Abstract
Introduction: Tai Chi (TC) has been often prescribed by geriatric clinicians to patients with a variety of neurological disorders. In the last 10 years, there has been an increase in the number of studies examining the effects of TC on brain morphology as assessed by neuroimaging including near infrared spectroscopy (NIRS) and structure and functional magnetic resonating imaging (sMRI & fMRI). Thus, the purpose of this scoping review is to evaluate how TC practice may affect the brain morphologically as assessed by neuroimaging techniques.
Methods: A comprehensive literature search was conducted using a variety of key words with different search engines to search from the last 10 years until May 2022. Studies were included if they investigated topographic brain responses after TC practice. A total of 24 original studies met the criteria and were included for the review process.
Results: The results showed increased oxygenation and volume of cortical grey matter, improved neural activity, and altered neural connectivity and homogeneity within and/or between different neural regions. These regions include the frontal, parietal, temporal, occipital lobes, cerebellum, basal ganglia, and/or limbic system. Such neural findings after TC practice are often associated with neurobehavioral improvements in cognition, memory, emotion, and functional integration and/or specialization.
Conclusions: TC is a promising exercise that is able to improve morphological capability and neurofunctional activity in the brain in both healthy people and patients with different medical diagnoses.
Keywords
TC exercise, Brain, Neuroimaging, Rehabilitation
Introduction
Clinically, mind-body exercises are frequently recommended by clinicians and mental health counselors among which Tai Chi (TC) is one of the most commonly used one [1]. As an ancient Chinese Martial art, TC integrates breathing, meditation, and body movement to achieve a great sense of inner peace and well-being in a calm, relaxed, and meditative way. During TC practice, the practitioner shifts their body weight or makes steps from one leg to the other through its coordinated and controlled slow, gentle, and graceful movements that emphasize smooth rotation of the trunk and arms as well as coordination between breathing and body part movements [2-4]. Its intensity is moderate and approximately equivalent to a walking speed of 6 kilometers or 3.7 miles per hour [5], but the intensity can vary depending on the training style, performance posture, and exercise parameters [6].
Currently, TC is recognized as an effective intervention for improving health, increasing physical performance and social participation, preventing falls, and enhancing posture for both the general population and for patients with neurological dysfunctions [1,2,5]. For example, TC has played a significant role in the recovery of patients who suffered from stroke, Parkinson disease, traumatic brain injury, and multiple sclerosis [6,7]. Because of its beneficial effects on health promotion and improvement of human dysfunctions including neurological disorders, TC has been considered as one of the most promising exercise programs that people with neurological diagnoses can practice to improve their physical and mental conditions [1,6]. Extensive research studies have demonstrated the beneficial effects of TC programs on different aspects, including flexibility, range of motion, muscle tone, strength, posture, balance, walking, psychological well-being, stress reduction, and quality of life [1,6].
In the last decade, an increasing number of studies have been conducted to investigate whether and how the human brain might respond to TC practice, assessed by using a variety of neuroimaging techniques which include the following. Functional near-infrared spectroscopy (FNIRS) is a cost-effective, wearable neuro-imaging technology that can safely assess the real-time brain activity during physical performance by monitor the hemodynamic response in the brain cortex using near-infrared light sources and detectors placed over the scalp of an individual [8]. Structural magnetic resonance imaging (sMRI) is a non-invasive imaging technique that can examine the morphological characterization of the brain in normal or pathological conditions [9]. Functional Magnetic Resonance Imaging (fMRI) is an imaging technique often used to assess two or more different states in an experimental functional condition in comparison to a control condition [10]. Magnetic resonance spectroscopy (MRS) is a companion MRI technique that is often used to non-invasively measure and evaluate the concentrations of different chemical components of the scanned tissue, and consequently provides metabolic and biochemical information within the tissues [11]. Also in fMRI, the voxel-mirrored homotopic connectivity (VMHC) is a method of resting state fMRI that is designed to directly compare the interhemispheric resting-state functional connectivity of two brain hemispheres and can be used to enquire and analyze functional homotopic (geometrically corresponding) connectivity and functional integration (VMHC) or specialization (¯VMHC) in each hemisphere or between two hemispheres [12]. Regional homogeneity (ReHo) and fractional amplitude of low-frequency fluctuations (fALFF), two different resting state fMRI parameter maps, have been introduced as well to study the brain. fMRI (ReHo) can be used to assess functional homogeneity between neural regions; increased homogeneity indicates improved functional integration, while decreased homogeneity indicates increased functional specialization between neural structures [10,12]. Further, fMRI (fALFF) can be used to assess local spontaneous neural activity of the brain [10,12]. Therefore, the purpose of this literature article was to review and examine if TC exercise might affect the brain as assessed through these neuroimaging techniques, and consequently to help healthcare professionals understand the possible implication of TC’s effect on morphology and neural activity of the human brain.
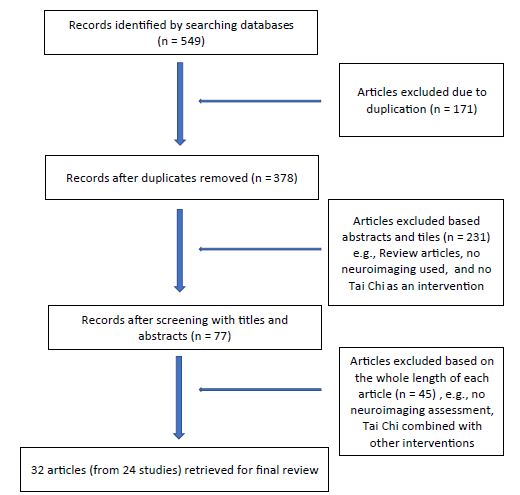
Figure 1: Flow chart of articles searched for analyses
Methods
Search Strategy
TC-related literature that investigated TC’s effects on morphological responses of the brain was searched. The following sources were included in the literature search process: Pubmed, Scopus, Medline (US National Library of Medicine), the Physiotherapy Evidence Database (PEDro), the Cochrane Controlled Trials Register (Cochrane Library), Cumulative Index of Nursing and Allied Health Literature (CINAHL), and the oversea English version of China National Knowledge Infrastructure (CNKI), up to May 2022. The search strategy used the following keywords and variations: Tai Ji, TC, TCh, TC Quan, Tai Ji Quan, Tai Ji Chuan, Chinese martial arts, Chinese fitness exercise, neuroimaging, functional near-infrared spectroscopy (FNIRS), magnetic resonance spectroscopy (MRS), magnetic resonance imaging (MRI), voxel-mirrored homotopic connectivity (VMHC), fMRI Regional homogeneity (ReHo), and fractional amplitude of low-frequency fluctuations (fALFF). Published reviews and all relevant studies and their reference lists were also reviewed manually in search for other pertinent publications.
Study Selection
Studies identified in the search were screened for inclusion. Articles that met the following criteria were selected: (1) studies investigating the effects of TC on brain response; (2) studies assessing the responses with FNIRS, sMRI, fMRI, MRS, and/or VHMC as the primary results; (3) participants were adults (age ≥18 years or older); (4) randomized control trials, single-group pre- and post- comparison, and cross-sectional studies comparing TC practitioners and non-practitioners; and (5) studies published in peer‐reviewed English or Chinese journals from last 10 years until May 2022.
