DOI: 10.31038/AFS.2022444
Abstract
The release of polycyclic aromatic hydrocarbons (PAHs), related to oil spills, can have devasting effects on the environment. In the presence of ultraviolet (UV) light, PAHs can be photoenhanced into more toxic compounds, leading to increased toxicity in aquatic organisms as measured by 24-h survival. PAHs cause a suite of physiological consequences particularly in the early stages of fish development due to increased surface area to volume ratios and nascent immune systems. This study compared the impacts of photoenhanced thin oil sheens on the survival and growth of larvae of three ecologically important coastal fish species: sheepshead minnows (Cyprinodon variegatus), red drum (Sciaenops ocellatus) and spotted seatrout (Cynoscion nebulosus). PAH + UV exposure increased the toxicity of thin oil sheens in all three species, with red drum demonstrating the highest sensitivity. An acute oil exposure at 1-2 days post hatch (dph) increased the rate of latent mortality and oxidative stress in sheepshead minnows. Non-photoenhanced oil caused a significant decrease in the growth of 1-2 dph sheepshead minnows and spotted seatrout. Results from this study reveal long-term effects of oil exposure on fish growth and survival, which could lead to better restoration and conservation outcomes for these vital estuarine species.
Introduction
Petroleum can enter waterways through large spills such as the April 2010 Deepwater Horizon (DWH) oil spill and the March 1989 Exxon Valdez shipping container oil spill [1]. More frequently, however, oil is released on smaller scales through industrial discharge, smaller vessel spills, runoff from impervious surfaces, and commercial port usage [2]. Oil pollution can cause physical and chemical changes to the coastal environment, as well as negatively impact the health of marine organisms [3].
Oil contains polycyclic aromatic hydrocarbons (PAHs), which are a group of organic molecules that are composed of fused benzene rings which allow them to be lipophilic, readily bioaccumulated, and have mutagenic and carcinogenic properties [4-7]. The effects of PAHs, especially those found in crude oil, on marine organisms have been extensively researched and the documented effects are widespread across a wide range of organisms [8-26]. Effects such as deformities, reduced growth and reproduction, genetic and behavioral changes, and altered physiological functions have been seen in invertebrates, as well as vertebrate species.
Although, PAHs alone have negative impacts on marine life, certain abiotic factors can increase the toxicity of PAHs. In particular, ultraviolet (UV) light has been shown to potentially make PAH compounds in fresh oil products up to 1000x more toxic than the original structure of the compound [27-33]. UV light can create enhanced toxicity via two mechanisms: photosensitization or photomodification [29]. Photosensitization occurs when an organism is exposed to UV light after biouptake of PAHs. PAH molecules within the organism’s tissues will absorb the UV light and promote electrons to enter an excited-state orbital. Alternatively, photomodification occurs when UV light oxidizes PAH molecules in the water column, forming more toxic molecules. The photomodified products can then be incorporated into the surrounding biota [29]. Studies of the interaction of UV light and PAHs in oil have documented increased toxicity and mortality in invertebrate and vertebrate larvae after short-term exposure [4,28,32-46]. Other documented effects of oil exposed larvae include damage to cell membranes through lipid peroxidation [30,44,47].
The present study used three larval estuarine fish, red drum, spotted seatrout, and sheepshead minnows, to examine and compare the effects of a co-exposure to PAH + UV light and PAH alone. Red drum, spotted seatrout, and sheepshead minnows are all ecologically important species, as they are prey items for larger fish, crustaceans, and wading birds [48,49]. In addition, red drum and spotted seatrout also have recreational and commercial significance among the Atlantic and Gulf of Mexico coasts [50-52]. Fish early life stages are particularly vulnerable to oil pollution because of the increased capacity for contaminant uptake due to greater surface area to volume ratios, increased metabolic rates, and less developed immune systems. In addition, many larval fish lack pigmentations and are often found at the surface making them particularly liable to UV light from sunlight, surface oil, and oil that has dispersed and dissolved in the water column [29,35,44,53,54].
Although photoenhanced oil effects on fish mortality are well known, few studies have sought to examine potential latent sublethal effects. While some research has focused on effects of water-accommodated fractions (WAF) of oil, thin oil surface sheens enhanced by UV light often are in direct contact with many newly hatched fish species. Therefore, this study’s primary aim was to compare the effects of thin oil sheens and UV light on the larval stages of red drum, spotted seatrout, and sheepshead minnows by examining endpoints of 24-hour mortality, latent mortality, growth metrics, and oxidative stress. The results of this study may be used to inform oil spill mitigation decisions and inform the assessment of impacts and ecosystem dynamics after an oil exposure.
Materials and Methods
Test Species
Red drum and spotted seatrout eggs (~12 h post fertilization (hpf)) were obtained from the Marine Resources Research Institute (MRRI), Mariculture division, of the SC Department of Natural Resources (SCDNR) in Charleston, SC during spawning months of April-May for spotted seatrout and July-October for red drum. The eggs were transferred to the NOAA National Centers for Coastal Ocean Science Charleston laboratory. After arrival, temperature, dissolved oxygen, and pH of the egg transfer water was measured, and aeration was provided. Viable eggs were transferred to four 10 -L tanks of seawater (35 ppt and 25°C) and allowed to hatch. The seawater was collected from Charleston Harbor, and polished via sand filtration, UV sterilization, and 5 µm nominal filtration. Adult sheepshead minnows were collected from a local tidal pond located on the Hollings Marine Laboratory property (N 32° 74′ 82.24”; W 79° 90′ 12.35”) using minnow traps. Adult fish were acclimated to laboratory conditions for 24 hours and then placed in spawning chambers within 75 -L aquariums (20 ppt and 25°C). Fish were fed Tetramin® fish flakes daily. Egg collection trays were used to retrieve eggs produced. Eggs were then transferred to shallow glass finger bowls and allowed to hatch. Larval sheepshead minnows were fed newly hatched brine shrimp (Artemia salina) prior to testing.
