Abstract
Purpose: Sedation is one of the essential interventions in ICU. The Richmond Agitation- Sedation Scale (RASS) has commonly been used in adult patients; however, no specific scale for pediatric patients is available. Thus, it is necessary to develop a simple sedation scale according to children’s developmental stages. The purpose of this study is to evaluate the efficacy of the RASS for sedation evaluation in pediatric patients.
Methods: The study included 1715 children admitted to a pediatric intensive care unit (PICU) between 2012 and 2016, where they received artificial respiration management under sedation. To assess the efficacy of the RASS, univariate and multivariate analyses were performed for determining the mean duration of stay in the PICU pre- and post-introduction of RASS, the number of days of artificial respiration management, and the number of adverse events. P-values <0.05 were considered statistically significant. All tests were performed using SPSS ver. 27.0J (IBM Corp., Armonk, NY).
Results: Analyses showed statistically significant differences in the number of ventilator-associated pneumonia (VAP) cases pre- and post-introduction of RASS (p=0.007 for univariate analysis; multivariate odds ratio=0.518, 95% confidence interval: 0.296–0.905, p=0.021). These results indicated that RASS introduction reduced the risk of VAP by one-half.
Conclusions: Appropriate use of sedatives contributes to improved patient outcomes, such as the prevention of VAP and reductions in the duration of artificial respiration management. Study results suggest that use of the RASS, an important measure in the VAP prevention bundle, can be effective in reducing the risk of VAP in pediatric patients.
Keywords
Richmond agitation sedation scale, Pediatric intensive care unit, Ventilator-associated pneumori, A retrospective cohort study
Introduction
Sedation is one of the essential interventions in ICU. To increase the patient’s comfort and reduce complications associated with sedation, it is essential to precisely set the target of sedation depth for each patient and appropriately maintain the particular depth [1-3]. For instance, excessively deep sedation would cause difficulty in ventilator weaning due to the atrophy and weakness of respiratory muscles, which may result in prolonging a ventilator fitting period or developing ventilator-related pneumonia (VAP) [4]. On the contrary, under a shallow sedation depth, a report shows that the case of accidental extubation in ventilators increases, followed by restlessness or agitation [5]. Therefore, establishing an objective “sedation scale,” a common standard among medical professionals in evaluating the sedation depth, should be mandatory. The use of the sedation scale is currently recommended in practice on patients under intensive care management, particularly during mechanical ventilation management [6]. Given this situation, the use of the Richmond Agitation Sedation Scale (hereinafter referred to as “RASS”) has been recently recommended, mainly for adult patients, as an appropriate sedation scale [7-9]. However, on the other hand, no recommendation has been made to use a specific sedation scale in the field of Pediatrics [10]. The cognitive and language abilities of pediatric patients are underdeveloped, in which the process of informed consent is often problematic. To make matters worse, those infants may suffer significant stress, not only from medical treatment or surroundings of a unique ICU environment but also from being separated from their family. Therefore, the management of pediatric patients in ICU is often challenging, which requires deeper sedation depth and a higher level of pain relief than those of adult patients [10]. However, the number of sedation scales assessed for their reliability and validity in pediatric patient management in mechanical ventilation is quite limited [11]; although the state behavioral scale (SBS) has been reported as a candidate for sedation scale of pediatric patients, no recommended sedation scale has been established in the treatment of pediatric patients as in its adult counterpart. Consequently, a simple and reliable scale in determining appropriate sedation depth according to each patient’s growth development stages is required. A pediatric hospital adopted the use of the Richmond Agitation-Sedation Scale (RASS) in 2014, and physicians and nurses have since determined optimal levels of sedation based on their patients’ scores. This study was based on children who underwent artificial respiration management in the pediatric intensive care unit (PICU), and evaluated the impact on clinical outcomes of using RASS to evaluate their sedation levels.
Design and Methods
Patients
This study included children admitted to the hospital’s PICU between 2012 and 2016. All children had received artificial respiration management under sedation during their PICU stay. We excluded patients who received muscle relaxants or who underwent artificial respiration management using high-frequency oscillatory ventilation or airway pressure release ventilation. The RASS was used to measure the level of sedation because its reliability and validity have been established in adults.
