Abstract
Urinary bladder cancer is the 10th most prevalent cancer globally, with rising incidence rates, particularly in developing countries. Accurate identification is essential for early diagnosis, prognosis, and treatment monitoring. Emmprin (CD147) has emerged as a potential biomarker linked to cell invasion and metastasis in bladder cancer. This study aimed to evaluate Emmprin expression in urothelial carcinoma and its correlation with histological grade and muscle invasion.
A cross-sectional observational study was conducted on 40 patients diagnosed with urothelial carcinoma. Immunohistochemical staining for Emmprin was performed on tissue sections, and its expression was correlated with tumor grade and muscle invasion. Among the participants, 85% were male, with an average age of 62.2 ± 9.6 years. High-grade urothelial carcinoma was observed in 70% of cases, and 50% had muscle-invasive disease. Emmprin expression was high in 52.5% of cases and low in 47.5%. High Emmprin expression showed a significant association with high-grade tumors (p<0.001) and muscle invasion (p=0.001). A moderate positive correlation was noted between Emmprin expression and tumor grade (rs=0.582, p<0.001) as well as muscle invasion (rs=0.538, p<0.001).
In conclusion, Emmprin overexpression is significantly linked to high-grade and muscle-invasive urothelial carcinoma. Its expression could serve as a valuable biomarker for assessing bladder cancer prognosis and progression, offering insights into tumor behavior and potential therapeutic targets.
Keywords
Emmprin, Urothelial carcinoma, Bladder cancer, Histological grade, Muscle invasion, Biomarker
Introduction
Urinary bladder cancer is the 10th most prevalent cancer globally, with an increasing incidence, particularly in developing countries. It is the 6th most common cancer in males and the 17th most common in females. The incidence rates in males are almost four times higher than in females. Although the incidence and mortality rates are higher in industrialized countries, they are also rising in regions such as Bangladesh, where factors like urbanization, industrialization, and tobacco consumption contribute to this trend [1,2,20].
The majority of bladder tumors are of epithelial origin, with urothelial carcinoma being the most frequent type, accounting for approximately 90% of all bladder cancers [3]. Urothelial carcinoma can be classified into two forms: non-muscle invasive and muscle-invasive. The non-muscle invasive form is characterized by a high recurrence rate (50-70%) and progression to muscle-invasive disease in about 10-20% of cases. In contrast, muscle-invasive bladder cancer (MIBC) presents with more aggressive behavior and a worse prognosis [4,5,17].
Currently, the diagnosis and prognosis of bladder cancer primarily rely on histological grading and staging. However, conventional prognostic factors such as tumor grade and stage are often insufficient to predict the disease’s behavior and clinical course accurately. This variability in patient outcomes highlights the need for reliable biomarkers to improve early diagnosis, prognostication, and therapeutic decision-making. Moreover, identifying molecular markers that can predict treatment responses and monitor recurrence remains an ongoing challenge [6-8,18,19].
Emmprin (CD147), an extracellular matrix metalloproteinase inducer, has gained attention as a potential biomarker for bladder cancer. It is overexpressed in several malignancies, including bladder cancer, where it plays a role in tumor proliferation, invasion, and metastasis. Emmprin expression has been shown to correlate with tumor grade and muscle invasion, suggesting that it could serve as a useful indicator of disease progression and prognosis [9-12,14,15].
The aim of this study was to evaluate the immunohistochemical expression of Emmprin in urothelial carcinoma of the bladder and to explore its correlation with histological grade and muscle invasion. We hypothesize that Emmprin expression is significantly correlated with the aggressiveness of the tumor, offering insights into its potential as a prognostic biomarker for bladder cancer.
Materials and Methods
Study Design and Ethical Considerations
This cross-sectional study was conducted at the Department of Pathology, Sir Salimullah Medical College (SSMC), and immunohistochemistry was performed at Bangabandhu Sheikh Mujib Medical University (BSMMU) from March 2022 to February 2024. Ethical approval (SSMC/2023/490, dated 25 February 2023) was obtained, and informed written consent was acquired from all participants.
Study Population
The cohort included 40 patients with histopathologically confirmed urothelial carcinoma of the bladder. Of the initial 45 cases, five were excluded due to inadequate tissue. Participants ranged from 45 to 85 years, with a mean age of 62.2 ± 9.6 years. Of the 40 participants, 85% were male, and 70% were smokers.
