertruyden JP, Nwakanma D, D’Alessandro U. Molecular-based isothermal tests for field diagnosis of malaria and their potential contribution to malaria elimination. J Antimicrob Chemother. 2014;70:2–13.
- Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:E63.
- Aydin-Schmidt B, Xu W, Gonzalez IJ, Polley SD, Bell D, Shakely D, et al. Loop mediated isothermal amplification (LAMP) accurately detects malaria dna from filter paper blood samples of low density parasitaemias. PLoS One. 2014;9:e103905.
- Hopkins H, Gonzalez IJ, Polley SD, Angutoko P, Ategeka J, Asiimwe C, et al. Highly sensitive detection of malaria parasitemia in a malaria-endemic setting: performance of a new loop-mediated isothermal amplification kit in a remote clinic in Uganda. J Infect Dis. 2013;208:645–52.
- Polley SD1, González IJ, Mohamed D, Daly R, Bowers K, et al. (2013) Clinical evaluation of a loop-mediated amplification kit for diagnosis of imported malaria. J Infect Dis 208: 637-644. [crossref]
- Cook J, Schmidt B, Gonzalez IJ, Bell D, Edlund E, Nassor MH, et al. Loop-mediated isothermal amplification (LAMP) for point-of-care detection of asymptomatic low-density malaria parasite carriers in Zanzibar. Malar J. 2015;14:43.
- FIND: Manual of standard operating procedures for malaria LAMP [http://www.finddiagnostics.org/export/sites/default/programs/malaria-afs/ docs/SOPs_LAMP_Malaria_AUG12.pdf]
- Cook J, Xu W, Msellem M, Vonk M, Bergstrom B, Gosling R, et al. Mass screening and treatment using a Plasmodium falciparum-specific rapid diagnostic test did not reduce malaria incidence in Zanzibar. J Infect Dis. 2014;211:1476–83.
- Morris U, Xu W, Msellem MI, Schwartz A, Abass A, Shakely D, et al. Characterising temporal trends in asymptomatic Plasmodium infections and transporter polymorphisms during transition from high to low transmission in Zanzibar, 2005–2013. Infect Genet Evol. 2015. doi:10.1016/j.meegid.2015.04.018.
- Cotter C, Sturrock HJ, Hsiang MS, Liu J, Phillips AA, Hwang J, et al. The changing epidemiology of malaria elimination: new strategies for new challenges. Lancet. 2013;382:900–11.
- Polley SD, Mori Y, Watson J, Perkins MD, Gonzalez IJ, Notomi T, et al. Mitochondrial DNA targets increase sensitivity of malaria detection using loop-mediated isothermal amplification. J Clin Microbiol. 2010;48:2866–71.
- Poon LL, Wong BW, Ma EH, Chan KH, Chow LM, Abeyewickreme W, et al. Sensitive and inexpensive molecular test for falciparum malaria: detecting Plasmodium falciparum DNA directly from heat-treated blood by loop-mediated isothermal amplification. Clin Chem. 2006;52:303–6.
- Mayxay M, Pukrittayakamee S, Chotivanich K, Looareesuwan S, White NJ. Persistence of Plasmodium falciparum HRP-2 in successfully treated acute falciparum malaria. Trans R Soc Trop Med Hyg. 2001;95:179–82.
- Sattabongkot J, Tsuboi T, Han ET, Bantuchai S, Buates S. Loop-mediated isothermal amplification assay for rapid diagnosis of malaria infections in an area of endemicity in Thailand. J Clin Microbiol. 2014;52:1471–7.
- Costa DC, Madureira AP, Amaral LC, Sanchez BA, Gomes LT, Fontes CJ, et al. Submicroscopic malaria parasite carriage: how reproducible are polymerase chain reaction-based methods? Mem Inst Oswaldo Cruz. 2014;109:21–8.
- Hsiang MS, Greenhouse B, Rosenthal PJ. Point of care testing for malaria using LAMP, loop mediated isothermal amplification. J Infect Dis. 2014;210:1167–9.
- Paris DH1, Imwong M, Faiz AM, Hasan M, Yunus EB, et al. (2007) Loop-mediated isothermal PCR (LAMP) for the diagnosis of falciparum malaria. Am J Trop Med Hyg 77: 972-976. [crossref]
- Abdul-Ghani R, Al-Mekhlafi AM, Karanis P. Loop-mediated isothermal amplification (LAMP) for malarial parasites of humans: would it come to clinical reality as a point-of-care test? Acta Trop. 2012;122:233–40.
- Hofmann N, Mwingira F, Shekalaghe S, Robinson LJ, Mueller I, Felger I. Ultra-sensitive detection of Plasmodium falciparum by amplification of multi-copy subtelomeric targets. PLoS Med. 2015;12:e1001788.
- Imwong M, Hanchana S, Malleret B, Renia L, Day NP, Dondorp A, et al. High throughput ultra-sensitive molecular techniques to quantify low density malaria parasitaemias. J Clin Microbiol. 2014;52:3303–9.
- Sutcliffe CG, Kobayashi T, Hamapumbu H, Shields T, Mharakurwa S, Thuma PE, et al. Reduced risk of malaria parasitemia following household screening and treatment: a cross-sectional and longitudinal cohort study. PLoS One. 2012;7:e31396.