Data Analysis
Univariate analysis was performed for the mean duration of stay in the PICU before and after the introduction of RASS, the number of days of artificial respiration management, the number of pneumonia cases associated with artificial respiration machines, and the number of unplanned extubations. Subsequently, multivariate analysis was conducted to confirm the efficacy of the RASS for each outcome measure, with the potential confounders found to be statistically significant in the univariate models included as covariates. P-values less than 0.05 were considered statistically significant. All tests were performed using SPSS ver. 27.0J (IBM Corp., Armonk, NY).
Ethical Considerations
This study was carried out in accordance with the Ethical Guidelines for Medical and Health Research Involving Human Subjects established by the Ministry of Health, Labor, and Welfare and was approved by the hospital’s Institutional Ethics Committee (Approval Number: 998). This hospital has been designated as the national research center for advanced and specialized medical care, and promotes the treatment and research of diseases during the reproductive cycle.
Findings
Patient Characteristics and Clinical Outcomes
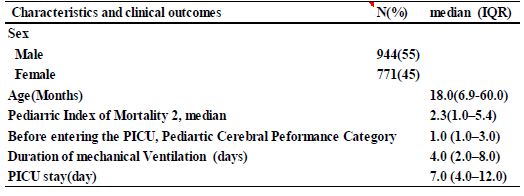
A total of 1715 patients were included in the study; their median age was 18 months (6–60 months). The median Proviral Integrations of Moloney virus 2 (PIM2) was 2.3 (1.0–5.4), the median Pediatric Cerebral Performance Category (PCPC) before admission to the PICU was 1.0 (1.0–3.0), the median number of days of artificial respiration management was 4.0 (2.0– 8.0), and the median PICU stay was 7.0 days (4.0–12.0) (Table 1).
Table 1: Patient characteristics and clinical outcomes(N=1715)
Midazolam and opioids were mainly used as analgesics and sedatives. Dexmedetomidine, ketamine, and phenobarbital were used as second-line drugs or adjuvants. The dose was adjusted as necessary according to the instructions of the on-site intensivist.
Verification of Effectiveness of Each Variable Pre-and Post-introduction of RASS
Study outcomes were compared for the periods pre- and post-introduction of the RASS in 2014. Changes in the number of days of artificial respiration management and in the duration of PICU stay were assessed using the Mann–Whitney U test for non-parametric variables. In addition, the chi-square test was used to compare changes in the number of cases of ventilator-associated pneumonia (VAP) and of unplanned extubations. There were no significant differences in the number of PICU days (p=0.296), artificial respiration management days (p=0.499), or number of unplanned extubations (p=0.456) pre- and post- introduction of RASS. In contrast, a statistically significant difference was observed in the number of VAPs (p=0.007) (Table 2).
Table 2: Verification of effectiveness of each variable pre-and post-introduction of RASS
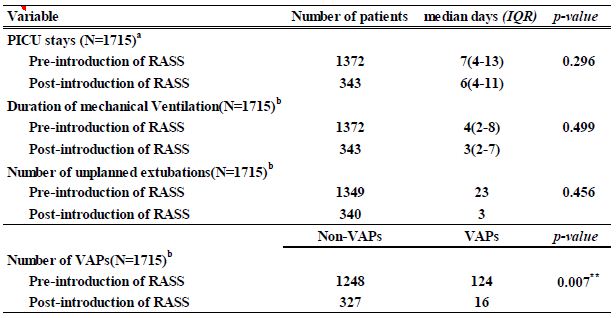
a Mann–Whitney U test
b the chi-square test
p < 0.05* p < 0.01**
Verification of Effectiveness for VAP Post-introduction of RASS
Multivariate analysis using logistic regression was performed for the number of VAPs, controlling for patient sex and age (months), PIM2, PCPC before admission to the PICU, the number of days of artificial respiration management and of ICU stay, and whether RASS was used as an assessment tool. A variable reduction technique, based on the likelihood ratio test, was used to select the covariates tested in the models. The results indicated that patient age, PIM2, PCPC, the number of days of artificial respiration management, and RASS introduction affected the number of VAPs. Specifically, the number of VAPs was significantly associated with patient age (odds ratio [OR]=1.010, 95% confidence interval [CI]: 1.000- 1.010, p=0.002), number of days of artificial respiration management (OR=1.05, 95% CI:– 1.040-1.060, p<0.001), PIM2 (OR=1.010, 95 % CI : 1.010-1.020, p<0.001), PCPC (OR=0.847, 95% CI: 0.717-1.000, p=0.04), and RASS introduction (OR=0.518, 95 %CI: 0.296-0.905, p=0.021). The Hosmer–Lemeshow test showed a goodness of fit for the logistic regression model (p=0.753). The discrimination rate between the predicted and actual values was 91.3% (Table 3).