Data Extraction
Initially, all identified articles were assessed independently by two reviewers by scanning the titles and abstracts to determine whether it met the predetermined eligibility criteria. When there was uncertainty or disagreement between the two reviewers, the lead author was involved in the discussion until a consensus decision was reached. Data extracted from each of these studies included study design, participant characteristics, exercise program characteristics, neuroimaging techniques, and morphological changes identified by these techniques.
Quality Assessment
The quality of all studies in this scoping review were assessed based on the type of study. Physiotherapy Evidence Database (PEDro) scale was used for randomized controlled trials [13]. Newcastle–Ottawa Scale (NOS) was conducted for cohort or cross-sectional studies [14].
Data Analysis
Study designs, participants’ characteristics, TC interventional parameters, neuroimaging, neurobehavior, and other functional assessments were all shown in Table 1. As the purpose of this review was to discuss TC’s effects on brain morphology changes in humans, neuroimaging data and their associations with neuroimaging changes in these included studies were extracted, summarized, and synthesized in Tables 2-4.
Table 1: Tai Chi Studies Assessed with Neuroimaging Techniques
|
Authors |
Research
Design |
Subjects | Interventional Parameters | Assessment instruments |
Quality Assessment |
| Shen, Watkins, Kahathuduwa, et al (2022)[15] | Single group Pre-and post- comparison | 12 postmenopausal females with osteoarthritis, 40-50 yrs old | Yang style 24 forms, 60 mins, 3/wk for 8 wks | rs-fMRI, Pain Visual analog scale, WOMAC, plasma metabolites | 6/10
|
| Cui, Tao, Yin et al (2021)[16]
|
RCT | 36 young healthy adults (18-25 y.o): 12 in each of 3 groups: TC, brisk walking, and usual care (as control | TC: Bafa Wubu,, 50-60 mins, 3/wk for 8 wks
|
rs-fMRI, Pain Visual analog scale, WOMAC, plasma metabolites
|
8/11
|
| Kong, Huang, Liu et al, (2021)[17]
|
RCT | IG – 24 with fibromyalgia
CG – 24 health subjects All subjects> 21 years old
|
TC; Yang style, 10 forms
60 mins each, 2/wk for 12 wks
|
fMRI
More-odd shifting task for cognitive flexibility
|
6/11
|
| Shen, Yin, Cui, et al, (2021)[18]
|
RCT | IG – n=12, TC (Yang style, 24 forms)
CG – n=12, brisk walking Young health adults (<25 y.o)
|
50-60 mins, 3/wk for 8 wks
|
fMRI and modified Flanker Test
|
7/11
|
| Xu, Zimmerman, Lazae et al (2020) [19] | Single group Pre-and post- comparison | 16 adult patients with major depression | 60 min each, 2/wk for 10 wks | fMRI
Beck Depression Inventory SF-36 |
5/10
|
| Adcock, Fankhauser et al (2020) [20] | RCT | IG – n =15 (77±6.4 y.o.)
· CG – n=16 (70.9±5.0 y.o.) · All healthy elderly individuals |
IG – TC + dancing +step-based cognitive games at home; 3/wk, 30-40 min each for 16 wks
· CG – normal daily living |
sMRI
Victoria Stroop test for cognition · Trail Making test for psychomotor speed and executive function; · Wechsler Memory Scale for memory |
7/11** |
| Yue, Zou, Mei et al (2020) [21]
Yue, Yu, Zhang et al (2020) [22] |
Cross-sectional | 42 healthy elderly females
· IG – TC, n=20 (62.9±2.38 y.o.) · CG – walking. n=22, (63.27±3.58 y.o.) · 90 min/each, 5/week, over 6 yrs
|
NA | fMRI (VHMC) | 7/10* |
| Chen et al (2020) [23] | Cross-sectional | TC – 22 (aged: 52.4 ±6.8; TC experience 14.6±8.6 y.o.;
Control – 18 (aged: 54.8 ±6.8)
All healthy adults |
NA | fMRI
Attention network test (ANT)
|
8/10* |
| Yang, Chen, Shao et al (2020) [24] | RCT | 13 TC vs 13 Control
All healthy elderly individuals |
TC: 45 min/each, 3/wk, for 8 wks
Yang style, 24-form Control: routine and general daily activity |
fNIRS
Flanker task test |
8/11** |
| Cui, Yin, Lyu et al (2019) [25] | RCT with 3 groups
|
36 young healthy college students (18-25 y.o.):
TC: 12 Brisk walking: 12 Control: 12
|
IG1 – TC: 8 hand movement techniques and 5 TC foot-works based on Yang-style TC.
IG2 – brisk walking Both TC and brisk walking groups: 60 min/each, 3/wk for 8 wks CG – routine daily activities |
sMRI and fMRI | 9/11** |
| Tsang et al (2019) [26] | Cross-sectional | 8 practitioners (over 7 years of experience) and 8 non-practitioners
All: 60-75 y.o. |
NA | fNIRS | 6/10* |
| Liu, Chen, Chen et al, (2019) [27]
Liu, Chen, Tu et al (2019) [28] |
RCT | IG1 – TC – n = 28
IG2 – BDJ – n = 29 IG3 – Stationary bike – n=27 CG – Health ed – n = 24 All (n = 108) 40-70 y.o. |
60 min each, 5/wk for 12 wks | sMRI and fMRI
Knee injury and osteoarthritis outcome score (KOOS) |
7/11** |
| Xie, et al (2019) [29] | Cross-sectional | 32 ordinary vs 25 long-term (>5 years) Chen-style TC practitioners (all 60-70 y.o.) | NA | fNIRS | 7/10* |
| Liu, Li, Liu, Sun et al (2020) [30]
Liu Li, Liu, Guo et al (2019) [31] |
Cross-sectional | 52 community-dwelling older adults (60-70 y.o.)
IG – TC – 26 (10 years or more TC experience) VG – 26 (non-TC practitioners, but matched in physical activity level)
|
Both groups were asked to accomplish a sequential risk-taking task | sMRI and fMRI
Beck Depression inventory NEO five-factor inventory Five facets mindfulness questionnaire, Mindful Attention Awareness Scale Barratt Impulsiveness Scale |
9/10* |
| Kong et al (2019) [32] | RCT | 21 patients with fibromyalgia (± 21 y.o.)
20 healthy matched participants |
TC – 60 min/each, 2/wk, 12 wks, Yang style
CG – no TC experience |
fMRI
Fibromyalgia Impact questionnaire (FIQR) |
6/11** |
| Wu, Tang, Goh et al (2018) [33] | RCT | Community living older adults (60-69 y.o.)
IG – n = 16 CG – n = 10 |
TC: 60 min each, 3/wk, 12 wks; Yang (10 min warm-up and 10 min cool down)
CG – telephone consultation biweekly without changing lifestyle |
fMRI | 7/11** |
| Port, Santaella et al (2018) [34] | Cross-sectional | 8 TC practitioners (>60 y.o.)
8 water aerobics practitioners (> 60 y.o.) |
NA | fMRI during attention time
· Stroop Word Color Task – SWCT · Working memory with N-back task |
7/10* |
| Zhou, Liao, Sreepada et al (2018) [35] | Single group pre-and post- comparison | 6 healthy elderly individuals (> 55 y.o.) | TC: 60 min each, ³ 2/wk, 12 wks | MRS
NAA: N-acetylaspartate; Cr: creatine PCr: phosphocreatine |
5/10* |
| Liu, Wu, Li, Guo (2018) [36] | Cross-sectional | IG – TC, n = 26 (10.44±5.48 yrs TC experience) (65.19±2.30 y.o.)