Initial PAH + UV Light Exposure
Fish larvae were used in experimental testing at 1-2 dph (Institutional Animal Care and Use Committee 2018-009). Eight to ten larvae were placed in 270 mL glass crystallizing dishes with 200 mL of 20 ppt (sheepshead minnows) or 35 ppt (red drum and spotted seatrout) seawater. Tests were run in two environmental incubators set at 25°C (Percival Scientific IntellusUltra C8) – one for PAH + UV exposures and one for PAH exposures. PAH + UV conditions were established using T5 AgroMax UV-A PLUS bulbs, whereas PAH conditions used cool-white fluorescent bulbs. PAH photoperiod was set at 16 h light:8 h dark while the PAH + UV photoperiod was set at 4 h UV:12 h light:8 h dark (red drum and seatrout) or 8 h UV:8 h light: 8 h dark (sheepshead minnow). Preliminary UV light threshold tests were conducted prior to the initial oil and UV light experiments to obtain the UV photoperiods for each fish species. Measures of irradiance (µW/cm2) of UV-A (λ= 380 nm) light exposures were taken using a miniature spectrometer (Ocean Optics Flame Series). A total integrated average dose was calculated for each 4 h and 8 h period of UV light by multiplying the average instantaneous irradiance measurements by the photoperiod in seconds.
Once placed in the incubator, fresh Louisiana Sweet Crude (LSC) oil was pipetted onto the surface of the water in the dishes containing the fish larvae to achieve an oil sheen. A range of sheen thicknesses (0.25, 0.5, 1.0, 2.0, and 4.0 µm) was tested dependent on sample number and a hypothesized estimated range that each species could tolerate. The equation (V=πr2h) was used to determine the volume (V) of oil needed to achieve the desired sheen thickness (h) using the radius (r) of the container; 1.42 µL, 2.84 µL, 5.67 µL, 11.34 µL, and 22.68 µL, respectively. Three to five replicates were used for each oil treatment and control. The test was run under static conditions with no renewal of the oil or seawater for 24 hours. After removal at the end of the test, if control fish mortality was ≤ 20%, all fish treatments were transferred for the grow-out phase. Water quality (temperature, pH, dissolved oxygen, and salinity) was measured from one replicate of each treatment at the end of the experiment.
Grow-out Phase Setup and Growth Measurements
Fish that survived the 24 h oil exposure were transferred to 200 mL of clean seawater in 237 mL polyethylene jars in the No UV incubator with a 16 h light:8 h dark light cycle. Each jar had a hole drilled in the lid for oxygen exchange. Water changes and feeding occurred daily. Spotted seatrout and red drum were initially fed rotifers followed by a mixture of rotifers and brine shrimp and then brine shrimp only. Sheepshead minnows and 12 dph spotted seatrout and red drum were fed brine shrimp ad libitum. Mortality was assessed daily, along with any behavior changes or any morphological deformities.
The fish grow out period was terminated after 30 days. Photo documentation was done utilizing Image-Pro Premier 9.2 64-bit software. Individual fish wet weight (mg) was recorded and the whole fish was frozen at -80°C for lipid peroxidation assay. Total body length (mm), total body depth (mm), and total ocular diameter (mm) were measured and recorded. The length was measured from the anterior to the posterior peduncle excluding the caudal fin. The depth was measured from dorsal to ventral behind the gill opening. The ocular diameter was measured across the maximum length of the retina.
Oxidative Stress: Lipid Peroxidation Assay
Lipid peroxidation activity was assessed using the malondialdehyde (MDA) method (modified from [55]). Whole fish tissues were kept frozen and homogenized on ice in potassium phosphate (K2PO4) buffer (1:4 wet weight to volume ratio) using a Pro Scientific model Pro 200 motor with a 20 mm x 150 mm stainless steel rod for a minimum of 30 sec. Samples were then centrifuged at 13,000 x g for 5 min at 4°C. Aliquots of 1400 µL of 0.375% thiobarbituric acid/15% trichloroacetic acid and 14 µL of 2% butylated hydroxytoluene were added to new microcentrifuge tubes along with 100 µL of the centrifuged supernatant for each sample, including 100 µL of K2PO4 buffer as a blank. A 10 mM stock solution of MDA was previously heated for 1 h at 50°C and allowed to cool to room temperature before generating standards. A secondary solution of 3200 µM MDA was used to prepare serial dilutions from 800 µM to 6.25 µM using K2PO4 buffer. All samples, standard tubes and the blank were vortexed and placed in a hot plate at 92°C for 15 minutes. Once heated, all tubes were centrifuged at room temp for 5 min at 13,000 x g. Aliquots of 300 µL supernatant for each sample, standard and a blank were loaded in triplicates in a clear Corning 96-well plate. Absorbance was measured with a Bio-Tek µQuant MQX200 microplate spectrophotometric reader at 532 nm in conjunction with Bio-Tek KC junior software. Absorbance readings for each sample and serial dilution were adjusted by subtracting from the blank value and the slope of the standard line was used to determine the amount of MDA in nmol/g (wet weight).