Table 3: Verification of effectiveness for VAP post-introduction of RASS (N=1715)
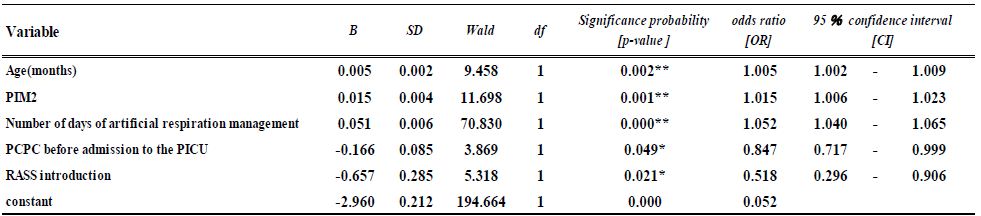
The Hosmer–Lemeshow test showed a goodness of fit for the logistic regression model (p=0.753) The discrimination rate between the predicted and actual values was 91.3%
p < 0.05* p < 0.01**
Discussion
Clinical sequelae of artificial respiratory management were compared for the periods before and after the RASS was introduced as an assessment tool in the PICU. After controlling for demographic and clinical factors, use of the RASS did not make a significant difference in the number of days patients spent in the PICU, the number of days of artificial respiration management, or the number of unplanned extubations. However, a statistically significant difference was observed in the number of VAPs, representing a 50% reduction in the risk of their occurrence. Analysis was performed to clarify the association between the number of VAPs and sex, age (months), PIM2, PCPC before admission to the PICU, the number of days of artificial respiration management and ICU stay, and the presence or absence of RASS. An appropriate use of sedatives contributes to improving outcomes in adults, such as prevention of VAP, reduction in the period of artificial respiration management, and improvement in survival [12-14]. The results of the present study demonstrated that using RASS to optimize sedation management significantly reduced the risk of VAP. Muscle weakness and functional impairments, such as cognitive/mental function disorder, are known complications after artificial respiratory management and have been labeled as ICU-acquired weaknesses [15]. To prevent these conditions, it is necessary to appropriately manage the risk factors in the acute phase, with particular attention to the appropriate management of sedatives. RASS assessments are thus an important component of the PICU VAP prevention bundle. The reliability and validity of the COMFORT scale, a sedative scale for children, has been established [16]. However, this scale cannot discriminate between analgesic and sedative effects as it assesses patients’ distress as well as pain. Furthermore, the results are represented as the total score of each item (range: 8–40 points), making it difficult to set target values in advance. The reliability and validity of the State Behavioral Scale have been established, a Japanese version is also being developed [17]; however, its application in the PICU is complicated by the number of evaluation items. Based on the above facts, we used the RASS to evaluate the patients’ level of sedation in the PICU. Previous studies have shown that the RASS is quick, intuitive, and an excellent tool for use in the PICU [18,19]. In adult patients, the RASS is used as part of the Confusion Assessment Method (CAM- ICU) for delirium evaluation of ICU patients. There is also a modified version, the pCAM-ICU, for children [9,20]. pCAM-ICU is to be evaluated using RASS. However, assessing the consciousness level by eye contact and gaze, as used in the RASS scoring system, can be difficult in infants. Evaluating sedation and excitement using RASS may improve delirium evaluation in the future.
Limitations and Future Tasks
This study has several limitations. First, this was a single-center study. Second, we did not have information to evaluate the effects of nurses’ and physicians’ clinical experience or skills in RASS assessments. In fact, it has been suggested that RASS can be an evaluation tool for pediatric patients through educational intervention [21]. Further, the validity of the RASS for children has not been adequately evaluated. The RASS is adapted for children by developing evaluation criteria according to their age and conscious levels, and the validity of this scale needs to be assessed with more patients.
Conclusions
The RASS is an important measure in the adult VAP prevention bundle. Results of the present study suggest that its use may also be effective in the PICU. Further studies are needed to verify our results.
Conflicts of Interest
This study received research funding from the policy-based Medical Services Foundation in 2017. The authors declare no conflict of interest.