CG – matched group (63.92±2.87 y.o.) (no TC experience) |
NA | fMRI | 8/10* |
| Wei et al (2017) [37]
Wei, Dong, Yang et al (2014) [38]
Wei, Xu, Fan et al (2013) [39] |
Cross-sectional | IG – TC, n = 22 (aged: 52.4 ±6.8; TC experience 14.6±8.6 years;
CG – n = 18 (aged: 54.8 ±6.8) |
NA | sMRI and fMRI
Attention network test (ANT)
|
7/10* |
| Tao, Liu, Liu et al (2017) [40]
Tao, Chen, Liu et al (2017) [41]
Tao, Liu, Egorova et al (2016) [42] |
RCT | TC-21 (62.38±4.55 y.o.)
BDJ-15 (62.33±3.88 y.o.) CG – 25 (59.76±4.83 y.o.) |
IG1 – TC: 60min, 5/wk, 12 wks; Yang-style, 24-form
IG2 – BDJ: 60min, 5/wk, 12 wks; CG – health education |
sMRI and fMRI (fALFF)
Wechsler Memory Scale (WMS)
|
9/11** |
| Zheng et al (2015) [43] | RCT | Community dwellers
IG – n = 17 (68.59 y.o.) CG – n = 17 (71.65 y.o.) |
IG – combined interventions: 3/week for 6 wks
1. cognitive training – 18 hrs 2. TC 18 hrs, Yang-24 3. Group counseling (6 90-min sessions) CG – two 120-min health-related lectures |
fMRI
Paired associative learning test (PALT) (to examine episodic memory)
Category Fluency test (CFT) (to examine speech production)
|
8/11** |
| Yin et al (2014) [44]
Li, Zhu, Yin, Niu et al (2014) [45] |
RCT | 45 older community-dwellers
IG – Multimodal intervention (TC + cognitive training + counseling – 26 CG – 19 |
Multimodal intervention include
IG 1 – TC (Yang style, 24 form, 60 min each, 3/wk for 6wks) + IG 2 – Cognitive training: (60 min each, 3/wk, for 6wks) + counseling (90,im each, 1/wk for 6 wks) CG – daily routine, 2 120-min healthcare education |
MRI
MoCA Associative Learning Test (ALT) Digital Span forward and Backward Tasks Category Fluency Test Train Making Test Social Support Rating Scale Satisfaction with Life Scale |
8/11** |
| Mortimer et al (2012) [46] | RCT | 120 community-living older adults (primary females) – 30 in each group
· IG1 – TC: 67.3±5.3 y.o., 19/30 females · IG2 – Walking: 67.8±5.0 y.o., 19/30 females · IG3 – Social: 67.9±6.5 y.o., 21/30 females · CG – No interventions: 68.2±6.5, 21/30 females
|
3/week for 40 wks
IG1: TC: 50 mins – 20min warm-up, 20 min TC and 10 min cool-down), 3 IG2: Walking: 50 mins – 10 warm-up, 30 min brisk walking, and 10 min cool-down in a 400-meter track IG3: Social interaction: 60 min for any topics CG: No interventions |
sMRI and fMRI | 9/11** |
CG: control group; fMRI: functional magnetic resonance imaging; IG: interventional group with Tai Chi as the intervention; MME: mini-mental status exam; MRS: magnetic resonance spectroscopy; NA: not applicable; RCT: randomized control trial; rsFC: resting state functional connectivity; sMRI: structural magnetic resonance imaging; TC: Tai Chi; y.o.: years old; min: minute(s); wk: week; wks: weeks; WOMAC: Western Ontario & McMaster Universities Osteoarthritis Index.*: Newcastle-Ottawa Scale assessment; **: PEDro scale assessment;
Results
Thirty-two articles from 24 studies were qualified for analysis (Table 1) [15-46]. There were 13 randomized control trials with 17 articles [16-18,20,24,25,27,28,32, 33,40-46], 8 cross-sectional studies with 12 articles [21-23,26,29-31,34,36-39], and 3 single group pre- and post- comparisons with 3 articles [15,19,35]. Among these 24 studies, 17 of them with 21 articles had elderly subjects who were 60 years and older [20-24,26,29-31,33-36,40-46], 4/24 studies with 8 articles had mixed age groups with subjects 21-70 years old [17,19,27,28,32,37-39], and 3 studies had healthy young subjects [16,18,25]. The majority of these studies used healthy subjects [15,16,18,20-31,33-46], but only four had subjects with a medical diagnosis of osteoarthritis [15], depression [19] or fibromyalgia [17,32]. Among 13 RCTs (17 articles), activities for the control groups or other intervention groups included normal daily activities [17,20,24,32], brisk walking [16,18,25,46], Baduanjin exercise [27,29,40-42], stationary biking [27, 29], health education [27,29,40-45] and social gathering interactions [46]. In 8 cross-sectional studies (12 articles), practitioners with 5 or more years of experiences in TC were compared with comparable subjects who walked every day [21, 22] or just non-TC practitioners [23,26,31,33,36-39].
With respect to quality assessment, 13 randomized controlled trials ranged from 6-9/11 in PEDro scale, indicating good to excellent studies [13]. Newcastle-Ottawa scale [14] showed 6-9 stars/10 in 8 cross-sectional studies (suggesting good to very good), and 5/10 in two sing-group cohort studies (indicating satisfactory), respectively.
Exercise Parameters Related to TC Effects on Neuroimaging Assessments
Based on 16 prospective studies (20 articles) in this review, including 13 randomized control trials (17 articles) and 3 pre-and post-intervention comparison studies (3 articles), the TC exercise parameters varied from study to study, but some of the parameters were commonly prescribed by many TC providers. The length of each TC practice session ranged from 30-40 minutes [20], 45 minutes [24], 50-60 minutes [15-19,27,28,32,33,35,40-46] with the most commonly used one being 50-60 minutes. The exercise frequencies were 2/week [17,19,32], 2-3/week [35], 3/week [15,16,18,20,24,25,33,43-46] and 5/week [27,28,40-42] with the most common one being 3 times a week. The duration for these studies varied from 6 weeks [43-45], 8 weeks [15,16,18,24,25], 10 weeks [19], 12 weeks [17,27,28,32,33,35,40-42], 16 weeks [20] to 40 weeks [46] with 12 weeks as the most commonly used. Put together, 60 minutes per session, 3 times a week for 12 weeks are the most commonly used TC parameters by TC researchers to investigate TC effects on the human brain. However, none of these 16 prospective studies did a follow-up after their TC interventions were completed, but the effects from a longer duration of TC practice are available from 8 cross-sectional studies (12 articles), in which subjects had been practicing TC for a minimum of 5 years and showed greater changes than the control groups [21-23,26,29-31,34,36-39].
TC Effects on Different Regions of Brain
As shown in Tables 1 and 2, TC practice is able to affect the whole brain by increasing total brain volume [46], the oxygenated hemoglobin (HbO2) in the motor cortex [29], and the white matter network connectivity locally and globally in the brain [21]. In each individual brain region, TC can affect many brain areas including the frontal, parietal, temporal, and occipital lobes, insula, basal ganglia, and cerebellum, among which the frontal lobe is the area that has been studied more than others (Table 2) [15-46].
