Abstract
Dentine sensitivity (DS) is a common painful condition affecting teeth. The exact mechanism of transmission of an environmental stimulus across dentine is not fully understood; currently the most accepted theory is the hydrodynamic theory as proposed initially by Brannstrom. Treatment has been concentrated on either reducing the dentine fluid flow by occlusion of tubule openings, or altering the pulpal sensory nerve activity preventing transmission of pain to the central nervous system.
Aims and Methods: The aims of the present in vitro study were to examine the dentine tubule occluding and penetrating properties of selected in-office desensitizing agents (varnishes and primers) using scanning electron microscopy and a dentine disc model.
Results: Of the products examined, the fluoride varnishes, Bifluoride 12 and Duraphat, and Cervitec (a chlorhexidine containing varnish) were effective in both occluding and penetrating the dentine tubules. The results from All Bond 2, One Step and Scotchbond primers were superior to those of HEMA group primers, Gluma 3, Gluma CPS and Solobond Plus.
Conclusions: These findings suggest a mechanism for the action of these potential desensitising agents and suggest that the tubule penetrating properties may play a role in the longevity of their retention on the tooth. Investigation of surface coverage and tubule penetrating characteristics are both necessary in order to fully evaluate in vitro the desensitising potential of agents claimed to reduce Dentine Sensitivity.
Introduction
Dentine sensitivity (DS) can be defined as a pain arising from exposed dentine typically in response to chemical, thermal, tactile, or osmotic stimuli, which cannot be explained as arising from any other form of dental defect or pathology [1]. Currently, the most accepted theory of stimulus transmission across dentine is the hydrodynamic theory [2] proposed initially by Brannstrom [3]. According to this theory, minute fluid shifts across dentine in either direction in response to thermal, tactile, chemical or osmotic stimuli can stimulate mechanoreceptors in or near the pulp, which in turn excite the pulp sensory nerves to cause pain.
Pashley [4] reported that there are two approaches in the treatment of DS, 1) partial or complete occlusion of the dentine tubules and 2) alteration of pulpal sensory nerve activity (SNA) at or near the pulpo-dentinal surface. If the hydrodynamic theory of intradental nerve stimulation is accepted then the treatment of DS through tubule occlusion is a feasible and reasonable approach.
Clinically, DS has been treated by numerous agents, in-office and over the counter (OTC), which have claimed to effectively reduce pain arising from exposed dentine. Laboratory evaluation of these desensitizing agents using the dentine disc model has been reported in several studies [5,6], although, Mordan et al. [7] have modified this model to establish a more precise methodology control to evaluate potential desensitizing agents.
The aims of the present study were to investigate the degree of dentine surface coverage and the extent of tubule occlusion and penetration of selected in-office varnishes and primers. A dentine disc model and qualitative scanning electron microscopy (SEM) were used.
Methods and Materials
Surgically extracted, carious free, unerupted third molars were fixed in 3% glutaraldehyde in 0.1M sodium cacodylate buffer (CAB) solution (pH 7,4) at 4°C for up to one week. 1mm thick dentine discs were obtained from the region below the crown and above the pulp using a Testbourne diamond saw and stored in 0.1 M CAB at 4°C until required.
Before use, the dentine discs were ultrasonicated for 30 seconds in distilled water to dislodge cutting debris. The smear layer was then removed using either 6% citric acid for two minutes or the appropriate etchant provided by the manufacturer and applied according to their instructions. The discs were rinsed in distilled water and marked on either side for orientation (Figure 1) before being broken into halves using dental pliers to provide control and test sections [7].
Figure 1: Marking of dentine discs used in the study
The selected desensitising agents and their ingredients, according to the manufacturer’s information, are shown in Tables 1a and 1b. During the study all the products were coded and at least two discs were used for each agent. The selected desensitising agents were applied onto the test halves according to manufacturer’s instructions. The test and control halves were then allowed to dry in a desiccator for at least 24 hours.