Specimen Collection and Processing
Specimens were fixed in 10% neutral buffered formalin, processed using standard histopathological methods, and stained with H&E. Tumor grading and staging followed the 2016 WHO guidelines.
Immunohistochemical Evaluation
Emmprin expression was evaluated by immunohistochemistry. Sections were cut (4 µm), deparaffinized, and antigen retrieval was performed. Primary antibody (Emmprin, 1: 100) was applied, followed by secondary antibody and DAB chromogen. Slides were analyzed by two pathologists at 400x magnification. An infiltrating duct carcinoma section served as a positive control.
Scoring of Emmprin Expression
Emmprin expression was scored based on the percentage of stained tumor cells and staining intensity:
- Percentage: 0 (<10%), 1 (10-24%), 2 (25-49%), 3 (50-74%), 4 (≥75%)
- Intensity: 0 (negative), 1 (weak), 2 (moderate), 3 (strong)
Final scores ranged from 0 to 7, with scores of 6-7 indicating strong Emmprin overexpression [16].
Ethical Issues
The study followed the Declaration of Helsinki (1975, revised 1983). Written informed consent was obtained from participants, and confidentiality was maintained by anonymizing data. Ethical standards for human research were strictly adhered to, with no animal experimentation involved.
Statistical Analysis
Data were analyzed using SPSS version 26. Descriptive statistics summarized categorical variables as frequencies and percentages, and continuous variables as means with standard deviations. The Chi-square test assessed categorical variables, while Spearman’s Rank Correlation Coefficient was used for continuous variables. A p-value of <0.05 was considered statistically significant.
Results
A total of 40 patients with histopathologically confirmed urothelial carcinoma were enrolled in the study. The age distribution of the patients was as follows: 8 patients (20.0%) were aged ≤50 years, 9 patients (22.5%) were aged 51-60 years, 18 patients (45.0%) were aged 61-70 years, and 5 patients (12.5%) were aged >70 years. The mean age of the cohort was 62.2 ± 9.6 years, with an age range spanning from 45 to 85 years. Regarding smoking status, 12 patients (30.0%) were non- smokers, while 28 patients (70.0%) were smokers (Table 1).
Table 1: Demographic and Smoking History of Study Participants.
|
Characteristic |
Category | Frequency |
Percentage (%) |
| Age Group (in years) |
≤50 |
8 | 20.0 |
| 51-60 | 9 |
22.5 |
|
|
61-70 |
18 | 45.0 | |
| >70 | 5 |
12.5 |
|
| Total |
40 |
100 |
|
| Mean ± SD |
62.2 ± 9.6 |
||
| Smoking History | Non-smoker |
12 |
30.0 |
| Smoker |
28 |
70.0 |
|
| Total |
40 |
100 |
Tumor localization revealed that 19 patients (47.5%) had urothelial carcinoma on the lateral wall, 11 patients (27.5%) on the posterior wall, 5 patients (12.5%) on the anterior wall, 2 patients (5%) on the trigone, 2 patients (5%) on the neck, and 1 patient (2.5%) on the dome. Histopathological grading showed that 28 patients (70.0%) had high-grade urothelial carcinoma, while 12 patients (30.0%) had low-grade urothelial carcinoma (Table 2).
Table 2: Distribution of Cases by Site, Grade, and Muscularis Propria Invasion (N=40).
|
Site |
Lateral wall | Posterior wall | Anterior wall | Trigone | Neck | Dome |
Total |
| Frequency |
19 |
11 | 5 | 2 | 2 | 1 | 40 |
|
Percentage(%) |
47.5 | 27.5 | 12.5 | 5 | 5 | 2.5 |
100 |
|
Muscularis propria invasion |
High grade | Low grade |
Total |
||||
| Present (MIBC) |
19(95%) |
1 (5%) |
20 |
||||
| Absent (NMIBC) |
9(45%) |
11(55%) |
20 |
||||
| Total |
28 |
12 |
40 |
||||
Muscularis propria invasion was observed in 20 cases (50.0%), while the remaining 20 cases (50.0%) did not exhibit muscularis propria invasion. Among the 20 patients with muscle-invasive bladder cancer (MIBC), 19 (95%) had high-grade tumors, and 1 (5%) had a low-grade tumor. In contrast, among the 20 patients with non-muscle- invasive bladder cancer (NMIBC), 9 (45%) were high-grade and 11 (55%) were low-grade (Table 2).