Chemical Analysis of Water Samples
Additional treatment containers without fish were setup for oil chemical analysis. Water samples from beneath the oil sheens (both PAH + UV and PAH) were collected via Teflon tubing siphons taped to the side of each glass crystallizing dish prior to the start of the experiment. Samples were analyzed for PAH concentrations according to methods detailed in [56]. Briefly, each sample was acidified to a pH of 2 using 18% hydrochloric acid (HCl) and then transferred to separatory funnels to undergo liquid/liquid extraction. The samples were spiked with isotopically labeled PAH internal standards, then solvent extracted three times, once with dichloromethane, once with 50:50 dichloromethane/hexane, and once with hexane. After extraction, samples were passed through GF/F paper containing anhydrous sodium sulfate, concentrated in a water bath, cleaned up using silica solid phase extraction (SPE) (Phenomenx Strata SI-1 Silica 500 mg/3 mL) and analyzed using gas chromatography/mass spectrometry (GC/MS). Samples were processed using an Agilent Technologies 6890/5973 GC/MS containing a DB17ms analytical column (Agilent J & W 60 m x 0.25 mm x 0.25 µm). Samples were introduced into the instrument through a split/splitless inlet operated in splitless mode. The mass spectrometer was operated in electron impact ionization (EI) and selected ion monitoring (SIM) modes. Samples were analyzed using Agilent Technologies MSD Chemstation Version E.02.02.1431 software. Total PAH (tPAH50) in this present study is reported as the sum of 50 parent and alkylated PAHs.
Statistical Analysis
Median lethal concentrations (LC50) and 10% lethal concentrations (LC10) were calculated from the 24 h exposures using SAS Probit Analysis (parametric data) [57] or Trimmed-Spearman Karber Analysis (nonparametric data) [58]. The lowest observable effect concentration (LOEC) values, 24 h exposure mortality, latent mortality, and all growth parameters (lengths, depths, ocular diameter, weights) were analyzed first using two-factor analysis of variance (ANOVA)(alpha=0.05) with interaction tests, the two factors being light treatment (UV or No UV) and PAH concentration. If no interactive effect was observed, a one–factor ANOVA (alpha=0.05) was used to further analyze the data. In the event that the assumptions of the parametric statistics were not met, a nonparametric Kruskall-Wallis (alpha=0.05) was used instead. Normality was assessed for all tests using histogram plots and a Shapiro Wilks test (alpha=0.05) and homogeneity of variance was assessed using a Levene’s test (alpha=0.05). Cook’s D and studentized residuals calculations were also used to determine cases of influential data within a specific endpoint. If a mortality endpoint or growth parameter measurement had a Cook’s D number > 4/n or a studentized residual number with an absolute value >3, the data point was deemed an influential observation and removed [59,60]. Lastly, a William’s Test for a Monotonic Trend was used to evaluate if there was a significant trend among the different light treatments and PAH concentrations for calculated MDA concentrations.
Results
Fish Mortality of Red Drum, Sheepshead Minnows, and Spotted Seatrout after 24 H Oil Exposure With and Without UV Light
The mean tPAH50 concentrations for each oil sheen thickness for all three species were calculated (Table 1). There was a positive correlation between the tPAH50 concentrations obtained from analysis of the water samples and the oil sheen thicknesses used in this study. Larval 1-2 dph red drum mortality was significantly affected (ANOVA; p=0.0388) at 14.47 µg/L (4.0 µm sheen) tPAH50 without UV light, however all oil treatments showed significant mortality with UV light (Figure 1). The LC10 value with No UV light was 1.35 µg/L tPAH50. When tested with UV light, survival was affected at significantly lower oil concentrations (≥3.0 µg/L tPAH50, 0.5 µm sheen) and the LC10 value was reduced to 0.59 µg/L tPAH50 (95% C.I. = 0.0002, 1.4942). UV light alone did not significantly affect fish survival (fluorescent light seawater control versus UV light seawater control). There was a significant interaction between oil and light for the overall ANOVA model (p<0.0001) and between the treatment levels (Figure 1).
Table 1: The measured mean tPAH50 (µg/L) for each oil sheen thickness. n is the number of samples used for each oil sheen thickness.
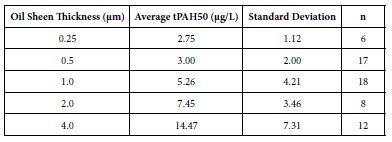
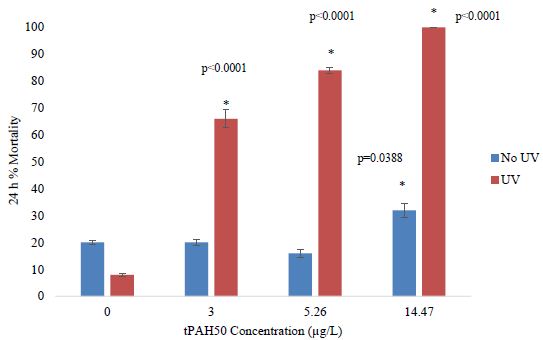
Figure 1: Percent mortality after 24 h for PAH and PAH + UV light treatments of 1-2 dph red drum (n=400) exposed to 4 hours of UV light and averaged oil sheen tPAH50 concentrations of 3.0 µg/L, 5.26 µg/L, and 14. 47 µg/L. Two influential data points were removed from the set. Asterisks and corresponding p-values for that treatment indicate a statistical significant difference from that light treatment’s control (0 µg/L).
In PAH + UV exposures, there was a significant effect (ANOVA; p=0.0095) on 1-2 dph spotted seatrout mortality at the highest concentration tested of 5.26 µg/L tPAH50 (1.0 µm sheen) (Figure 2). Additionally, for PAH and PAH + UV treatments below 5.26 µg/L, no significant effect was observed within 24 h on 1-2 dph spotted seatrout survival and there was no significant interaction between light and oil factors.
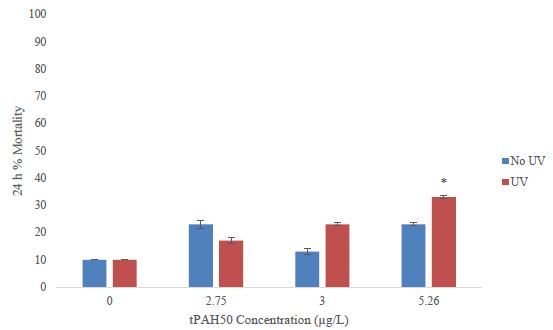
Figure 2: Percent mortality after 24 h for the PAH and PAH + UV light treatments of 1-2 dph spotted seatrout (n=240) exposed to 4 hours of UV light and averaged oil sheen tPAH50 concentrations of 2.75 µg/L, 3.0 µg/L, and 5.26 µg/L. The asterisk and corresponding p-value indicate a statistical significant difference from the UV control (0 µg/L) treatment.
