Abstract
Chamomilla has long been recognized in traditional medicine for its established uses in herbal medicine and homeopathy. It is commonly recommended for treating respiratory, hepatic, gastrointestinal, and mental disorders. Additionally, it exhibits sedative, antiseptic, antiemetic, and anti-inflammatory properties and is frequently used to address issues related to teething in young patients. Despite its widespread use, scientific validation is essential to enhance the credibility of this medicine. In vitro studies offer a valuable approach for assessing the impact of homeopathic medicines on cellular functions, including cytotoxicity and cytokine secretion. Cell viability is typically evaluated through assays such as MTT, which measures cellular metabolic activity and provides insight into the proportion of viable cells following exposure to specific compounds. In the case of mesenchymal stem cells (MSCs) exposed to Matricaria chamomilla D3, the goal is to determine whether the homeopathic remedy affects cell survival or induces cytotoxicity. MSCs are known for secreting various cytokines that regulate inflammatory responses and promote tissue regeneration. Exposure to Matricaria chamomilla D3 may influence cytokine secretion, potentially altering the inflammatory response. This study evaluated the in vitro toxicity of injectable Chamomilla D3 in human mesenchymal stem cells, along with its potential anti-inflammatory effects, as evidenced by the reduction in the pro-inflammatory cytokine IL-8. The findings suggest that homeopathic Chamomilla D3 exhibits in vitro anti-inflammatory activity.
Keywords
Homeopathy, Vegetal, Complementary medicine
Introduction
Matricaria chamomilla, also known as chamomile, is a globally distributed plant [2]. It has a rich history in herbal medicine and homeopathy, with indications for treating various diseases [1]. Matricaria chamomilla is a versatile plant with multiple uses in folk medicine. It treats respiratory, hepatic, gastrointestinal, and mental alterations like stress and anxiety. It is also used as a sedative, antiseptic, and antiemetic, among others reported.
The phytochemistry, biological, and pharmacological properties of Matricaria chamomilla extracts are extensively characterized and systematically documented within herbal medicine. Its phytochemical composition encompasses over 120 bioactive compounds, including essential oils, terpenoids, and phenolic substances such as phenolic acids, flavonoids, and coumarins. These compounds impart a range of well-documented activities, including antioxidant, antibacterial, antifungal, antiparasitic, insecticidal, antidiabetic, anticancer, anti-inflammatory, antidepressant, antipyretic, anti-allergic, and analgesic effects [3].
According to the Homeopathic Medical Material, Matricaria chamomilla is indicated for various clinical manifestations such as irritability and hypersensitivity. Additionally, it may be relevant for treating otitis and diarrhea, especially in children, and for conditions associated with teething and gastrointestinal disturbances [4]. described effects such as anti-inflammatory and antispasmodic activities, among others, that support alleviating clinical symptoms. These effects may significantly improve the previously mentioned signs and symptoms [5].
The present study aimed to evaluate the anti-inflammatory activity of Matricaria chamomilla prepared according to the homeopathic pharmacopeia, specifically at a D3 potency and a concentration of 8 µL/mL. This evaluation focused on releasing the inflammatory cytokine Interleukin 8 (IL-8) and assessed the viability of healthy mesenchymal stem cells exposed to the medicine Matricaria chamomilla.
Materials and Methods
MTT Assay
The injectable homeopathic medicinal product Chamomilla D3 was tested on human mesenchymal stem cells (MSC) by the MTT test at a concentration of 8 µL/mL. Cell culture was performed in 75 cm2 flasks until reaching 80% confluence. Human mesenchymal stem cells in culture were trypsinized and distributed in a 96-well plate. After this process, the cells were incubated for 24 hours at 37°C in a 5% CO₂ environment. The test substance was prepared at 8 µL/mL and distributed into the designated wells. After 24-hour incubation, the culture medium was withdrawn and discarded. A volume of 50 μL of medium supplemented with 20% FBS was added, followed by 50 μL of medium containing the diluted test substance. The cells were incubated for an additional 48 hours in a CO₂ incubator, maintained at 5% CO₂ and a temperature of 37°C. After this period, the treatment medium was discarded, and 100 µL of the MTT solution was added to each well. The plate was covered with aluminum foil and incubated for 4 hours in an oven. Subsequently, MTT was removed, and 100 µL of DMSO was added to each well. The optical density was measured at 570 nm ± 10 nm using a plate reader. After this assay, the cytokine levels released upon exposure to the medicine were measured.