Emmprin expression was categorized as low in 19 patients (47.5%) and high in 21 patients (52.5%) (Table 3). Among the 12 cases with low- grade urothelial carcinoma (LGUC), only 1 case (8.3%) demonstrated high Emmprin expression. In contrast, 20 of the 28 cases (71.4%) with high-grade urothelial carcinoma (HGUC) exhibited high Emmprin expression. Statistical analysis using the Chi-square test revealed that high-grade urothelial carcinoma had significantly higher Emmprin expression compared to low-grade urothelial carcinoma (p<0.001) (Table 3).
Table 3: Association of Histological Grading and Muscle Invasion with Emmprin Expression in Urothelial Carcinoma of the Urinary Bladder (n=40).
|
Criteria |
Total Cases (n) | Low Emmprin (n, %) | High Emmprin (n, %) | p-value | Correlation (rs) |
|
Grading |
<0.001 |
0.582 |
|||
| Low Grade |
12 |
11 (91.7%) | 1 (8.3%) | ||
|
High Grade |
28 | 8 (28.6%) |
20 (71.4%) |
||
| Muscle Invasion |
0.001 |
0.538 |
|||
| Absent |
20 |
15 (75.0%) |
5 (25.0%) |
||
| Present |
20 |
4 (20.0%) |
16 (80.0%) |
||
| Total |
40 |
19 (47.5%) |
21 (52.5%) |
When considering muscle invasion, 5 patients (25.0%) without muscle invasion exhibited high Emmprin expression, whereas 16 patients (80.0%) with muscle invasion showed high Emmprin expression. The Chi-square test revealed a significant association between muscle invasion and increased Emmprin expression in muscle-invasive bladder cancer (MIBC) compared to non-muscle- invasive bladder cancer (NMIBC) (p=0.001) (Table 3).
Additionally, a moderate positive correlation was found between tumor grading and Emmprin expression (rs=0.582, p<0.001) (Figure 1). There was also a significant positive correlation between muscle invasion and Emmprin expression (rs=0.538, p<0.001) (Figure 2).
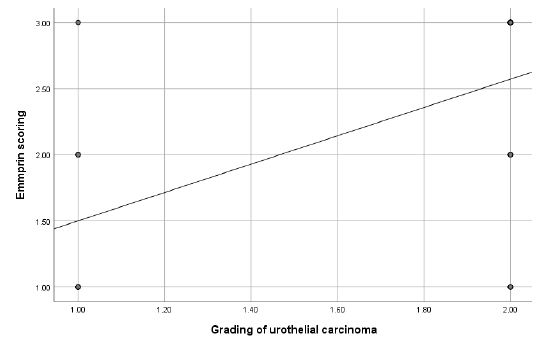
Figure 1: Scatter plot diagram showing relationship between grading of urothelial carcinoma and emmprin score.
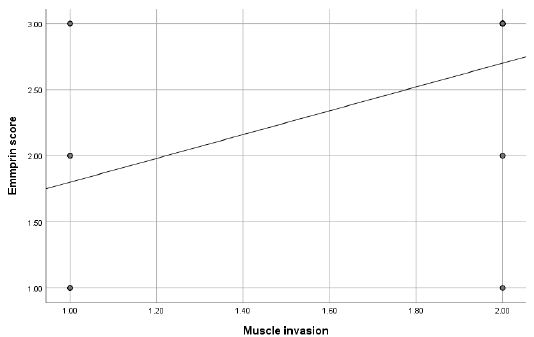
Figure 2: Scatter plot diagram showing relationship between muscle invasion and emmprin score.
This table presents the age distribution and smoking history of 40 participants. The age groups are divided as ≤50, 51-60, 61-70, and >70 years. The largest group (45%) was in the 61-70 age range. The mean age of participants was 62.2 ± 9.6 years. In terms of smoking history, 70% of participants were smokers, while 30% were non-smokers.
This combined table provides two sets of data for 40 cases: the distribution by anatomical site and by grade and muscularis propria invasion. The first section shows the distribution of cases across six sites, with the lateral wall being the most commonly affected site (47.5%). The second section presents the distribution of cases based on grade and muscularis propria invasion, with 19 high-grade cases having muscularis propria invasion (MIBC) and 9 high-grade cases without invasion (NMIBC). The total number of cases is 40 for both sections).
Statistical tests: Chi-square test (Grading & Muscle Invasion), Spearman’s correlation (Grading & Muscle Invasion with Emmprin expression). Significance: p<0.05. A significant association was found between Grading and Muscle Invasion with Emmprin expression (p<0.001 for grading, p=0.001 for muscle invasion). Positive correlations were observed between Emmprin expression and Grading (rs=0.582), as well as Muscle Invasion (rs=0.538), indicating higher Emmprin expression correlates with more aggressive tumor characteristics.