For 1-2 dph sheepshead minnows, there was a significant increase (ANOVA; p=0.0295) in mortality at the highest exposure dose, 7.45 µg/L tPAH50 (Figure 3), for the PAH + UV treatments. There was no significant effect on survival after a 24 h exposure to oil sheen thicknesses ≤ 2.0 µm (7.45 µg/L tPAH50) (Figure 3). No significant effect was observed at any oil sheen concentration in the PAH treatments. The 24h LC50 value for PAH + UV exposure was 6.80 µg/L (95% C.I. = 6.34, 7.29).
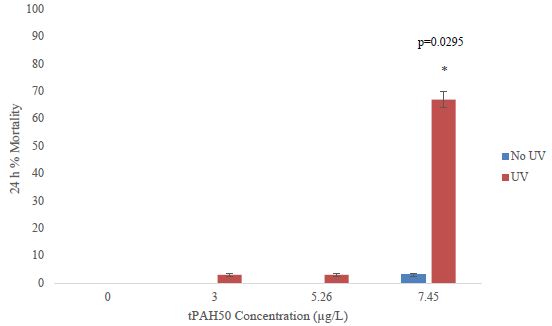
Figure 3: Percent mortality after 24 h for the PAH and PAH + UV light treatments of 1-2 dph sheepshead minnows (n=240) exposed to 8 hours of UV light and averaged oil sheen tPAH50 concentrations of 3.0 µg/L, 5.26 µg/L, and 7.45 µg/L. The asterisk and corresponding p-value indicates a statistical significant difference from the UV control (0 µg/L) treatment.
When comparing PAH + UV effects in all three species, 1-2 dph red drum had a LOEC of 3.0 µg/L and a LC10 value of 0.59 µg/L (95% C.I. = 0.0002, 1.4942), 1-2 dph spotted seatrout had a LOEC of 5.26 µg/L and a LC10 value of 0.75 µg/L (95% C.I. = ND), and 1-2 dph sheepshead minnows had a LOEC of 7.45 µg/L and a LC10 value of 5.41 µg/L (95% C.I. = -1063.83, 6.47). Exposure to UV light increased the toxicity of oil in all species. Larval red drum were the most sensitive species tested among the three.
Latent Mortality after Initial 24 H Oil Exposure with and without UV Light
To examine the latent effects of a 24 h oil and/or UV exposure, surviving fish were moved to clean seawater and mortality was reassessed after 7 days. The number of surviving fish varied among treatments. In larval sheepshead minnows that were grown out in fresh seawater, a higher mortality was observed among the PAH + UV light treatments versus the PAH treatments compared to the controls, however, there was no significant difference calculated (Figure 4). In other larval fish examined for latent mortality, the spotted seatrout displayed 96% mortality in all treatments at and above 3.0 µg/L. In larval red drum, 100% mortality occurred in all treatments including the controls after 5 days, preventing growth measurements.
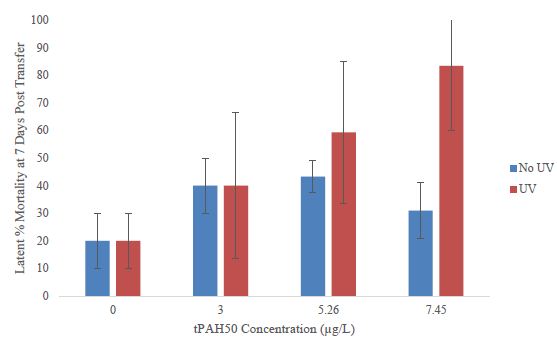
Figure 4: Latent percent mortality at 7 days post transfer to clean seawater for the PAH and PAH + UV light treatments of sheepshead minnows (n=178) previously exposed at 1-2 dph to 24 hours of averaged tPAH50 concentrations of 3.0 µg/L, 5.26 µg/L, and 7.45 µg/L and 8 hours of UV light.
Growth of Sheepshead Minnows and Spotted Seatrout after Initial 24 H Oil Exposure with and without UV Light
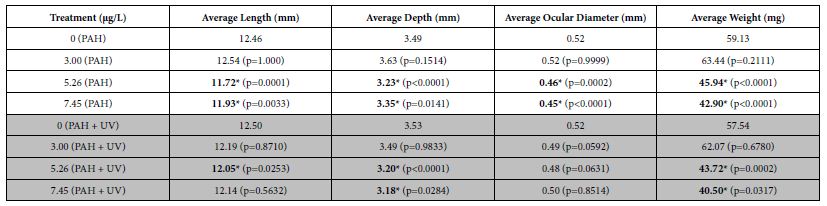
Average lengths, depths, ocular diameters, and weights were determined for fish at 30-31 dph to assess effects of short-term PAH + UV light exposure on growth. An initial 24 h oil exposure at 1-2 dph yielded significant effects on sheepshead minnow growth. Average length, depth, ocular diameter and weight at 30-31 dph were all significantly reduced in PAH treatments at tPAH50 concentrations of 5.26 µg/L and greater (Table 2). Similar results were seen with the PAH + UV treatments, although ocular diameter was not significantly different from the control.
Table 2: Mean growth measurements for the PAH and PAH + UV light treatments of 30-31 dph sheepshead minnows (n=129) exposed at 1-2 dph to 24 hours of average tPAH50 concentrations of 3.0 µg/L, 5.26 µg/L, and 7.45 µg/L and 8 hours of UV light. Six influential data points were removed. Bolded numbers with an asterisk indicate a statistical significant difference from that light treatment’s control. There were significant differences from the controls among the 5.26 µg/L and 7.45 µg/L PAH concentrations in all growth measures but there was no significant interactive effect between light and PAH concentration.