Table 1a: Formulation and manufacturer information of test agents: Varnishes
|
Test agent |
Manufacturer |
Active ingredients |
| Bifluoride 12 | Voco GMBH, 27457, Cuxhaven, Germany | Fluoride varnish made of synthetic resin. Sodium and calcium fluoride |
| Cervitec | Vivadent Ets. Shaan
Liechtenstein, Germany |
1 g contains: 0.010 g chlorhexidine, 0.010 g thymol Polyvinyl Butyral (varnish) Ethanol, ethyl acetate |
| Duraphat | Previously Rhone-Poulenc Rover GMBH, Nattermannallee 150829, Koln, Germany (Current Manufacturer Colgate Palmolive Company, USA) | An alcoholic suspension of natural resins containing 5% NaF |
Table 1b: Formulation and manufacturer information of test agents: Primers
|
Test agent |
Manufacturer |
Active ingredients |
| Hema-Seal G | Germipherne Corporation
Ontario, Canada |
HEMA, Glutaraldehyde, Sodium fluoride, Water |
| Gluma 3 | Bayer Dental
Leverkusen, Germany |
Aqueous solution of gluteral (Glutaraldehyde), HEMA |
| Gluma CPS | Bayer Dental
Leverkusen, Germany |
36,1% HEMA, 5,1% Glutaraldehyde, 58,8% Water |
| Solobond Plus
Universal Bonding Agent |
Voco,
Cuxhaven, Germany |
Methacrylate Acetone |
| All Bond 2
Universal Adhesive System Dual cured |
Bisco Inc.
Itasca, IL, 60143 Illinois, USA |
N-phenylglycine-glycine methacrylate and bisphenyl dimethacrylate. |
| Scotchbond
Multi-purposed Dental Adhesive System |
3M Dental Products
Saint Paul, MN 55144 1000, USA |
HEMA and BIS-GMA |
| One Step
Universal Dental Adhesive System |
Bisco Inc.
Itasca, IL 60143 Illinois, USA |
BPDM monomer dissolved in an acetone solution |
A separate series of discs was prepared following the protocol described above, but both control and test halves were carefully fractured into quarters providing longitudinal surfaces for examination of the tubule lumen contents.
After drying in the desiccator, the discs were attached to SEM stubs and sputter coated using a Polaron E5000 sputter coater (Polaron U.K.) with a layer of gold/palladium. The specimens were viewed in a FEI/Philips XL30 FEG SEM (FEI, Eindhoven, Netherlands) at a working distance of 10 mm.
Micrographs were taken from selected fields in the central portion of each half disc on either side of the fractured edge and the test surfaces were only compared with the corresponding controls. Comparison of the test products’ ability to block and penetrate the dentinal tubules was also subjectively assessed using the micrographs.
Results
An example of a control disc with both surface and fractured profiles can be observed in Figure 2a showing open dentinal tubules in both views (surface and fractured).
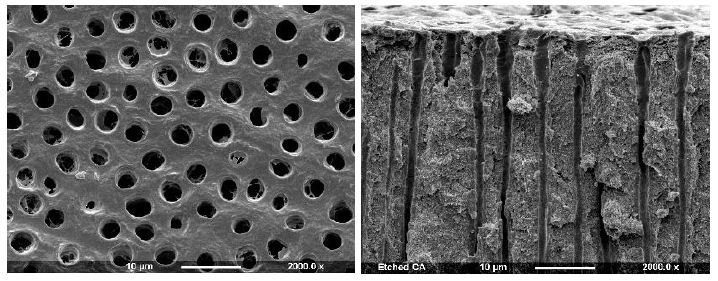
Figure 2a: Control Surface and Fracture
Varnishes
After application to the dentine disc, Cervitec (containing chlorhexidine) was observed to form a uniform layer that covered the whole dentine surface (Figure 2b). No tubule orifices, however were apparent. Fracturing the disc revealed the presence of a thick, textured layer, which covered the tubule orifices. There was some penetration of the product into the tubule lumen (arrows). Duraphat (containing fluoride) provided an uneven crystalline layer that covered the dentine surface and left tubule orifices visible (Figure 2c). Upon fracturing the dentine disc, a thin, amorphous surface layer was evident, although the varnish was usually present within the tubules, where penetration occurred it appeared to coat the lumen occupying the whole tubule diameter. When Bifluoride 12 (containing fluoride) was applied to the dentine disc, the surface was observed to be covered with small, irregular crystal-like structures and the tubule orifices were covered (Figure 2d). After fracturing the disc a thick, rough layer was observed which occluded the tubule orifices and occluded the tubules with plugs that occupied most of the tubule lumen (arrow).