X-axis: Tumor grading (Low grade vs. High grade), Y-axis (Vertical): Emmprin score (Scale indicating levels of Emmprin expression). Each point represents an individual case, plotted based on tumor grading and corresponding Emmprin score. Trend Line Shows the positive correlation between tumor grading and Emmprin expression, where higher tumor grades tend to have higher Emmprin scores. A moderate positive correlation was found between tumor grading and Emmprin expression (rs=0.582, p<0.001), suggesting that higher tumor grade is associated with higher Emmprin expression.
X-axis: Muscle invasion status. Y-axis: Emmprin score (Scale indicating levels of Emmprin expression). Each point represents an individual case, plotted based on muscle invasion status and corresponding Emmprin score. Trend Line Shows the positive correlation between muscle invasion and Emmprin expression. The presence of muscle invasion tends to be associated with higher Emmprin scores. A moderate positive correlation was observed between muscle invasion and Emmprin expression (rs=0.538, p<0.001), indicating that higher Emmprin expression is linked with the presence of muscle invasion (Figures 3 and 4).
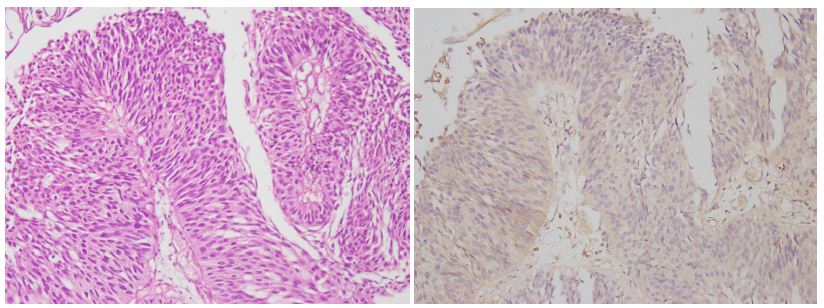
Figure 3: The photomicrograph shows a tissue section from a low-grade urothelial carcinoma (case no-39). The first panel features H&E staining, highlighting neoplastic cell structures with mild atypia. The second panel shows low Emmprin expression, indicating less aggressive tumor behavior, with a magnification of 200X.
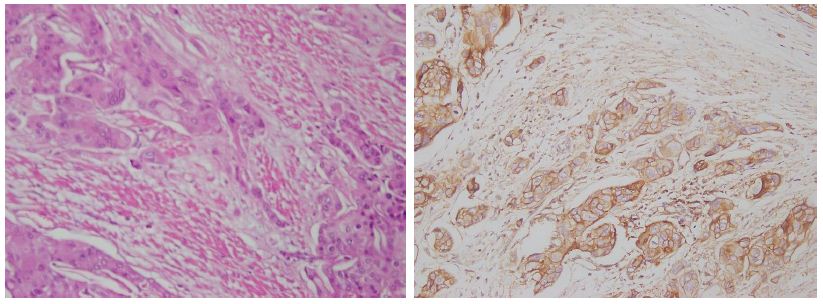
Figure 4: The photomicrograph shows a tissue section from a high-grade muscularis propria invasive urothelial carcinoma (case no-29). The first panel displays H&E staining, highlighting abnormal cellular structures, while the second panel shows high Emmprin expression, indicating tumor aggressiveness. The magnification is 200X.
Discussion
The present study provides significant insights into the role of Emmprin expression in the pathogenesis of bladder cancer (BC). We found a marked correlation between elevated Emmprin levels and both high-grade urothelial carcinoma (HGUC) and muscle-invasive bladder cancer (MIBC), supporting its potential as a prognostic biomarker in BC. These findings offer an important contribution to the understanding of BC aggressiveness and may have clinical implications for guiding therapeutic decisions.
Our study revealed that Emmprin expression was significantly higher in HGUC compared to low-grade urothelial carcinoma (LGUC), which is consistent with previous research suggesting that Emmprin overexpression is associated with tumor progression and invasiveness [6,13,21]. The correlation between Emmprin expression and tumor grade (rs=0.582, p<0.001) reinforces the notion that Emmprin could serve as an indicator of tumor aggressiveness, as high- grade tumors are often associated with poorer outcomes. This finding aligns with other studies that have established emmprin as a key player in promoting the malignant phenotype of various cancers, including bladder cancer [21].