References
- Brook AD, Ahrens TS, Schaiff R, Prentice D, Sherman D, et al. (1999) Effect of a nursing implemented sedation protocol on the duration of mechanical ventilation. Critical Care Medicine 27: 2609-2615. [crossref]
- Deeter KH, King MA, Ridling D, Irby GL, Lynn AM, et al. (2011) Successful implementation of a pediatric sedation protocol for mechanically ventilated patients. Critical Care Medicine 39: 683-688. [crossref]
- Smith HAB, Gangopadhyay M, Goben CM, Jacobowski NL, Chestnut MH, et al. (2017) Delirium and benzodiazepines associated with prolonged ICU stay in critically ill infants and young children. Critical Care Medicine 45: 1427-1435. [crossref]
- Rello J, Diaz E, Roque M, Vallés J (1999) Risk factors for developing pneumonia within 48 hours of intubation. American Journal of Respiratory and Critical Care Medicine 159: 1742-1746. [crossref]
- da Silva PS, Fonseca MCM (2012) Unplanned endotracheal extubations in the intensive care unit: systematic review, critical appraisal, and evidence-based recommendations. Anesthesia & Analgesia 114: 1003-1014. [crossref]
- Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, et al. (2013) Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Critical Care Medicine, 41, 263-306. [crossref]
- Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, et al. (2003) Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) The Journal of the American Medical Association 289: 2983-2991. [crossref]
- Sesseler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PM, et al. (2002) The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. American Journal of Respiratory and Critical Care Medicine 166: 1338-1344. [crossref]
- Smith HAB, Brink E, Fuchs DC, Ely EW, Pandharipande PP (2013) Pediatric delirium: monitoring and management in the pediatric intensive care unit. Pediatric Clinics of North American 60: 741–760. [crossref]
- Vet NJ, Ista E, de Wildt SN, van Dijk M, Tibboel D, et al. (2013) Optimal sedation in pediatric intensive care patients: a systematic Intensive Care Medicine 39: 1524-1534. [crossref]
- Playfor S, Jenkins I, Boyles C, Choonara I, Davies G (2006) United Kingdom Paediatric Intensive Care Society Sedation, Analgesia and Neuromuscular Blockade Working Group. (2006) Consensus guidelines on sedation and analgesia in critically ill children. Intensive Care Medicine 32: 1125-1136.
- Girard TD, Kress JP, Fuchs BD, Thomason JWW, Schweickert WD, et al. (2008) Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomized controlled trial. Lancet 371: 126-134. [crossref]
- Robinson BR, Mueller EW, Henson K, Branson RD, Barsoum S, et al. (2008) An analgesia-delirium-sedation protocol for critically ill trauma patients reduces ventilator days and hospital length of stay. The Journal Trauma 65: 517-526. [crossref]
- Nakatani A, Tazaki O, Yoshiya K, Asai T, Nishio S, et al. (2017) Examination on reduction of incident rate after introduction of RASS. Journal of the Japanese Society of Intensive Care Medicine 24: 47-48. (In Japanese).
- Needham DM (2008) Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. The Journal of the American Medical Association 300: 1685-1690. [crossref]
- Ambuel B, Hamlett K. W, Marx C. M, and Blumer J. L. (1992) Assessing distress in pediatric intensive care environments: the COMFORT scale. Journal of Pediatric Psychology 17: 95-109.[crossref]
- Hoshino H, Sakuramoto H, Matsuishi Y, Shimojo N, Enomoto Y, et al. (2019) Development of the Japanese version of the State Behavioral Scale for critically ill children. Acute Medicine & Surgery 6: 101-108. [crossref]
- Kerson AG, DeMaria R, Mauer E, Joyce C, Gerber LM, et al. (2016) Validity of the Richmond Agitation Sedation Scale (RASS) in critically ill children. Journal Intensive Care 4. [crossref]
- Haraguchi M, Ide K, Miura M, Hayashi Y, Oho K, et al. (2017) Nurse- physician inter-rater agreement on the Richmond Agitation Sedation Scale in critically ill infants and children. Journal of the Japanese Society of Intensive Care Medicine, 24(Suppl), 172. (In Japan)
- Traube C, Silver G, Kearney J, Patel A, Atkinson TM, et al. (2014) Cornell Assessment of Pediatric Delirium: a valid, rapid, observational tool for screening delirium in the PICU. Critical Care Medicine 42: 656-663. [crossref]
- Kihlstrom M, J, Ashley P, Edge A, P, Cherry K, M, Paul J, et al. (2018) Multi-modal Educational Curriculum to Improve Richmond Agitation- sedation Scale Inter-rater Reliability in Pediatric Patients. Pediatric Quality & Safety 3. [crossref]