Cytokine Dosing
After reaching cellular confluence, cultured human mesenchymal stem cells were subjected to trypsinization and plated in 96-well plates. After 24 hours of incubation at 37°C with 5% CO₂, the culture medium was removed, and the wells were washed with PBS. Subsequently, 200 μg/mL of LPS (lipopolysaccharides from *Escherichia coli* O55: B5 – Sigma Aldrich), diluted in an antibiotic-free medium, was added in wells of positive control group and treatment group. Negative control group did not receive LPS, only culture medium. A subsequent incubation was performed for an additional 24 hours. Following the induction period of cellular inflammation with LPS, the medium was removed from the plate, and the wells were washed with PBS. The medicine was added at a final concentration of 8 μL/mL in treatment group. In negative and positive control groups, culture medium was added. The plate was then incubated for 24 hours under the previously described conditions.
After the treatment period, the supernatant was removed, and serum-free culture medium was added for 24 hours. The supernatant was collected for IL-8 analysis. IL-8 levels were measured using flow cytometry with the FACS VIA BD™ cytometer.
Results and Discussion
The present study assessed the cytotoxicity of the injectable homeopathic medicine Chamomilla D3 in human mesenchymal stem cells. The analysis revealed that, at the tested concentration, the material exhibited no cytotoxic potential (Table 1 and Figure 1).
Table 1: Cell viability obtained from the control and treated groups (Chamomilla 8 μl/mL) after MTT testing.
|
Cell viability(%) |
|
|
Control |
Chamomilla 8µl/mL |
| 94 |
93 |
|
101 |
93 |
| 102 |
95 |
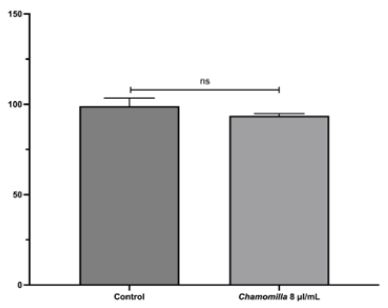
Figure 1: Cell viability (%) of the control and treated groups (Chamomilla 8 μl/mL): ns = no statistical difference.
Following the initial analysis, the release of the inflammation marker IL-8 by MSCs was evaluated both in response to LPS exposure and after treatment with the medicine. It was observed that LPS induced the release of IL-8, demonstrating its effectiveness in stimulating inflammation within the cell culture environment (see Figure 2). Additionally, cells previously “inflamed” by LPS were subsequently “treated” with the homeopathic medicine Chamomilla D3. The results demonstrated a significant reduction in the inflammation marker IL-8 following treatment. A similar pattern was observed in the control group, which consisted of cells maintained in a culture medium without LPS induction or Chamomilla D3 treatment (see Figure 2).
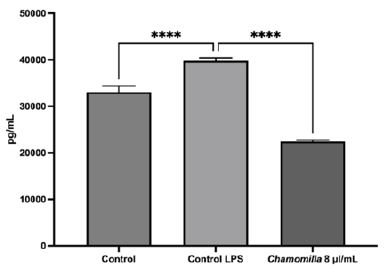
Figure 2: The results of the cytokine dosage test are presented in pg/mL for the following groups: the control group without LPS addition, the control group with LPS addition, and the treated group with Chamomilla at 8 μl/mL.
Homeopathy is frequently investigated due to its low likelihood of toxicity and minimal risk of causing side effects. According to Millstine [6], homeopathy can benefit the treatment of specific clinical conditions by potentially providing symptom relief.
Homeopathic Chamomilla is known for its anti-inflammatory effects, which can aid in treating various conditions [10]. Studies have demonstrated that the compounds in Chamomilla, such as flavonoids and terpenoids, possess properties that aid in reducing inflammation and alleviating related symptoms. As noted by Amsterdam [7], Chamomilla is also recognized for its calming and relaxing effects, which can contribute to alleviating stress and anxiety – factors often associated with inflammatory processes in the body.