Additionally, our data demonstrated that Emmprin expression was significantly higher in MIBC compared to NMIBC, with a positive correlation between muscle invasion and Emmprin levels (rs=0.538, p<0.001). These results are in line with findings from Xue et al. [21] and Wittschieber et al. [13], who also reported a strong association between emmprin expression and the invasive potential of tumors. The ability to distinguish MIBC from NMIBC based on Emmprin expression could offer a valuable tool for predicting disease progression, allowing clinicians to identify patients who may benefit from more aggressive treatment strategies.
While our study contributes valuable insights into the prognostic value of Emmprin in BC, it is not without limitations. The sample size of 40 patients may not fully represent the heterogeneity of the BC population, limiting the generalizability of the results. Furthermore, the retrospective nature of the study, relying on archival tissue samples, introduces potential biases in patient selection. Larger, multicenter, and prospective studies are needed to validate these findings and explore the broader applicability of Emmprin as a biomarker in BC. It would also be valuable to assess the molecular mechanisms underlying emmprin’s role in BC, including its interaction with other key players in the tumor microenvironment, such as extracellular matrix components and immune cells [11,15].
Another limitation is the lack of longitudinal data, which prevents us from drawing definitive conclusions regarding the prognostic utility of Emmprin in predicting clinical outcomes, such as recurrence, metastasis, and overall survival. Future studies should aim to follow patients prospectively to assess whether Emmprin expression correlates with patient prognosis and treatment response over time.
Despite these limitations, our findings suggest that Emmprin expression could serve as a useful biomarker for predicting tumor grade and muscle invasion in BC. This could aid in identifying patients at high risk for aggressive disease, thus informing treatment decisions and enabling personalized therapeutic approaches. Further research is warranted to investigate the potential of emmprin as a therapeutic target in BC, as targeting Emmprin may hold promise for improving patient outcomes [13,15,21].
To conclude, this study provides compelling evidence that elevated emmprin expression is correlated with high-grade and muscle- invasive bladder cancer. These results suggest that Emmprin could be a valuable prognostic marker, offering potential clinical utility in predicting disease aggressiveness and guiding treatment decisions. However, additional studies with larger cohorts and longitudinal follow-up are necessary to validate these findings and to elucidate the underlying mechanisms by which Emmprin contributes to bladder cancer progression [6,13,21].
Conclusion
The findings of this study establish a significant positive correlation between Emmprin expression and both histological grade and muscle invasion in urothelial carcinoma, highlighting its potential as a key biomarker for bladder cancer aggressiveness. Elevated emmprin levels may not only serve as a prognostic indicator but also represent a promising therapeutic target for chemotherapy, paving the way for novel, targeted treatment strategies aimed at improving clinical outcomes in bladder cancer management. These results underscore the potential of Emmprin as a critical player in bladder cancer progression, warranting further investigation into its molecular mechanisms and therapeutic targeting.
Acknowledgement
The authors would like to express their heartfelt gratitude to Dr. Md Zahirul Islam, Lecturer, Department of Pathology, Sher-e-Bangla Medical College, Barisal, for his invaluable support throughout the research process. We are also deeply thankful to all the doctors from the Department of Urology, Sir Salimullah Medical College, whose unwavering assistance was instrumental in making this study possible. Special acknowledgment goes to the patients, from whom specimens were collected, and their attendants, for their active participation and cooperation in this study.
Contribution of the Authors
Shamim Ahamed conceptualized the study, designed the methodology, and revised the manuscript. Nafisa Abedin, as the corresponding author, led the data analysis, and manuscript drafting. Jannat Ara and Ummey Qoraiman Tahira contributed to data collection, analysis, and drafting. Sayeed Kishwara Kashfi and Shahana Sultana supervised the methodology and reviewed the manuscript. Mst Rubeyatul Jannat and Mashrufa Rahman assisted with literature review, statistical analysis, and manuscript revisions. Shahnaj Begum provided senior supervision and final approval of the manuscript.
Data Availability Statement
The data that support the findings of this study are fully available and can be obtained from the corresponding author, Nafisa Abedin, upon request.
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Funding Statement
No specific funding was received for this study.
Conflict of Interest Disclosure
The authors declare no conflict of interest.
Ethics Approval Statement
Ethical approval was obtained from the appropriate institutional ethics committee.
Patient Consent Statement
Informed consent was obtained from all patients included in this study.
Permission to Reproduce Material from Other Sources
Permission was obtained where necessary.