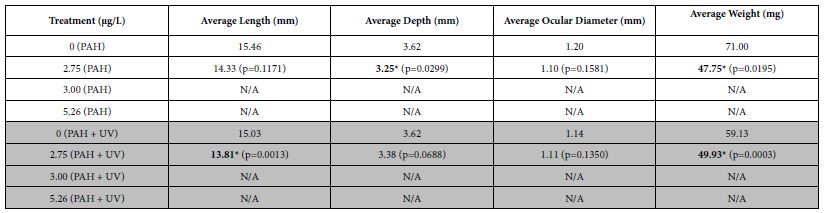
When spotted seatrout were exposed at 1-2 dph to an initial 24 h oil exposure, significant decreases in average size (length and depth) and weight were seen after 30 days at tPAH50 concentrations of 2.75 µg/L (PAH + UV and PAH) compared to its control (Table 3). Overall, for both PAH + UV and PAH treatments, sheepshead minnows and spotted seatrout had decreased growth as PAH concentration increased with effects threshold starting at 5.26 µg/L and 2.75 µg/L, respectively. Growth measurements for red drum were unavailable due to 100% latent mortality 5 days after the transfer.
Table 3: Mean growth measurements for the PAH and PAH + UV light treatments of 30-31 dph spotted seatrout (n=31) exposed at 1-2 dph to 24 hours of average tPAH50 concentrations of 2.75 µg/L, 3.00 µg/L, and 5.26 µg/L and 4 hours of UV light. N/A indicates almost 100% latent mortality in that treatment after the initial exposure of oil and light. One influential data point was removed. Bolded numbers with an asterisk indicate a statistical significant difference the No UV control. There were differences between the controls and the 2.75 µg/L PAH concentration for No UV and UV treatments but there was no significant interactive effect of light and PAH concentration.
Oxidative Stress of Sheepshead Minnows after Initial 24 H Oil Exposure with or without UV Light
Oxidative stress was only assessed in larval sheepshead minnows due to the high latent motality in larval red drum and spotted seatrout. There was a significant upward trend (William’s Test; p<0.0001) of MDA concentrations in the PAH + UV light treatments of 30-31 dph sheepshead minnows exposed as larvae but none in the PAH light treatment (Figure 5).
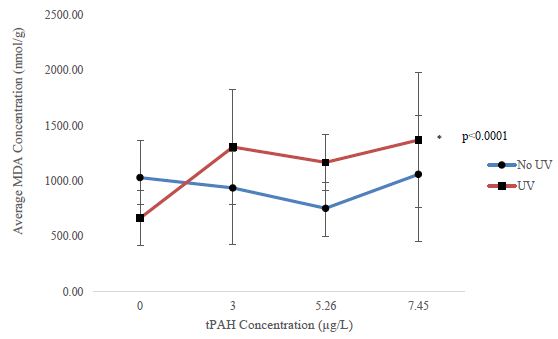
Figure 5: Mean MDA concentration for the PAH and PAH + UV light treatments of 30-31 dph sheepshead minnows (n=129) exposed at 1-2 dph to 24 hours of averaged tPAH50 concentrations of 3.0 µg/L, 5.26 µg/L, and 7.45 µg/L and 8 hours of UV light. Three influential data points were removed.
Discussion
Sheepshead minnows were most resilient to oil exposures with and without UV light, compared to spotted seatrout and red drum. Both of the latter species have reproductive strategies which include releasing thousands of eggs with each spawn, followed by high larval mortality [50,61]. This spawning strategy makes these species more sensitive to variable environmental factors, and made it difficult to use these species for toxicity assessment in a laboratory environment. Another factor that contributes to greater resilience in sheepshead minnows is the buoyancy of each larval species. Both red drum and spotted seatrout obtain nutrients through a yolk sac the first three days of life making them more positively buoyant than sheepshead minnows. This strategy makes the former species more likely to have an exposure to UV light at the surface. The fragility of the red drum and seatrout larvae has ecological importance, since these fishes are managed by NOAA, targeted by recreational fishers and are among the most sensitive to oil [62]. Increased large external stressor mortality, such as an oil spill, could lead to potential reductions in population size.
24 h Mortality
All three species, red drum (LC10 = 0.59 µg/L), spotted seatrout (LC10 = 0.75 µg/L), and sheepshead minnows (LC10 = 5.41 µg/L) exhibited PAH + UV light enhanced mortality in a 24 h acute exposure. The differences in species sensitivity are most likely due to life history strategies and habitat utilization. Estuaries are known to be harsh environments to extreme variation in environmental conditions and long-term accumulation of pollutants. Sheepshead minnows have adapted biological mechanisms such as the ability to withstand hypoxic and heavily contaminated conditions, as well as large temperature changes [63]. This demonstrates their tolerance to these dynamic estuarine conditions, making it the most resilient among the three. Although an LC50 value could not be calculated due to treatment mortality ≥ 50 % in red drum larvae, the significant mortality threshold (<3.0 µg/L) and LC10 value (0.59 µg/L) were similar to findings in [35]. [35], though using HEWAFs instead of oil sheens, observed an LC50 value of 3.42 µg/L for 1-2 dph red drum larvae exposed to PAH + UV, with significant mortality in PAH concentrations at and above 3.13 µg/L. This present study found an LC50 value could not be calculated for 1-2 dph spotted seatrout at the concentrations tested, however the LC10 value (0.75 µg/L) was within the range of effects reported by [35], which determined an LC50 value of 0.827 µg/L. Differences in LC50 value sensitivity and threshold mortality among the studies could be due to many different factors such as: experimental setup variance, UV exposure time, wavelength of UV light, intensity of UV, oil exposure type, concentration, and water conditions, such as temperature or salinity[42,44,64,65]. For example, preliminary UV threshold testing with red drum and spotted seatrout obtained from the SCNDR revealed <100% treatment survival at 4 hours of UV exposure (23.80 ± 17.22 µW/cm2). [35] used 6 h durations of natural UV allowing more time and UV wavelengths for photoenhancement to occur, potentially causing the mortality to be different than the mortality at 4 hours observed in this study. Additionally, the larvae used for each of these studies came from two different individual adult broodstock populations with potentially distinct environmental adaptations and sensitivities.