Table 2: Effects of Tai Chi on Brain Assessed with Neuroimaging Techniques
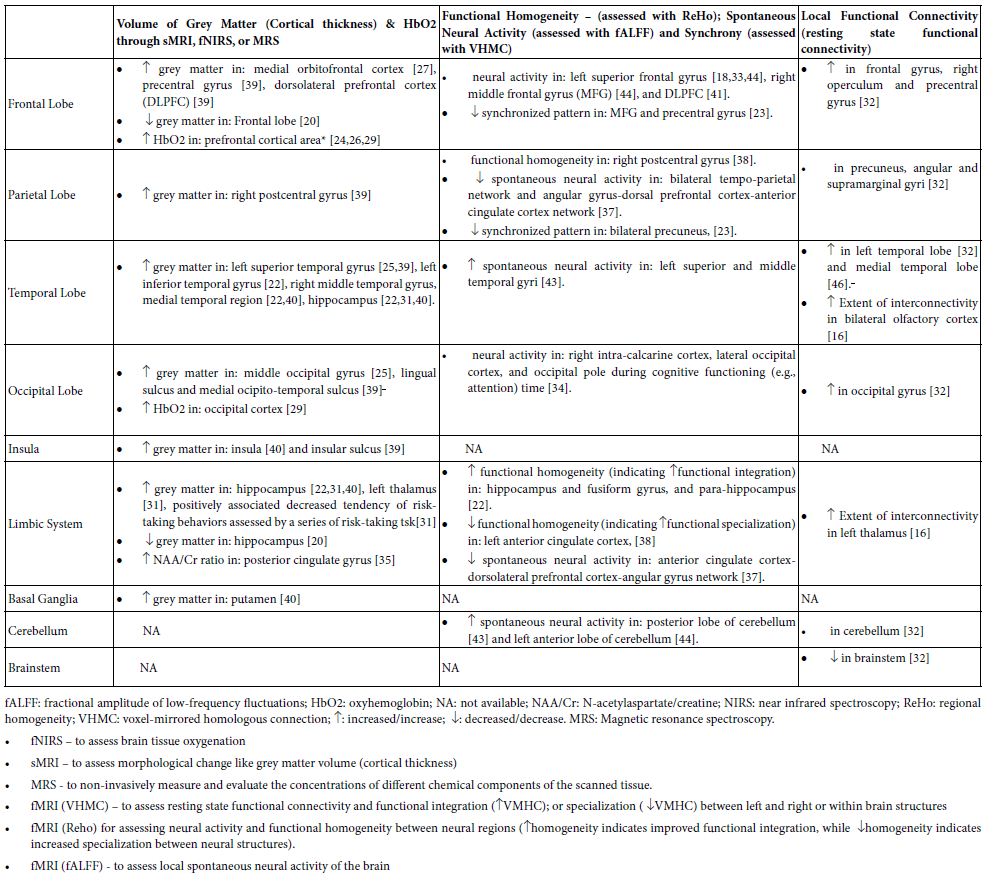
Frontal Lobe
TC practice is able to affect many regions of the frontal lobe (Table 2) by increasing 1) grey volume in the medial orbital prefrontal cortex [27], precentral gyrus [38], and dorsolateral prefrontal cortex (DLPFC) [39]; 2) oxyhemoglobin (HbO2) in the prefrontal cortical area [24,26,29]; and 3) neural activity in the left superior frontal gyrus [18,33;44]; right middle frontal gyrus (MFG) [44], and DLPFC [41]. The increased neural activity in the left superior frontal gyrus [33] and the dorsolateral prefrontal cortex [41] have been found to be associated positively and respectively with decreased error-making rates in switch/non-switch tasks [33], or with improved memory [41] in older community dwellers.
Further, increased connectivity was also reported locally in the frontal gyrus, right operculum, and precentral gyrus [32]. Decreased synchronized pattern in MFG and precentral gyrus as assessed by the VHMC technique was also found [23].
Parietal Lobe
The right postcentral gyrus shows increased cortical thickness and improved neural integration as indicated by increased functional homogeneity, which are positively associated with practitioners’ time length of TC experience and improvement of cognitive attention [39]. Increased functional connectivities were identified locally in precuneus, angular, and supramarginal gyri [32], and the middle frontal gyrus [25]. Decreased synchrony, as indicated by decreased VMHC, was seen in the precuneus, which is correlated with years of TC practice experience [23]
Temporal Lobe
Increased thickness of the cortex was identified in the left superior temporal gyrus [25,39], left inferior temporal gyrus [22], right middle temporal gyrus, and medial temporal region [22,40]. More spontaneous neural activities through fALFF assessment were detected in the left superior and middle temporal gyri [43]. The rsFC fMRI technique exerted greater functional connectivity locally in the left temporal lobe [32], medial temporal lobe [45], and bilateral primary olfactory cortex (in the lower temporal lobe) [16].
Occipital Lobe
Randomized controlled trials showed that 8-week TC practice can enhance the volume of grey matter in the middle occipital gyrus [25] and a 12-week TC program can increase resting state functional connectivity in the occipital gyrus [32]. Moreover, cross-sectional studies revealed that TC practitioners with a minimum of 5-year experience demonstrated 1) more volume of grey matter in the lingual sulcus and medial occipito-temporal sulcus [39], 2) increased HbO2 in occipital cortex [29], and 3) less activation of the right intra-calcarine cortex, lateral occipital cortex, and occipital pole during cognitive functioning (e.g., attention) time [34].
Insula and Limbic System
Grey matter can become thicker in the insula [39,40], hippocampus [20,31,40] and left thalamus [31]. Increase of the NAA/Cr (N-acetyl aspartate/creatine) ratio, a biomarker of brain functionality, was found in posterior cingulate gyrus [35]. Functional homogeneity was increased in the hippocampus, fusiform gyrus, and para-hippocampus [22], but decreased in the left ACC which is associated with years of TC experience [38]. Also increased extent of interconnectivity was identified within the left thalamus [16].
Basal Ganglia, Thalamus, Cerebellum, and Brainstem
After TC practice, neuroimaging techniques showed more grey matter in the putamen [40], and more spontaneous neural activity in the anterior and posterior lobes of the cerebellum [43,44], but decreased neural activity in brainstem [32].
Inter-regional Connectivity
As shown in Table 3, changes of inter-regional functional connectivity after TC practice were identified between different brain regions, among which the frontal lobe [15,19,25,27,30,32,37,42,45] has many more structures to make such inter-regional connections than any other neural lobes or brain areas, followed by the temporal lobe [19,42,45], limbic system [17,19,27,32,37], parietal lobe [19,25,37], insula [19], basal ganglia [30], and brainstem [32]. In the frontal lobe, the following structures, including superior, middle, and inferior frontal gyri, dorsolateral prefrontal cortex, medial prefrontal cortex, medial orbito-frontal cortex, and supplementary motor cortex, have either increased or decreased inter-regional connectivity with structures outside the frontal lobe (Table 3). In the temporal lobe and limbic system, the superior temporal gyrus, medial temporal lobe, hippocampus, cingulate gyrus, amygdala, and hypothalamus are involved in the TC-caused inter-regional connectivity. Further, the superior parietal lobule and angular gyrus in the parietal lobe, the ventral striatum in basal ganglia, the insula, and the ventral tegmentum and periaqueduct grey in the brainstem are involved as well (Table 3). Many of these connectivities are increased after TC exercise as seen in the left column of Table 3, while some of these connectivities are decreased in the right column of the table.