Furthermore, regarding the anti-inflammatory properties of homeopathic Chamomilla, this article supports and validates the study by Scabello and Gardin [8], which examines injectable dynamized medicines available in Brazil. The authors noted that Chamomilla harmonizes the excessive action of the soul organization over the vital force, particularly within the digestive and menstrual spheres, and addresses general inflammation, per the principles of Anthroposophical Medicine.
In the homeopathic form, Chamomilla was identified as one of the ten most frequently used medicines for treating migraines, as highlighted and reviewed by Santos. Migraines are types of headaches that impair the patient’s quality of life.
Another property attributed to a medicine based on Matricaria chamomilla is its relaxation and analgesic effects. These effects were demonstrated in the study by Jyothis [9], which experimentally evaluated its impact on the central nervous system. As a result, a significant reduction in locomotor activity was observed, indicating muscle relaxation, analgesic effects, and anticonvulsant activity. Pinto [12] also reported relaxation effects in animals subjected to stress and depression.
The antibacterial and fungicidal actions of Matricaria chamomilla were emphasized in the study by, which explored its various aspects and properties. The study noted that its compounds impart sedative attributes, support digestion, and exhibit antimicrobial effects against bacteria and fungi [13].
Conclusion
The present study demonstrated the low in vitro toxicity of injectable Chamomilla D3 in human mesenchymal stem cells. Additionally, it suggested a potential anti-inflammatory action, as evidenced by a reduction in the levels of the pro-inflammatory cytokine IL-8. However, further studies are needed to confirm the homeopathic indications of its compounds in their homeopathic form and establish Chamomilla‘s in vivo anti-inflammatory activity.
References
- Reis LS, Pardo PE, Oba E, Kronka Sdo N, Frazatti-Gallina NM (2006) Matricaria chamomilla CH12 decreases handling stress in Nelore calves. J Vet Sci. [crossref]
- El Mihyaoui A, Esteves da Silva JCG, Charfi S, Candela Castillo ME, Lamarti A, Arnao MB (2022) Chamomile (Matricaria chamomilla L.): A Review of Ethnomedicinal Use, Phytochemistry and Pharmacological Uses. Life (Basel) [crossref]
- Santos ARF, da C, Cruz JH, de A, Guênes GMT, Oliveira Filho A Ade, Alves M ASG (2020) Matricaria chamomilla L: pharmacological properties. Archives Of Health Investigation, 8(12)
- Lathoud JA (2017) Studies of Homeopathic Materia Medica. Ed. Organon. 3rd edition. Sao Paulo.
- Fernanda Michel Tavares CANTO(a) Oswaldo de Castro COSTA NETO(a) Jéssica Muniz LOUREIRO(a) Guido Artemio MARAÑÓN-VÁSQUEZ(a) Daniele Masterson Tavares Pereira FERREIRA(b) Lucianne Cople MAIA(a) Matheus Melo PITHON(c) (2022) Efficacy of treatments used to relieve signs and symptoms associated with teething: a systematic review. Oral Res. [crossref]
- Millstine D (2023, December) Homeopathy. MSD Manual.
- Amsterdam JD, Li Y, Soeller I, Rockwell K, Mao JJ, Shults J (2009) A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. [crossref]
- Scabello RT, Gardin NE (2015) Potentized injectable medicines available in Brazil: indications based on homotoxicology and possibilities of use according to anthroposophic medicine. Arte Med Ampl
- JYOTHIS, AB Ram. A Study on Analgesic activity of Matricaria chamomilla.
- Srivastava JK, Shankar E, Gupta S (2010) Chamomile: A herbal medicine of the past with bright future. Mol Med Report. [crossref]
- Amsterdam JD, Li Y, Soeller I, Rockwell K, Mao JJ, Shults J (2009) A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol.
- Pinto SAG, Bohland E, de Paula Coelho C, de Azevedo Morgulis MSF, Bonamin LV (2008) An animal model for the study of Chamomilla in stress and depression: pilot study. Homeopathy [crossref]
- Singh O, Khanam Z, Misra N, Srivastava MK (2011) Chamomile (Matricaria chamomilla L.): an overview. Pharmacognosy Reviews.