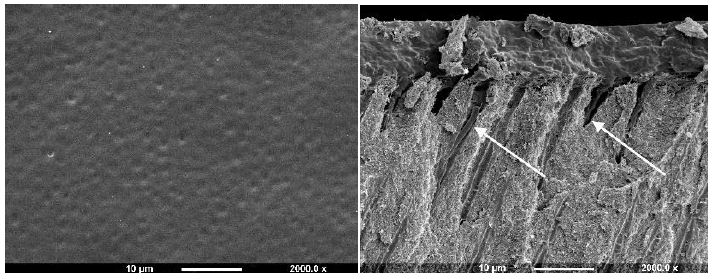
Figure 2b: Cervitec varnish Surface and Fracture views
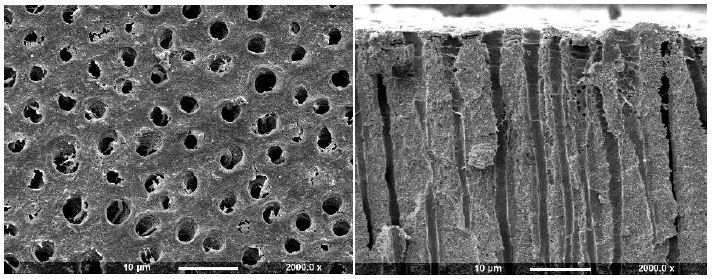
Figure 2c: Duraphat Varnish (Surface and Fractured views)

Figure 2d: BiFluoride 12 Varnish (Surface and Fractured views)
Primers
The Gluma group of primers (Gluma 3 and Gluma CPS) as well as Hema-Seal G and Solobond Plus primer, whose principal active ingredients are glutaraldehyde and 2- hydroxyethylmethacrylate (HEMA), appeared to produce similar deposits on the dentine surface, partially occluding the tubule openings (Figures 3a-3f).
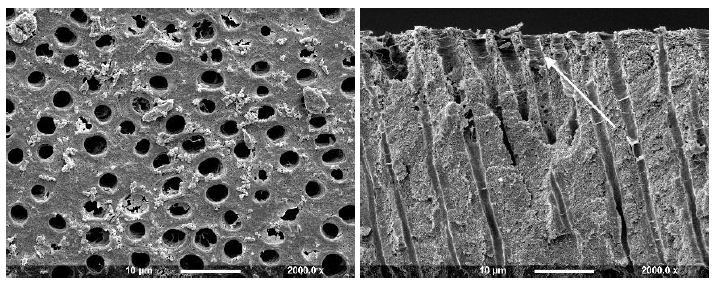
Figure 3a: Hema-seal G Primer (Surface and Fractured views)
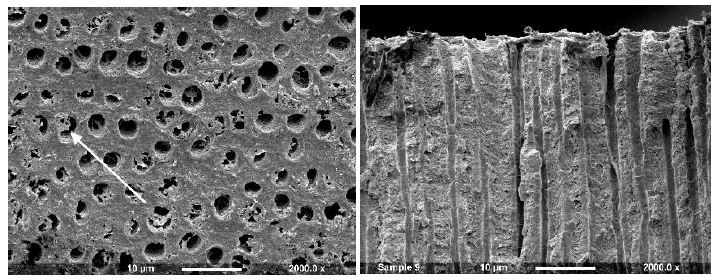
Figure 3b: Gluma 3 Primer (Surface and Fractured views)
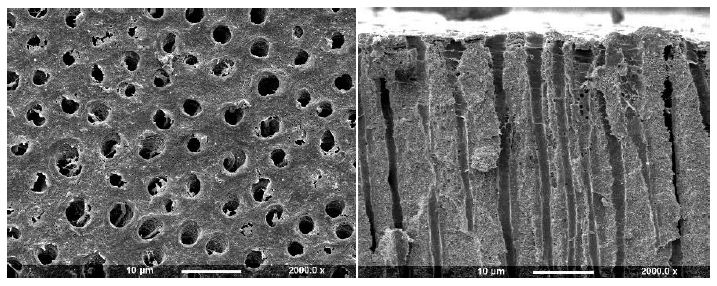
Figure 3c: Gluma CPS Primer (Surface and Fractured views)
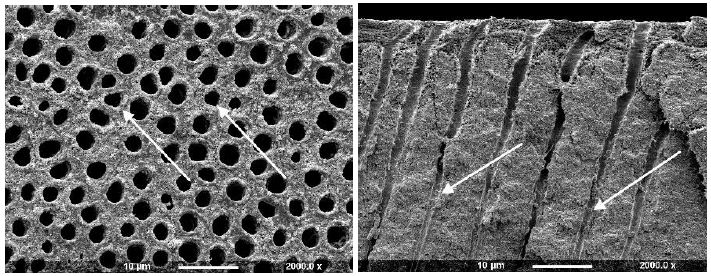
Figure 3d: Solobond Plus (Surface and Fractured views)
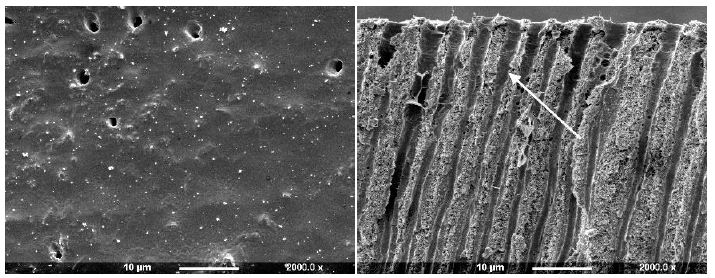
Figure 3e: All Bond 2 (Surface and Fractured views)
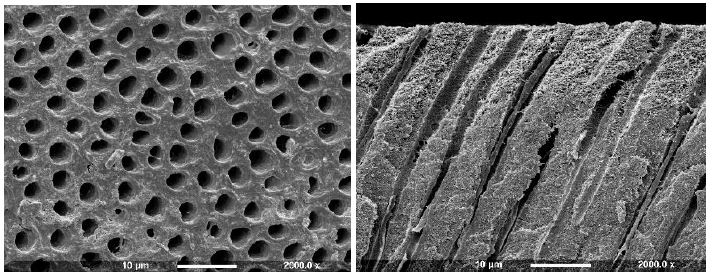
Figure 3f: Scotchbond (Surface and Fractured views)
Application of a Hema-Seal G primer resulted in limited deposition on the dentine disc with small deposits partially occluding the tubule orifices (Figure 3a). After comparison with the control half of the disc, it was observed that there was some apparent further etching. When the dentine disc was fractured the tubule orifices appeared unsealed with no sign of material deposit although some tubules appeared widened (arrow). The Gluma 3 primer produced partial occlusion of some of the tubule orifices with irregularly shaped deposits, usually observed on the surface and at the edges of the tubule openings (Figure 3b arrow). When fractured, tubule lumens free of deposits were observed. After treatment with Gluma CPS there were sparse irregular deposits on the dentine surface, some of which appeared to partially occlude the tubule orifices, whereas others were observed on the tubule periphery (Figure 3c). Fracture of the disc revealed little or no tubule occlusion or penetration.
Solobond Plus Universal Adhesive System was applied on a dentine disc etched with Vococid. The dentine surface appeared rough, and the tubule orifices were patent (Figure 3d). Some particles were noticed on the dentine around, but not occluding, the tubules (arrow). When fractured the tubules, small deposits 20-30µm were observed deeper into the tubules, although the tubules were open and widened towards the dentine surface fractured compared to the control disk (example 2a).