Latent Mortality
All species tested exhibited latent mortality after the initial exposure. Latent mortality after oil exposures is an often overlooked and underestimated endpoint despite the importance it may play in assessing development, survival, and population structure later in life [66]. [67] found a 25-77% reduction in survival of larval bay anchovies 6 days following a 24 h exposure to Macondo source oil. Similarly, larval coral reef fish exposed to low doses of PAHs in oil for 24 hours showed a significant increase in latent mortality [68]. In a pilot study performed by [69], pink salmon embryos exposed to Exxon Valdez oil, released into the wild, and then recaptured 2 years later exhibited a 15% reduction in survival. Although these three other studies did not test with an added UV factor, the findings from this present study were similar in that those fish exposed to thin oil sheens led to an increase in mortality several days to a week after exposure and decreased survival rates. With the added UV factor in this present study, it was demonstrated that fish exposed to thin oil sheens and UV light in one event could lead to a greater increased mortality rate in higher oil sheen exposures weeks later. This could potentially lead to reductions in survival rates over years like those seen in [69], and also changes in population structure.
Growth Parameters
Exposures to oil sheens for a 24 h period at and above 5.26 µg/L at 1-2 dph (UV and No UV light) had an impact on the lengths, weights, depths, and ocular diameters 30 days later in sheepshead minnows. Significant decreases in average size (length and depth) and weight were seen 30 days after exposure to tPAH50 concentrations of 2.75 µg/L (UV and No UV light) in spotted seatrout. Similar to the results found in this study, [70] found that sheepshead minnow larvae experienced significantly reduced lengths (5-13% reduction) and wet weights (13-35% reduction) with a long term exposure to LSC oil in sediment. Reduced wet weight has also been seen after a 28-day exposure to Macondo source oil in inland silversides [71].
In this present study, ocular diameter in sheepshead minnows was also affected but only in the 5.26 µg/L and 7.45 µg/L PAH treatments. Research conducted by [72] and [15] both found that the length of the retina and lens area, respectively, were smaller in oil treated larval zebrafish. [22] also found that there was a 11% and 15% decrease in lens diameter of 11 dph red drum and 8 dph sheepshead minnows, respectively, after a 24 h oil exposure prior to hatch.
No reductions in growth were observed between light treatments. This may be explained by the concentration of napthalenes and fluorenes in LSC and MC252 oil, which have low potential for phototoxicity [8,29,73]. [29] found that phototoxic compounds of oil appear to be restricted to specific PAHs with three to five fused benzene rings. LSC and MC252 oil have been shown to have a high concentration of naphthalenes and fluorenes, PAHs with only two benzene rings [8,73], suggesting the main oil components in this study have a low potential to become phototoxic. LSC contains relatively- high concentrations of phenanthrene, a compound with three benzene rings, thus, while photoenhanced toxicity was observed in the initial 24 h of LSC oil sheen exposures, the increased effect did not extend to long term effects on fish growth. Several studies, including [10,12,17,69,74], found that weathered oil, which typically contains more degradation, metabolites, and PAHs with three or more rings, is more likely to cause sublethal effects such as cardiac, ocular, and circulatory defects. Future studies should consider using crude oils containing higher proportions of PAHs with three or more rings to explore the interactive effect between PAH + UV on growth. Another potential cause of no reductions of growth after an exposure to PAH + UV light is the mechanism of action. Parent PAHs are typically those with three to four benzene rings and those that could be uptaken through bioaccumulation and photomodification mode of action and cause the associated cardiac effects. In this present study, if the mode of action that occurred was photosensitization, reactive products that were created within the larval fish’s body caused oxidative damage rather than associated growth effects. Therefore, growth may be a less relevant toxic endpoint for oil and UV exposures.
Although anecdotal, and not specifically recorded as an endpoint, throughout the grow-out phases for all species, there was noticeable swimming and behavioral impairments, such as swimming in circles and trouble maintaining position in the water column that lasted until the conclusion of the grow-out phase or became precursors to mortality. [75] saw a similar trend in larval fish which were previously exposed to oil and then removed to clean water. They started to exhibit melanosis, less mobility, reduced startle response, erratic swimming patterns, and loss of equilibrium. [21] also saw decreased swimming performance in 25 day old mahi-mahi exposed to comparable PAH concentrations (1.2 µg/L) upon hatching. Research conducted by [76] shows that these types of behaviors are indicative of narcosis and typical of high short-term naphthalene dominated oil exposures, such as MC252 oil. Fish from this present study may have dealt with the same type of narcosis. This reveals that fish that do not experience significant mortality initially may still succumb to behavioral changes later on in life with the potential to affect prey-predator dynamics [11]. Future studies may consider tracking fish behavior to support this claim.