Table 3: Tai Chi Effects on Inter-Regional Resting-State Functional Connectivity
|
Increased Connectivity between |
Decreased Connectivity between |
|
|
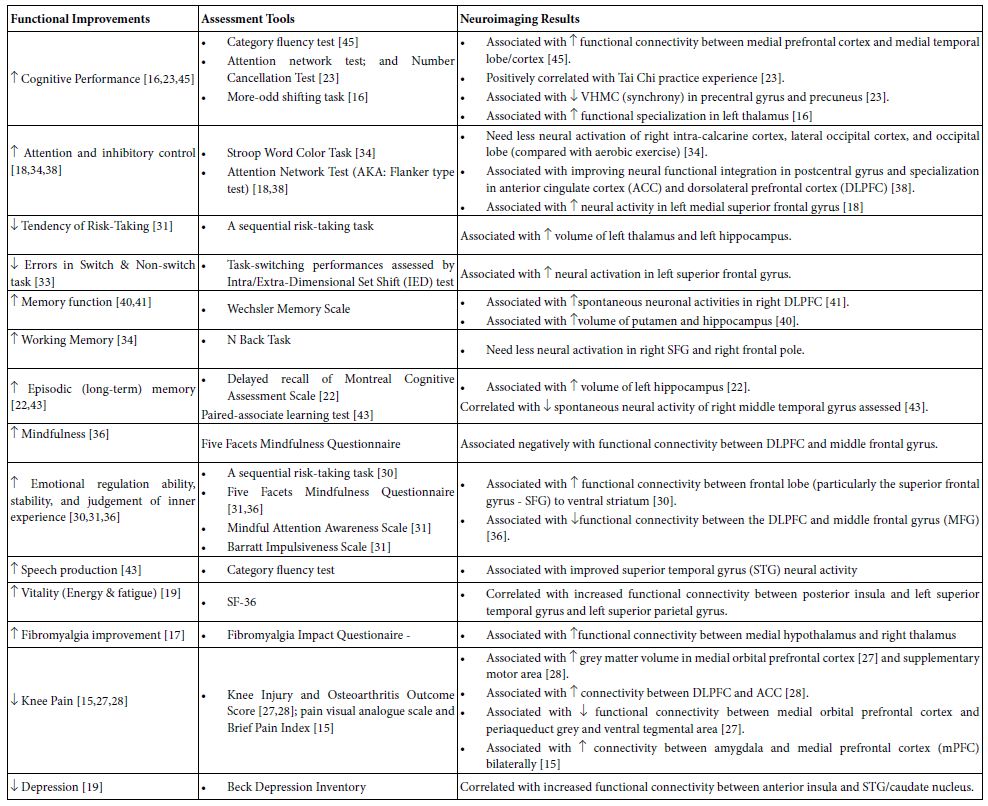
Neuropsychological Functional Assessments
Neuropsychological functions were assessed in some studies and their associations with TC-caused neuroimaging changes in the brain were presented in Table 4. These studies used a variety of instruments to assess neuropsychological functions and to see how they may associate with neuroimaging changes after the TC intervention. As seen in Table 4, changes of inter-regional neural connectivity and cortical thickness (grey matter volume) were found to be associated with neuropsychological improvements such as cognition [16,18, 22, 23, 30, 31, 33, 34, 36, 38,40,41,43,45], vitality [19], depression [19], pain [15,27,28], and even the overall aspects of the fibromyalgia [17]. The cognition-related improvements may include general cognitive performance [16,23,45], attention and increased inhibitory control during attention [18,34,38], decreased errors in switch and non-switch task [33] and decreased tendency of risk-taking [31], different memory performance [22,34,40,41,43], mindfulness [36], emotional stability and judgement of inner experience [30,31.36], and speech production [43]. For examples, emotion regulation ability [30], cognitive performance [45], depression [19] and vitality [19] improvements are positively and respectively correlated with increased connectivities between the left superior frontal cortex and ventral striatum [30], between the medial prefrontal cortex and medial temporal lobe [45], between the right ACC and superior temporal gyrus [19], or between the right posterior insula and both the left superior temporal gyrus and left superior parietal gyrus [19]. Other the other hand, decreased tendency of risk-taking behavior [31] seems to parallel with increased cortical thickness in the hippocampus and thalamus [31]. In patients with knee arthritis, decreased knee pain was positively associated 1) with increased volume of grey matter in the medial orbitofrontal cortex [24] and supplementary motor cortex [28], 2) with increased connectivity between the dorsolateral prefrontal cortex (DLPFC) and anterior cingulate cortex (ACC) [27,28], but 3) with decreased connectivity in the medial orbito-frontal cortex and both periaqueduct grey and ventral tegmental area [27,28]. In addition, patients with fibromyalgia have significant improvement in functional, overall, and symptoms aspects as assessed with the Fibromyalgia Impact Questionnaire after 12-week TC practice. The improvement is positively correlated with the increased connectivity between the medial hypothalamus and the right thalamus [17].
Table 4: Function Improvements in Association with Neuroimaging Results after Tai Chi (TC) Practice
Discussion
Local Neural Responses in Different Brain Areas following TC Practice
In the frontal lobe, increased grey matter thickness, spontaneous neural activities, and/or local connectivities of the medial prefrontal cortex, dorsolateral prefrontal cortex (DLPFC), medial orbitofrontal cortex (mOFC), precentral gyrus, superior and middle frontal gyri, and frontal operculum were all reported in TC practitioners (Table 2). Functionally, the medial prefrontal cortex is responsible for memory and decision making [47], the mOFC is for goal-directed decision-making [48], DLPFC is for top-down attentional and cognitive control [49], the precentral gyrus is the primary center for voluntary motor movement [50], the superior frontal gyrus is for self-awareness of individual personality [50], and the right middle frontal gyrus is a convergence site of attention networks for higher order cognition and motor-related information processing [51]. The frontal operculum plays an important role in a network controlling the process of cognitive tasks [52]. With these together, TC practice might be able to improve neuropsychological and related neuromuscular behaviors such as memory, attention, cognitive attention, decision-making, sensorimotor integration, motor execution and control, and individual self-awareness through influence on these structures in the frontal lobe. How these different structures within the frontal lobe work in a coordinated way is not clear, but an improved functional specialization in frontal lobe structures might be an explanation. For instance, a VHMC study showed reduced homogeneity in the middle frontal gyrus and precentral gyrus [23], which may indicate an increased functional specialization of these two structures after TC practice.
In the parietal lobe, the right post-central gyrus is the only area that showed increased cortical thickness and spontaneous neural activity [38,39], while increased local connectivities were seen in the precuneus and inferior parietal lobule (angular and supramarginal gyri) [32]. Functional considerations of these structures, like the post-central gyrus for primary somatosensory processing, the precuneus for executive function, default network of self-consciousness, and mental imagery strategies and episodic memory retrieval [53], and inferior parietal lobule for recognition memory, language, and perception of emotion [54], may indicate that TC practice is able to improve sensory integration of higher-order motor execution, memory, emotion, and mental imagery strategies for motor actions [32,37,38]. Further, these TC studies [32,37,38] showed that TC experience is positively associated with increased neural activity in the right post-central gyrus [38], but negatively with decreased synchrony in bilateral precuneus as indicated by decreased VMHC value [23]. So, it is possible to assume that the longer one practices TC, the more improved general sensory is shown in certain parts (e.g., the left side) of the body, which might be through decreased synchrony between the left and right precuneus. However, whether and how such an assumption is holdable surely needs more future studies.