After application of All Bond 2 to the dentine it was apparent that the surface was covered with an almost uniform layer sealing the tubule orifices (Figure 3e). Fracture of the dentine disc revealed the presence of a thin layer covering the tubule orifices which appeared to coat the inner walls of the dentine tubule to a varying depth (arrow). Treatment with Scotchbond following etching with maleic acid 10% for 15 seconds demonstrated that some of the ttubule orifices were evident, but greatly reduced in diameter (Figure 3f). The fractured view revealed a degree of product penetration within the tubule lumen which appeared coated (Figure 3f fractured view). One Step produced an even layer which covered the whole dentine surface and no tubule orifices were obvious (Figure 3g). Fracturing the dentine disc showed a thin layer covering the tubule orifices and a considerable degree of agent penetration within the tubule lumen (arrow).
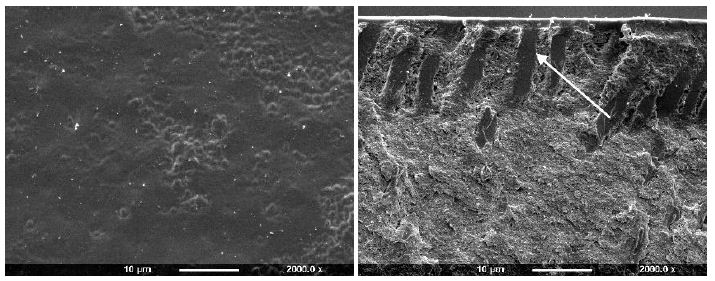
Figure 3g: One Step (Surface and Fractured views)
Subjective assessment of the test products’ ability to both occlude and penetrate the dentinal tubules was recorded as shown in Table 2. These results would suggest that the fluoride varnishes (Bifluoride 12 and Duraphat, and Cervitec) were effective in both occluding and penetrating the dentine tubules. It was also evident that All Bond 2, One Step and Scotchbond primers were superior to those of the HEMA group primers (Hema-Seal G, Gluma 3, Gluma CPS) and Solobond Plus
Table 2: Summary of the test products’ tubule occluding and penetrating ability
|
Test agents |
Degree of tubule occlusion |
Degree of dentine surface coverage |
Degree of tubule lumen penetration |
| Bifluoride 12 |
+++ |
+++ |
+++ |
| Cervitec |
+++ |
+++ |
+++ |
| Duraphat |
+++ |
+++ |
++ |
| Hema-Seal G |
+ |
+ |
0 |
| Gluma 3 Primer |
+ |
+ |
0 |
| Gluma CPS |
+ |
++ |
0 |
| Solobond Plus |
+ |
+ |
0 |
| All Bond 2 |
+++ |
+++ |
+++ |
| Scotchbond |
++ |
++ |
+++ |
| One Step |
+++ |
+++ |
+++ |
Key:
+++ Most tubules occluded/maximum surface coverage/good penetration
++ Some tubule occlusion/some surface coverage/some penetration
+ Few tubules occluded/little surface coverage/little penetration
0 No occlusion/coverage/penetration.
Discussion
Currently, the most accepted mechanism of stimulus transmission across dentine is the hydrodynamic theory, which proposes that rapid shifts of fluid movement in either direction within the dentine tubules may stimulate mechano-receptors in or near the pulp to excite the pulpal nerve and cause pain. This theory leads to the concept of dentine tubule occlusion as a method of dentine desensitization [2,3].
The use of the dentine disc model has been proved to be a reliable method for the initial in vitro screening of tubule occluding properties of potential desensitizing agents [5,7,8]. The dentine disc would appear to be the method of choice since it is easy to use, reproducible, provides a flat surface for elemental analysis and may be correlated with fluid flow research [7]. Due to the differences in the size, orientation, density and diameter of the dentine tubules throughout the tooth [9], the discs were obtained from the same region of the tooth, below the crown and above the root canal. One half of each dentine disc provided the control and the other half the test portion, and only those tubules from the central region of the disc, on either side of the fractured edge, were examined and all the observations on the test side were compared with the control half of the same disc. All the tested agents were applied according to the manufacturer’s instruction in an attempt to mimic the clinical situation.