Oxidative Stress
MDA is one of the byproducts seen from an increase in free radicals and therefore, is often used as a biomarker to measure damage due to oxygen radical formation. Mean MDA concentrations for 30-31 dph sheepshead minnows exposed to PAH + UV at 1-2 dph were higher for all UV plus oil treatments when compared to fish exposed to oil treatments alone. Moreover, the highest mean levels of MDA production occurred in the 2 µm UV exposed sheen (tPAH50 concentration of 7.45 µg/L) and there was a significant upward increasing trend of MDA concentrations in the UV treatments. Ultimately, this demonstrates that fish had undergone oxidative stress and the effects remained 30 days after the exposure was ended. Similarly, [75] found there was increased lipid peroxidation occurring in pink salmon gill tissue in fish exposed to oil, UV, and oil plus UV. [77] also found that bluegill sunfish (40-55 g) exposed to concentrations of a PAH plus UV, produced a higher concentration of MDA than any other of their treatment groups tested. PAHs accumulate in fish tissues through passive diffusion across the gills, absorption through the skin, and through ingestion [44]. Since 1-2 dph sheepshead minnows have little to no pigment, UV light is easily able to penetrate a larval body, interact with PAHs, create reactive oxygen species, and cause chain reactions with the potential to damage cell membranes through various modes of action [30,78-81]. Cell membrane damage has physiological and immune health implications, such as latent mortality and impaired growth, as observed in this present study. These effects play a role in the survival and fitness of early life-stages and consequently, could have the potential to impact community structure if population dynamics are shifted due to increased early life mortality.
Conclusions
The effects of thin oil sheens demonstrated in this study occurred at environmentally relevant concentrations and are important due to the observed associated consequences of exposure to PAHs from thin sheens rather than WAFs. LOEC concentrations ranged from 3.0 (0.5 µm) to 7.45 (2.0 µm) µg/L tPAH50 for the species tested, which are well within in the lower range of tPAH50 concentrations reported during the Deepwater Horizon oil spill and effect concentrations in other studies [35,82]. The UV-A (λ=380 nm) instantaneous light readings measured in this study ranged from 7.74 µW/cm2 to 41.55 µW/cm2 with a total average of 23.80 ± 17.22 µW/cm2 and a total integrated average dose of 685.44 mW s/cm2 for 8 hours and 342.72 mW s/cm2 for 4 hours [35]. These measurements are relatively lower when compared to other studies, but the integrated 8 hour dose still falls within the range of UV light measurements encountered in Gulf of Mexico surface waters during Deepwater Horizon oil spill [83].
The implications of larval exposure to PAH + UV related toxicity are important to understand because they may have an effect on individual fish, population changes and ultimately, community structure. As demonstrated by this study, a combination of thin oil sheens and UV can have acute mortality effects on sheepshead minnows, red drum and spotted seatrout and latent mortality effects on sheepshead minnows. Exposure to oil sheens alone can still impact physiological processes that result in oxidative stress in sheepshead minnows and decreased growth in sheepshead minnows and spotted seatrout. Small changes that decrease larval fish survival and fitness can detrimentally impact the interconnected predator- prey dynamic of an ecosystem and even impact human activities such as recreational fishing.
This present study demonstrates that some, but not all, estuarine fish acutely exposed to oil have immediate and long-term consequences for survival and growth associated with short-term exposure. Co-exposure of oil with UV light significantly increased oil toxicity for all three species tested. Short-term (24 h) oil exposures induced sublethal effects 30 days later on fish growth (reduced lengths, depths, weights, and ocular diameters) and increased oxidative stress. The oil rainbow sheens, and water concentrations used for this research are similar to environmentally relevant concentrations that can be seen in estuarine waterways and therefore, the results from this present study represent possible outcomes for larval fish exposed to combinations of UV light and oil sheens or oil sheens alone in their first few days of life.
Acknowledgements
This project would not have been possible without the generous provision of red drum and spotted sea trout eggs from Aaron Watson and staff at the SC Department of Natural Resources Mariculture Division. Graduate student support for Danielle Beers was provided by the College of Charleston and the Slocum Lunz Foundation. We appreciate the assistance of the NCCOS Ecotoxicology Branch staff who provided support for this project including Pete Key, Blaine West, and James Daugomah. The NOAA, National Ocean Service does not approve, recommend, or endorse any proprietary product or material mentioned in this publication. The use of larval fish species for this project was approved under the College of Charleston’s Institutional Animal Care and Use Committee (2018-009).
Statements and Declarations
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Declarations of Interest
The authors have no personal/financial interest or belief that would affect their objectivity.
Ethical Approval
The use of larval fish was approved under the College of Charleston’s Institutional Animal Care and Use Committee (2018-009). All authors followed ethical and professional standards in the completion of this research study.
Consent to Participate
All authors consent to participate in this research study.
Consent to Publish
All authors consent to have this manuscript published.
References
- Incardona JP, Swarts TL, Edmunds RC, Linbo TL, Aquilina-Beck A, et al. (2013) Exxon Valdez to Deepwater Horizon: Comparable toxicity of both crude oils to fish early life stages. Aquat Toxicol 142-143: 303-316.
- Transportation Research Board and National Research Council (2003) Oil in the Sea III: Inputs, Fates and Effects. Washington, DC: The National Academies Press.
- Yuewen D, Adzigbli L (2018) Assessing the impact of oil spills on marine organisms. J Oceanogr Mar Res 6: 1.
- Alloy M, Baxter D, Stieglitz J, Mager E, Hoenig R, et al. (2016) Ultraviolet radiation enhances the toxicity of Deepwater Horizon oil to mahi-mahi (Coryphaena hippurus) embryos. Environ Sci Technol 50: 2011- 2017. [crossref]
- Baumard P, Budzinski H, Garrigues P, Sorbe JC, Burgeot Y, et al. (1998) Concentrations of PAHs (polycyclic aromatic hydrocarbons) in various marine organisms in relation to those in sediments and to trophic level. Mar Pollut Bull 36: 951-960.
- Weinstein JE (1996) Anthropogenic impacts on salt marshes-A review. In: (eds) Sustainable Development in the Southeastern Coastal Zone, eds. Vernberg FJ, Vernberg WB, Siewicki T, 20:135-170. Columbia, SC: Belle W. Baruch Library of Marine Science University of South Carolina.