The temporal lobe and its superior, middle, and inferior temporal gyri showed increased volume of grey matter [22,25,39,40],spontaneous neural activity [43], and local functional connectivity [32] after TC practice. The same changes were also seen in the medial temporal lobe [22,40,45]. Comparatively the left temporal lobe showed more changes [22,25,32,39,43] than the right one (mainly the right middle temporal gyrus) [40]. The temporal lobe is functionally responsible for emotion, memory, and awareness of special sensation, and the left (dominant) side is more involved in language understanding [55,56]. Thus, it is understandable that TC could be a good exercise choice to improve emotion, memory, auditory and visual sensory, and even language perception. The medial temporal lobe that includes the hippocampus and para-hippocampus will be discussed in the paragraph of the limbic system below.
The occipital lobe showed increased oxyhemoglobin (HbO2), total hemoglobin (cHb) [29] and local connectivity [32] following TC intervention. Also, increased thickness of grey matter was noticed in the middle occipital gyrus [25], lingual sulcus, and medial occipito-temporal gyrus [42]. With consideration of functions of the occipital lobe [57], reactions to TC practice in the middle occipital gyrus and occipital cortex in general may hint that the occipital lobe could be morphologically changed to some extent [36,39], and likely participate in improving cognition and anti-memory decline [26,29], as well as improving spatial recognition and perception of objects [57] after TC practice.
In parts of the limbic system, studies demonstrated increased 1) grey matter in the insula [39,40], medial temporal gyrus [40], left thalamus [31], and hippocampus [22,31,40]; 2) increased spontaneous neural activity in hippocampus [22], fusiform gyrus [22], and para-hippocampus [22]; 3) increased extent of interconnectivity in left thalamus [16]; 4) increment of N-acetylaspartate/creatine ratio (indicating neuronal growth) in posterior cingulate gyrus [35]; and 5) increased functional specialization in anterior cingulate cortex [38]. On the other hand, decreased grey matter was found decreased [20] and decreased neural connectivity was identified in the anterior cingulate cortex and dorsolateral prefrontal cortex-angular gyrus network [37]. The insula has been regarded as a limbic system structure in respect to visceral sensation and autonomic control, but it also takes part in functions of pain processing, empathy, social cognition, attention, and decision making [60]. The medial temporal lobe (including hippocampus, para-hippocampus, and amygdala) is associated with emotion learning and behavior, as well as memory encoding, consolidation, storage, and retrieval [59,60], particularly for episodic and spatial memory [60]. A decrease in size of the posterior cingulate gyrus has been reported to play a role in cognition by influencing attentional focus by ‘tuning’ whole-brain metastability [61]. Additionally, the fusiform gyrus is responsible for object and face recognition [62] and semantic memory [63], and the thalamus is a relay hub for multiple sensory information and even memory [64,65]. However, reduction of grey matter in the hippocampus was recently reported when TC intervention was combined in an exercise program (30-40 minutes per total session) including TC-inspired exercise, dancing, and cognitive game [20], in which the TC time for each session was not long enough [20]. With respect to these studies about TC effects on the limbic system [20,22,31,35,39,40], generally speaking, they may indicate that TC is likely able to improve the practitioners’ emotion, memory, visceral and somatosensory capability, decision making, pain processing, and attention. TC may also be able to subsequently reduce the cognitive decline through the limbic system including the medial temporal lobe [66] if the TC practitioners have practiced over 5 years [22,31] or have practiced in longer duration (60 minutes each) on a daily basis [38].
In the basal ganglia and cerebellum, responses to TC exercise include increased grey matter in the putamen [40], and increased neural activity [43,44] and local connectivity in the cerebellum [32]. Literature has suggested that the putamen is functionally responsible for movement execution, working memory [67], and cognition [68]. Besides motor learning and coordination, the cerebellum is also for cognition and emotional processing (particularly the posterior cerebellar lobe) [69]. Injury to the cerebellum may cause cerebellar cognitive affective syndrome [70]. This information indicates that TC may improve motor execution, working memory and cognition through the putamen and cerebellum.
Inter-regional Connectivity after TC Practice
In addition to increased functional connectivity in each individual brain region (Table 2), there are also many inter-regional connectivities influenced by TC practice (Table 3, among which there are more increased such connectivities (see the left column of Table 3) than those decreased (see the right column of Table 3). We speculate that the increased inter-regional connectivities may suggest functional integration of different brain regions while the decreased inter-regional connectivities may indicate functional specialization of different regions. With consideration of morphological changes in each individual brain region after TC exercise, these individual brain regions might be able to functionally respond to TC practice differently depending on functions executed by these regions.
Functional Consideration
Given involved brain regions detailed in Tables 2 and 4, we can easily see that many of them are functionally and positively associated with neuropsychological behaviors. These behavior improvements were assessed with different neuropsychological instruments. For examples, following TC interventions, positive correlations were reported in improvement of 1) reduced risk-taking behavior as assessed by a series of risk-taking tasks [31]; 2) decreased error-making assessed with the switch-non-switch task [41]; 3) cognitive performance assessed by Category fluency test and attention network test [23,45]; 4) emotional regulation and stability assessed by Five Facets Mindfulness Questionnaire and Barratt Impulsiveness scale [31,36]; 5) depression by Beck Depression Inventory [19]; 6) vitality (energy and fatigue) assessed with SF-36 [19]; 7) memory as assessed by Wechsler Memory Scale [40,41] including working memory and episodic (long-term) memory [22,43]; and even knee pain as assessed by Knee Injury & Osteoarthritis Outcome Score [15,27,28]. These findings suggest that TC can be utilized not only as a physical but also a cognitive exercise, which may work by modulating both the local regional morphologies and inter-regional brain connectivity networks to improve the brain’s neural functions.
Study Limitations
There are several limitations that should be mentioned. First, due to the barrier to resources in non-English language, we were not able to access articles that were published in non-English literatures. Second, 10 out of 21 qualified studies are randomized control trials, but others include 8 cross-sectional studies, 2 single group pre- and post-TC comparisons, and 1 single-case report may reduce the level of evidence for this review study. Third, seed-based analysis is often used in resting state MRI in which a neural region of interest (ROI) is selected to determine how other regions interested by the investigators may correlate to the ROI. However, the obvious downside of such a method is that it depends on the investigators’ assumption for the ROI selection [71]. If a different ROI was picked, the involved brain regions might vary.
Conclusions
In the last 10 years, as neuroimaging techniques develop, more morphological changes of the human brain after TC practice have been investigated and identified in the frontal, temporal, parietal, and occipital lobes, insula, limbic system, basal ganglia, cerebellum, and brainstem, with the frontal and temporal lobes having more changes than other regions. These changes include increased cortical thickness or grey matter volume, altered local spontaneous neural activity, as well as changed inter-regional functional connectivities. Also, many of these changes are associated with improvements of many neuropsychological behaviors such as cognitive attention, memory, depression, vitality, risk-taking task, error-making tests, and even pain reduction. All of these imply that TC can be a great exercise program to improve the practitioners’ neural dysfunctions. However, so far, many brain structures have been found to be affected by TC exercise, but why and how only these structures are involved in response to TC practice are still not fully understood. Future studies are needed to assess how the structures are involved and how functionally some of these structures are integrated and/or specialized post-TC interventions.
Author Contributions
All five authors had substantially contributed to the conception and design of the article and interpreting the relevant literature. HL drafted the article and revised it critically with YS, SA, CN and CH for important intellectual content.