Cervitec is a chlorhexidine containing varnish, marketed in Europe, which possesses very good dentine covering and tubule occluding properties. Chlorhexidine may act as an antiseptic in the dentine tubule lumen and reduce the number of microbes penetrating the open tubules of sensitive dentine, reducing possible pulp inflammation.
Duraphat has been studied in vivo [10-18] and proved to be effective in alleviating DS short-term. The main ingredient of Duraphat is sodium fluoride (NaF), which can precipitate onto the dentine surface [19] and may contribute its tubule occluding potential, along with its fluid texture which allows penetration into the tubule lumen. Fluoride is also reported to have potential for reducing sensitivity, perhaps acting on the SNA, and the effect of the combined sensory and physical actions may account for the widespread clinical success of Duraphat.
Bifluoride12 appears to seal the dentine surface with a crystal-like deposit in an amorphous matrix that penetrates the tubule lumen. It contains sodium and calcium fluoride, which probably account for the presence of deposits. Although the plugs penetrating the tubules do not completely occupy the tubule lumen, they appear to reduce the tubule radius and this, in association with the desensitising potential of the fluoride, may contribute to a reduction in DS in the clinical environment.
The Gluma group primers (Gluma 3, Gluma CPS), Hema-Seal G and Solobond Plus primer contain mainly glutaraldehyde and HEMA. They appeared to produce similar deposits on the dentine disc surface, partially occluding the tubule orifices. Fracturing did not reveal any degree of deposit penetration. Dondi Dall’ Orologio et al. [20] reported significant reduction of DS following application of Gluma 3 attributed to a possible reaction of glutaraldehyde with the dentinal fluid proteins, precipitation and thus a partial or complete obturation of dentine tubules. The mode of action of HEMA is still unknown. Probably, the partial occlusion of tubule openings shown in the present study may explain the reduction in DS. Hema-Seal G also contains sodium fluoride. Thus any reduction in DS may be attributed either to the partial occlusion of dentine tubule orifices or to the action of sodium fluoride which forms crystals of calcium fluoride reducing the radius of dentine tubules [19]. However, a degree of ‘over etching’ was observed following application of Hema-Seal G, indicating that, when applied to the dentine surface in vivo and in conjunction with acidic dietary intake, it might result in an increase in dentine permeability.
All-Bond 2 primers formed a layer that appeared to fully cover the dentine surface and also penetrated the tubule lumen to some depth. This is in accordance with Tay et al. [21] who also showed penetration of the tubule lumen. Furthermore, Ianzano et al. [22] and Gillam et al. [23] reported that All-Bond 2 primers were effective in vivo in reducing DS, although different evaluation techniques were employed by these investigators. All-Bond 2, therefore, may be useful in the in-office treatment of DS although its effectiveness may be relatively short-lived. One Step was a relatively new product (at the time of evaluation) and so far there are no studies evaluating its desensitizing or occluding properties although it is the same as All Bond 2. As with All-Bond 2, One Step may be of some clinical value given that it’s occluding and penetrating properties are satisfactory and it is also easily applied. Scotchbond has been reported to be effective in reducing DS [24] although it is possible that the primer was subsequently covered with a light-cured resin. In the present study only, the primer was applied onto the dentine disc surface and the results showed a degree of surface coverage although some tubules with reduced diameters were also evident. This may be due to the application procedure or the etching period. It is possible that longer application period or thicker layer would completely obscure the tubule openings. However, the fracture of the disc revealed penetration of the product within the tubules rendering this agent a potential desensitiser.
This study was based on a well-established dentine disc model which has been modified to provide greater control in the preparation and analysis of surface deposits. In this respect, the method may be considered an improvement over previously reported studies. Difficulties may still arise, however, in the interpretation of the results particularly with regard to fracturing the disc halves, which is relatively technique sensitive, and the exact plane of fracture is somewhat unpredictable. Nevertheless, if the control and the test disc sections are carefully compared, the penetrating potential of the tested agents can be satisfactory evaluated as indicated in this present study. The dentine disc model although useful for the initial in vitro assessment of different agents may not reflect the in vivo situation and the results of this in vitro study should be extrapolated to the in vivo situation with caution. Factors such as pulpal pressure, the oral environment (saliva, gingival fluid) and patient’s habits (vigorous toothbrushing, acidic dietary intake) may also influence the retention of these products on the dentine surface. The presence of dentinal fluid within the tubules and its rate of movement in vivo may influence the formation of deposits on the dentine surface despite efforts to keep the exposed dentine [15]. Vongsavan and Matthews [25] reported that the penetration of different molecules into the dentine tubules may be greater in vivo than in vitro.