Clinical Trial Registration
Not applicable.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. [crossref]
- Ploeg M, Aben KK, Kiemeney LA (2009) The present and future burden of urinary bladder cancer in the World J Urol. [crossref]
- Netto GJ, Amin MB (2020) The lower urinary tract & male genital In: Robbins & Cotran Pathologic Basis of Disease. 10th ed.
- Zang Y, Li X, Cheng Y, Qi F, Yang N (2020) An overview of patients with urothelial bladder cancer over the past two decades: A Surveillance, Epidemiology, and End Results (SEER) Ann Transl Med. [crossref]
- Hemdan T (2016) Prognostic and Predictive Factors in Bladder Cancer [Doctoral dissertation]. Acta Universitatis Upsaliensis.
- Zhong WD, Chen QB, Ye YK, Han ZD, Bi XC, Dai QS, et (2010) Extracellular matrix metalloproteinase inducer expression has an impact on survival in human bladder cancer. Cancer Epidemiol. [crossref]
- El-Rehim A, Mohamed D, El-Maqsoud A, Reda NM, El-Hamid A, Mohamed A, et (2013) Expression of extracellular matrix metalloproteinase inducer and fascin in urinary bladder cancer: correlation with clinicopathological characteristics. Mol Clin Oncol. [crossref]
- Ye F, Wang L, Castillo-Martin M, McBride R, Galsky MD, Zhu J, et (2014) Biomarkers for bladder cancer management: present and future. Am J Clin Exp Urol. [crossref]
- Han ZD, He HC, Bi XC, Qin WJ, Dai QS, Zou J, et (2010) Expression and clinical significance of CD147 in genitourinary carcinomas. J Surg Res. [crossref]
- Landras A, Reger de Moura C, Jouenne F, Lebbe C, Menashi S, Mourah S (2019) CD147 is a promising target of tumor progression and a prognostic Cancers. [crossref]
- Li H, Xu Y, Li H (2017) CD147 as a novel biomarker for predicting the prognosis and clinicopathological features of bladder cancer: a meta-analysis. Oncotarget. [crossref]
- Hambalie LA, Rahaju AS, Mastutik G (2021) The Correlation of EMMPRIN and EGFR Overexpression toward Muscle Invasiveness in Urothelial Carcinoma of Indian J Forensic Med Toxicol.
- Wittschieber D, Stenzinger A, Klauschen F, Stephan C, Jung K, Erbersdobler A, et (2011) Decreased RECK and Increased EMMPRIN expression in urothelial carcinoma of the bladder are associated with tumor aggressiveness. Pathobiology. [crossref]
- Peng J, Jiang H, Guo J, Huang J, Yuan Q, Xie J, et (2020) CD147 expression is associated with tumor proliferation in bladder cancer via GSDMD. Biomed Res Int. [2020]. [crossref]
- Hemdan T, Malmström PU, Jahnson S, Segersten U (2015) Emmprin expression predicts response and survival following cisplatin-containing chemotherapy for bladder cancer: A validation study. J Urol. [crossref]
- Monteiro LS, Delgado ML, Ricardo S, Garcez F, Amaral B, Pacheco JJ, et (2014) EMMPRIN Expression in Oral Squamous Cell Carcinomas: Correlation with Tumor Proliferation and Patient Survival. Biomed Res Int. [crossref]
- Sasikumar S, Wijayarathna KSN, Karunaratne KAMS, Gobi U, Pathmeswaran A, Abeygunasekera AM (2016) Pathological Characteristics of Primary Bladder Carcinoma Treated at a Tertiary Care Hospital and Changing Demographics of Bladder Cancer in Sri Adv Urol. [crossref]
- Rahman P, Khan KH, Afroz S, Rahman MM, Rashid JS (2022) Association of Ki-67 expression in radical cystectomy specimens of infiltrating urothelial carcinoma with histopathological Bangabandhu Sheikh Mujib Med Univ J.
- Sadaf A, Rahman MZ, Bhattacharjee P, Ahamad MSU, Nasreen S (2021) Significance of vascular endothelial growth factor expression in the bladder urothelial carcinoma and its association with tumor grade and Iran J Pathol. [crossref]
- Hussain SMA (2013) Comprehensive update on cancer scenario of South Asian J Cancer. [crossref]
- Xue YJ, Lu Q, Sun ZX (2010) CD147 overexpression is a prognostic factor and potential therapeutic target in bladder cancer. Med Oncol. [crossref]