References
- Symms M, Jager HR, Schmierer K, Yousry TA (2004) A review of structural magnetic resonance neuroimaging. J Neurol Neurosurg Psychiatry 75: 1235-1244. [crossref]
- Ayaz H, Izzetoglu M, Izzetoglu K, Onaral B (2019) The use of functional near-infrared spectroscopy in neuroergonomics. In: H. Ayaz, F. Dehais: Neuroergonomics: The brain at Work and in Everyday Life. Academic Press 17-25.
- Liu H (2019) Tai Chi. In Chapter 39: Complementary and Integrative Therapies. (In: Umphred’s Neurological Rehabilitaion.7th Lazaro RT, Reina-Guerra SG, Quiben Q). Page 1071-1074. Mosby; Maryland Heights, Missouri.
- Lan C, Chen SY, Lai JS, Wong AMK (2013) Tai Chi Chuan in medicine and health promotion. Evid Based Complement Altern Med 2013: 502131. [crossref]
- Zhou D, Shepard RJ, Plyley MJ, Davis GM (1984) Cardiorespiratory and metabolic responses during TC Chuan exercise. Can J Appl Sport Sci 9: 7-10. [crossref]
- Liu H, Connors M, Grando V, Liu H (2012) Tai Chi intervention for older adults using assistive devices in a senior living community: A pilot study. Int J Ther Rehab 19: 136-142.
- Bonifonte P (2004) T’Ai Chi for Seniors: How to Gain Flexibility, Strength, and Inner Peace. New Page Book, Franklin Lakes, NJ.
- Liu H, Frank A (2010) Tai Chi as a Balance Improvement Exercise for Older Adults: A Systematic Review. J Geriat Phys Ther 33: 103-109. [crossref]
- Winser SJ, Tsang WWN, Krishnamurthy K, Kannan P (2018) Does Tai Chi improve balance and reduce falls incidence in neurological disorders? A systematic review and meta-analysis. Clin Rehabil 32: 1157-1168. [crossref]
- Glover GH (2011) Overview of functional magnetic resonance imaging. Neurosurg Clin N Am 22: 1333-1339. [crossref]
- Soares DP, Law M (2009) Magnetic resonance spectroscopy of the brain: review of metabolites and clinical applications. Clin Radiol 64: 12-21. [crossref]
- Wei J, Wei SB, Yang RX, Yang Lu, Yin Q, et al. (2018) Voxel-mirrored homotopic connectivity of resting state functional magnetic resonance imaging in blepharospasm. Front Psychol 9: 1620. [crossref]
- Verhagen AP, de Vet HCW, de Bie RA, kessels AG, Boers M, et al. (1998) The Delphi list: a criteria list for quality assessment of randomised clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 51: 1235-1241. [crossref]
- Wells G, Shea B, O’Connell D, et al. (2017) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis.
- Shen CL, Watkins BA, Kahathuduwa C, Chyu MC, Zabet-Moghaddam M, et al. (2022) Tai Chi improves brain functional connectivity and plasma lysophosphatidylcholines in postmenopausal women with knee osteoarthritis: An exploratory pilot study. Front Med 8: 775344. [crossref]
- Cui L, Tao S, Yin HC, Shen QQ, Wang Y, et al. (2021) TCChuan Alters Brain Functional Network Plasticity and Promotes Cognitive Flexibility. Front Psychol 22: 665419. [crossref]
- Kong J, Huang YT, Liu J, Yu SY, Ming C, et al. (2021) Altered functional connectivity between hypothalamus and limbic system inMol Med 14: 17. [crossref]
- Shen QQ, Yin HC, Cui L, Zhang JY, Wang DL, et al. (2021) The Potential Advantages ofTCChuan in Promoting Inhibitory Control and Spontaneous Neural Activity in Young Adults. Front Behav Neurosci 15: 747733.
- Xu A, Zimmerman CS, Lazar SW, Ma Y, Kerr CE (2020) Distinct insular functional connectivity changes related to mood and fatigue improvements I major depression disorder following Tai Chi training: a pilot study. Front Integra Neurosci 14: 25. [crossref]
- Adcock M, Fankhauser M, Post J, Lutz K, Zizlsperger L, et al. (2020) Effects of an In-home multicomponent exergame training on physical functions, cognition, and brain volume of older adults: A randomized controlled trial. Front Med 6: 321.
- Yue C, Zou L, Mei J, Moore D, Herold F, et al. (2020) Tai Chi training evokes significant changes in brain white matter network in older women. Healthcare 8: 57.
- Yue C, Yu Q, Zhang Y, Herold F, Mei J, et al. (2020) Regular Tai Chi practice is associated with improved memory as well as structural and functional alterations of the hippocampus in the elderly. Front Aging Neurosci 12: 586770. [crossref]
- Chen LZ, Yuan X, Zhang Y, Zhang S, Zou L, et al. (2020) Brain functional specialization is enhanced among Tai Chi Chuan practitioners. Arch Phys Med Rehabil 101: 1176-1182. [crossref]
- Yang Y, Chen T, Shao M, Yan S, Yue GH, et al. (2020) Effects of Tai Chi Chuan on inhibitory control in elderly women: An fNIRs study. Front Human Neurosci 13: 476. [crossref]
- Cui L, Yin HC, Lvu SJ, Shen QQ, Wang Y, et al. (2019) Tai Chi Chuan vs General aerobic exercise in brain plasticity: a multimodal MRI study. Sci Rep 9: 17264.
- Tsang WWN, Chan KK, Cheng CN, Hu FSF, Mak CTK, et al. (2019) Tai Chi practice on prefrontal oxygenation levels in older adults: a pilot study. Complement Ther Med 42: 132-136. [crossref]
- Liu J, Chen L, Chen X, Hu K, Tu Y, et al. (2019) Modulatory effects of different exercise modalities on the functional connectivity of the periaqueductal grey and ventral tegmental area in patients with knee osteoarthritis: a randomized multimodal magnetic resonance imaging study. British J Anaesthesia 123: 506-518. [crossref]
- Liu J, Chen L, Tu Y, Chen X, Hu K, et al. (2019) Different exercise modalities relieve pain syndrome in patients with knee osteoarthritis and modulate the dorsolateral prefrontal cortex: A multiple mode MRI study. Brain Behav Immunity 82: 253-263. [crossref]
- Xie X, Zhang M, Huo C, Xu G, Li Z, et al. (2019) Tai Chi Chuan exercise related change in brain function as assessed by functio0nal near-infrared spectroscopy. Sci Rep 9: 13198. [crossref]
- Liu Z, Li L, Liu S, Sun Y, Li S, et al. (2020) Reduced feelings of regret and enhanced fronto-striated connectivity in elders with long-term Tai Chi experience. Soc Cogn Affect Neurosci 15: 861-873.