All the agents introduced in the present study, appear to be clinically applicable since they are easily applied, and some appear to possess both occluding and penetrating ability. Varnishes and primers, (together with sealants which were not reported on in this study), because of the relative ease of application, may provide a useful treatment option for the practicing dentist in the alleviation of DS. Attention should be paid to the mode of their application, which may be technique sensitive, e.g. All Bond 2 and Scotchbond where a vigorous air blast may remove a great amount of the agent leaving uncovered dentine tubules. Tubule penetration properties are important because even when the agent is removed from the dentine surface, plugs of the material may remain within the tubule lumen, which may either maintain the desensitizing effect of the agent or provide the patient with temporary relief until natural tubule occlusion occurs. Both short and long term clinical studies are required to determine whether the in vitro potential of these agents can be extrapolated in the clinical environment.
The majority of in vitro studies have evaluated the desensitizing properties of agents using descriptive methods of dentine surface assessment. Although some authors claimed that their methods of assessing the amount of tubule occlusion were quantitative in nature [6,26] in many cases they were semi-quantitative with no measuring involved. Quantitative studies using digital image analysis and SEM would provide a more accurate result [27] however, most of the products in this study resulted in total surface coverage, negating the need for measurement. Studies including fractures of dentine discs are of benefit in the investigation of the desensitizing potential as there may be blockage within the tubule which is not evident from the surface. Elemental analysis was not within the scope of this study, but further examination of some of the surface products would be interesting.
Conclusions
The results of this study suggest that, of the agents tested, the varnishes, All-Bond 2 primer, Scotchbond primer, and One Step appear to have both occluding and penetrating properties. However, agents which have been observed to be effective in vivo but with no significant tubule occluding or penetrating properties as demonstrated in the present study may employ other mechanism/s which cannot be simulated in this in vitro model. Moreover, the results obtained following the application of all the agents tested in the present study may be affected by the application technique(s) employed to apply them on the dentine surface, although every effort was made to follow the manufacturer’s instructions for clinical application.
References
- Addy M, Mostafa P, Absi EG, Adams D (1985) Cervical dentine hypersensitivity. Etiology and management with particular reference to dentifrices. In: Rowe, N. H. (ed): Proceedings of symposium on hypersensitive dentine Origin and Management, University of Michigan, (ed) E. & S, Livingston Limited, Edinburgh & London, pp: 147-167. [crossref]
- Gillam DG (1995) Mechanisms of stimulus transmission across dentin–a review. J West Soc Periodontol Periodontal Abstr 43(2): 53-65. [crossref]
- Brannstrom M (1962) The elicitation of pain in human dentine and pulp by chemical stimuli. Arch Oral Biol 7: 59-62. [crossref]
- Pashley DH (1985) Strategies for clinical evaluation of drugs and/or devices for the alleviation of hypersensitive dentin. In: Rowe NH, editor. Proceedings of Symposium on Hypersensitive Dentin. Origin and Management. Ann Arbor: Univ Michigan. p.65-88 [crossref]
- Greenhill JD, Pashley DH (1981) The effects of desensitizing agents on the hydraulic conductance of human dentin in vitro. J Dent Res 60: 686-98 [crossref]