- Liu S, Li L, Liu Z, Guo X (2019) Long term Tai Chi experience promotes emotional stability and slows gray matter atrophy for elders. Front Psychol 10: 91. [crossref]
- Kong j, Wolcott E, Wang Z, Jorgenson K, Harvey WF, et al. (2019) Altered resting state functional connectivity of the cognitive control network in fibromyalgia and the modulation effect of intervention. Brain Imaging Behav 13: 482-492. [crossref]
- Wu MT, Tang PF, Goh JOS, Chou TL, Chang YK, et al. (2018) Task-switching performance improvements after Tai Chi Chuan training are associated with greater prefrontal activation in older adults. Front Aging Neurosci 10: 280. [crossref]
- Port AP, Santaella DF, Lacerda SS, Speciali DS, balardin JB, et al. (2018) Cognition and brain function in elderly Tai Chi practitioners: A case-control study. Explore 14: 352-356. [crossref]
- Zhou M, Liao H, Sreepada LP, Ladner JR, Balschi JA, et al. (2018) Tai Chi improves brain metabolism and muscular energetics in older adults. J Neuroimaging 28: 359-364. [crossref]
- Liu Z, Wu Y, Li L, Guo X (2018) Functional connectivity within the executive control network mediates the effects of long-term Tai Chi exercise on elders’ emotion regulation. Front Aging Neurosci 10: 315. [crossref]
- Wei GX, Gong XQ, Yang Z, Zuo XN (2017) Mind-body practice changes fractional amplitude of low frequency fluctuations in intrinsic control networks. Front Psychol 8: 1049. [crossref]
- Wei GX, Dong HM, Yang Z, Luo J, Zuo XN (2014) Tai Chi Chuan optimizes the functional organization of the intrinsic human brain architecture in older adults. Front Aging Neurosci 6: 74. [crossref]
- Wei GX, Xu T, Fan FM, Dong HM, Jiang LL, et al. (2013) Can Taichi reshape the brain? A brain morphometry study. PLosOne 8: e61038. [crossref]
- Tao J, Liu J, Liu W, Huang J, Xue X, et al. (2017) Tai Chi Chuan and Baduanjin increases grey matter volume in older adults: a brain imaging study. J Alzheimers Dis 60: 389-400. [crossref]
- Tao J, Chen X, Liu J, Egorova N, Xue X, et al. (2017) Tai Chi Chuan and Baduanjin mind-body training changes resting-state low-frequency fluctuations in the frontal lobe of older adults: A resting-state fMRI study. Front Human Neurosci 11: 514. [crossref]
- Tao J, Liu J, Egorova N, Chen X, Sun S, et al. (2016) Increased hippocampus-medial prefrontal cortex resting-state functional connectivity and memory function after Tai Chi Chuan practice in elder adults. Front Aging Neurosci 8: 25. [crossref]
- Zheng Z, Zhu X, Yin S, Wang B, Niu Y, et al. (2015) Combined cognitive-psychological-physical intervention induces reorganization of intrinsic functional brain architecture in older adults. Neural Plasticity 2015: 713104.
- Yin SF, Zhu XY, Li R, Niu Y, Wang B, et al. (2014) Intervention-induced enhancement in intrinsic brain activity in healthy older adults. Rep 4: 7309. [crossref]
- Li R, Zhu X, Yin S, Niu Y, Zheng Z, et al. (2014) Multimodal intervention in older adults improves resting-state functional connectivity between the medial prefrontal cortex and medial temporal lobe. Front Aging Neurosci 6: 39. [crossref]
- Mortimer JA, Ding D, Borenstein AR, Carli CD, Guo Q, et al. (2012) Changes in brain volume and cognition in a randomized trial of exercise and social interaction in a community-based sample of non-demented Chinese elders. J Alzheimer’s Dis 30: 757-766. [crossref]
- Euston DR, Gruber AJ, McNaughton BL (2012) The role of medial prefrontal cortex in memory and decision making. Neuron 76: 1057-1070. [crossref]
- Gourley SL, Zimmermann KS, Allen AG, Taylor GR (2016) The medial orbitofrontal cortex regulates sensitivity to outcome value. J Neurosci 36: 4600-4613. [crossref]
- Brosnan MB, Wiegand I (2017) The dorsolateral prefrontal cortex, a dynamic cortical area to enhance top-down attentional control. J Neurosci 37: 3445-3446. [crossref]
- Petrides M, Pandya DN. The Frontal Cortex. In: Mai JK, Paxinos G: The Human Nervous System (3rd). Academic Press, Cambridge, MA. 2012. Page 988-1011.
- Japee S, Holiday K, Satyshur MD, Mukai I, Ungerlieder LG (2015) A role of right middle frontal gyrus in reorienting of attention: a case study. Front Syst Neurosci 9: 23. [crossref]
- Higo T, Nars RB, Boorman ED, Buch ER, Rushworth MFS, et al. (2011) Distributed and causal influence of frontal operculum in task control. Proc Natl Acad Sci USA 108: 4230-4235. [crossref]
- Cavanna AE, Trimble MT (2006) The precuneus: a review of its functional anatomy and behavioral correlates. Brain 129: 563-584. [crossref]
- O’Connor AR, Han S, Dobbins IG (2010) The inferior parietal lobule and recognition memory: Expectancy violation or successful retrieval? J Neurosci 30: 2924-2934. [crossref]
- Kiernan JA (2012) Anatomy of the temporal lobe. Epilepsy Res Treat 2012: 176157.
- Aversi-Ferreira TA, Tamaishi-Watanabe BH, Magri MPF, Ferreira RAGMFA (2019) Neuropsychology of the temporal lobe – Laria’s and contemporary conceptions. Dement Neuropsychol 13: 251-258. [crossref]
- Renier LA, Anurova I, De Volder AG, Carlson S, Vanmeter J, et al. (2010) Preserved functional specialization for spatial processing in the middle occipital gyrus of the early blind. Neuron 68: 138-148. [crossref]
- Uddin LQ, Nomi JS, Hebert-Seropian B, Ghaziri J, Boucher O (2017) Structures and function of the human insula. Clin. Neurophysiol 34: 300-306. [crossref]
- Squire LR, Stark CEL, Clark RE (2004) The medial temporal lobe. Annu Rev Neurosci 27: 279-306. [crossref]
- Cutsuridis V, Yoshida M (2017) Memory processes in medial temporal lobe: experimental, theoretical and computational approaches. Front Syst Neurosci 11: 00019.
- Leech R, Sharp DJ (2014) The role of the posterior cingulate cortex in cognition and disease. Brain 137: 12-32. [crossref]
- Weiner KS, Zilles K (2016) The anatomical and functional specialization of the fusiform gyrus. Neuropsychol 83: 48-62. [crossref]
- Ding JH, Chen K, Chen Y, Fang Y, Yang Q, et al. (2016) The left fusiform gyrus is a critical region contributing to the core behavioral profile of semantic dementia. Front Hum Neurosci 10: 215. [crossref]
- Fama R, Sullivan EV (2015) Thalamic structures and associated cognitive functions: relations with age and aging. Neurosci Biobehav Rev 54: 29-37. [crossref]
- Wolff M, Vann SD (2019) The cognitive thalamus as a gateway tp mental representations. J Neurosci 39: 3-14. [crossref]
- Ferreira LK, Diniz BS, Forlenza OV, Busatto GF, Zanetti MV, et al. (2011) Neurostructural predictors of Alzheimer’s disease: A meta-analysis of VBM studies. Neurogiol Aging 32: 1733-1741. [crossref]
- Arsalidou M, Duerden EG, Taylor MJ (2013) The center of the brain: Topographical model of motor, cognitive, affective, and somatosensory functions of the basal ganglia. Human Brain Mapp 34: 3031-3054. [crossref]
- Middleton FA, Strick PL (2002) Basal ganglia “projections” to the prefrontal cortex of the primate. Cereb Cortex 12: 926-935. [crossref]
- Stoodley CJ, Valera EM, Schmahmann JD (2012) Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage 59: 1560-1570. [crossref]
- Schmahmann JD (2004) Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiat Clin Neurosci 16: 367-378. [crossref]
- Lee MH, Smyser CD, Shimony JS (2013) Resting-state fMRI: a review of methods and clinical applications. Am J Neuroradiol 34: 1866-1872. [crossref]