- Absi EG, Addy M, Adams D (1995) Dentine hypersensitivity: uptake of toothpastes onto dentine and effects of brushing, washing and dietary acid–SEM in vitro study. J Oral Rehabil 22(3): 175-82. [crossref]
- Mordan NJ, Barber PM, Gillam DG (1997) The dentine disc. A review of its applicability as a model for the in vitro testing of dentine hypersensitivity J Oral Rehabil 24(2): 148-156. [crossref]
- Ling TY, Gillam DG, Barber PM, Mordan NJ, Critchell J (1997) An investigation of potential desensitizing agents in the dentine disc model: a scanning electron microscopy study. J Oral Rehabil 24(3): 191-203. [crossref]
- Pashley DH, Leibach JG, Horner JA (1987) The effects of burnishing NaF/kaolin/glycerin paste on dentin permeability. J Periodontol 58(1): 19-23. [crossref]
- Kerns DG, Scheidt MJ, Pashley DH, Horner JA, Strong SL, Van Dyke TE (1991) Dentinal tubule occlusion and root hypersensitivity. J Periodontol 62(7): 421-8. [crossref]
- Kim S (1986) Hypersensitive teeth: desensitization of pulpal sensory nerves. J Endod 12(10): 482-5. [crossref]
- Sena FJ (1990) Dentinal permeability in assessing therapeutic agents. Dent Clin North Am 34(3): 475-90. [crossref]
- Knight NN, Lie T, Clark SM, Adams DF (1993) Hypersensitive dentin: testing of procedures for mechanical and chemical obliteration of dentinal tubuli. J Periodontol 64(5): 366-73. [crossref]
- Jain P, Vargas MA, Denehy GE, Boyer DB (1997) Dentin desensitizing agents: SEM and X-ray microanalysis assessment. Am J Dent 10(1): 21-6. [crossref]
- Dragolich WE, Pashley DH, Brennan WA, O’Neal RB, Horner JA, Van Dyke TE (1993) An in vitro study of dentinal tubule occlusion by ferric oxalate. J Periodontol 64(11): 1045-51. [crossref]
- Gwinnett AJ (1988) Aluminium oxalate for dentin bonding. A SEM study. Amer J Dent 1, 5-8. [crossref]
- Ellingsen JE, Rölla G (1987) Treatment of dentin with stannous fluoride – SEM and electron microprobe study. Scand J Dent Res 15, 281-286. [crossref]
- Hansen EK (1992) Dentin hypersensitivity treated with a fluoride-containing varnish or a light-cured glass-ionomer liner. Scand J Dent Res 100(6): 305-9. [crossref]
- Addy M, Mostafa P (1989) Dentine hypersensitivity. II. Effects produced by the uptake in vitro of toothpastes onto dentine. J Oral Rehabil 16(1): 35-48. [crossref]
- Dondi dall’Orologio G, Malferrari S (1993) Desensitizing effects of Gluma and Gluma 2000 on hypersensitive dentin. Am J Dent 6(6): 283-6. [crossref]
- Tay FR, Gwinnett AJ, Pang KM, Wei SH (1994) Structural evidence of a sealed tissue interface with a total-etch wet-bonding technique in vivo. J Dent Res 73(3): 629-36. [crossref]
- Ianzano JA, Gwinnett AJ, Westbay G (1993) Polymeric sealing of dentinal tubules to control sensitivity: preliminary observations. Periodontal Clin Investig 15, 13-16. [crossref]
- Gillam DG, Coventry JF, Manning RH, Newman HN, Bulman JS (1997) Comparison of two desensitizing agents for the treatment of cervical dentine sensitivity. Endod Dent Traumatol 13: 36-39. [crossref]
- Copeland JS (1985) Simplified remedy for root sensitivity. Northwest Dent 64(6): 13-4. [Crossref]
- Vongsavan N, Matthews B (1991) The permeability of cat dentine in vivo and in vitro. Arch Oral Biol 36(9): 641-646. [crossref]
- McAndrew R, Kourkouta S (1995) Effects of toothbrushing prior and/or subsequent to dietary acid application on smear layer formation and the patency of dentinal tubules: an SEM study. J Periodontol 66(6): 443-8. [crossref]
- Ahmed TR, Mordan NJ, Gilthorpe MS, Gillam DG (2005) In vitro quantification of changes in human dentine tubule parameters using SEM and digital analysis. J Oral Rehabil 32(8): 589-97. [crossref]