- Xue W, Warshawsky D (2005) Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: a review. Toxicol Appl Pharmacol 206: 73-93. [crossref]
- Almeda R, Wambaugh Z, Wang Z, Hyatt C, Liu Z, et al. (2013a) Interactions between zooplankton and crude oil: toxic effects and bioaccumulation of polycyclic aromatic hydrocarbons. PLoS one 8: e67212.
- Almeda R, Baca S, Hyatt C, Buskey E (2014) Ingestion and sublethal effects of physically and chemically dispersed crude oil on marine planktonic copepods. Ecotoxicology 23: 988-1003.
- Carls MG, Rice SD, Hose JE (1999) Sensitivity of fish embryos to weathered crude oil: Part 1. Low-level exposure during incubation causes malformations, genetic damage, and mortality in larval pacific herring (Clupea pallasi). Environ Toxicol Chem 18: 481-493.
- Carvalho PSM, Kalil DCB, Novelli GAA, Bainy ACD, Fraga APM (2008) Effects of naphthalene and phenanthrene on visual and prey capture endpoints during early stages of the dourado Salminus brasiliensis. Mar Environ Res 66: 205-207. [crossref]
- Diamante G, Muller GAS, Menjivar-Cervantes N, Xu EG, Volz DC, et al. (2017) Developmental toxicity of hydroxylated chrysene metabolites in zebrafish embryos. Aquat Toxicol 189: 77-86. [crossref]
- Dubansky B, Whitehead A, Miller JT, Rice CD, Galvez G (2013) Multitissue molecular, genomic, and developmental effects of the Deepwater Horizon oil spill on resident gulf killifish (Fundulus grandis). Environ Sci Technol 47: 5074-5082. [crossref]
- Frometa J, DeLorenzo ME, Pisarski E, Etnoyer PJ (2017) Toxicity of oil and dispersant on the deep water gorgonian octocoral Swiftia exserta, with implications for the effects of the Deepwater Horizon oil spill. Mar Pollut Bull 122: 91-99.
- Huang L, Wang C, Zhang Y, Wu M, Zuo Z (2013) Phenanthrene causes ocular developmental toxicity in zebrafish embryos and the possible mechanisms involved. J Hazard Mater 261: 172-180. [crossref]
- Huang L, Zuo Z, Zhang Y, Wu M, Lin JJ, et al. (2014) Use of toxicogenmoics to predict the potential toxic effect of benzo(a)pyrene on zebrafish embryos: Ocular developmental toxicity. Chemosphere 108: 55-61. [crossref]
- Incardona JP, Collier TK, Scholz NL (2004) Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol Appl Pharmacol 196: 191-205. [crossref]
- Incardona JP, Gardner LD, Lindo TL, Brown TL, Esbaugh AJ, et al. (2014) Deepwater Horizon crude oil impacts the developing hearts of large predatory pelagic fish. Proc Nat Acad Sci U.S.A. 111: 1510-1518.
- Johansen JL, Esbaugh AJ (2017) Sustained impairment of respiratory function and swim performance following acute oil exposure in a coastal marine fish. Aquat Toxicol 187: 82-89. [crossref]
- Khursigara AJ, Perrichon P, Bautista NM, Burggren WW, Esbaugh AJ (2017) Cardiac function and survival are affected by crude oil in larval red drum, Sciaenops ocellatus. Sci Total Environ 579: 797-804. [crossref]
- Mager EM, Esbaugh AJ, Stieglitz JD, Hoenig R, Bodinier C, et al. (2014) Acute embryonic or juvenile exposure to Deepwater Horizon crude oil impairs the swimming performance of mahi-mahi (Coryphaena hippurus). Environ Sci Technol 48: 7053-7061. [crossref]
- Magnuson JT, Khursigara AJ, Allmon EB, Esbaugh AJ, Roberts AP (2018) Effects of Deepwater Horizon crude oil on ocular development in two estuarine fish species, red drum (Sciaenops ocellatus) and sheepshead minnow (Cyprinodon variegatus). Ecotoxicol Environ Saf 166: 186-191. [crossref]
- Rice SD, Thomas RE, Carls MG, Heintz RA, Wetheimer AC, et al. (2001) Impacts to pink salmon following the Exxon Valdez oil spill: Persistence, toxicity, sensitivity and controversy. Rev Fish Sci 9: 165-211.
- Whitehead A, Dubansky B, Bodinier C, Garcia TI, Miles S, et al. (2012) Genomic and physiological footprint of the Deepwater Horizon oil spill on resident marsh fishes. Proc Natl Acad Sci U.S.A. 109: 20298-20302. [crossref]
- Xu EG, Mager EM, Grosell M, Pasparakis C, Schlenker LS, et al. (2016) Time- and oil- dependent transcriptomic and physiological responses to Deepwater Horizon oil in mahi-mahi (Coryphaena hippurus) embryos and larvae. Environ Sci Technol 50: 7842-7851.
- Xu EG, Khursigara AJ, Magnuson J, Hazar ES, Hardiman G, et al. (2017) Larval red drum (Sciaenops ocellatus) sublethal exposure to weathered Deepwater Horizon crude oil: Developmental and transcriptomic consequences. Environ Sci Technol 51: 10162- 10172. [crossref]
- Ankley GT, Erickson RJ, Sheedy BR, Kosian PA, Mattson VR, et al. (1997) Evaluation of models for predicting the phototoxic potency of polycyclic aromatic hydrocarbons. Aquat Toxicol 37: 37-50.
- Arfsten DP, Schaeffer DJ, Mulveny DC (1996) The effects of near ultraviolet radiation on the toxic effects of polycyclic aromatic hydrocarbons in animals and plants: a review. Ecotoxicol Environ Saf 33:1-24. [crossref]
- Barron MG, Kaaihue L (2001) Potential for photoenhanced toxicity of spilled oil in Prince William Sound and Gulf of Alaska waters. Mar Pollut Bull 43: 86-92. [crossref]
- Landrum PF, Giesy JP, Oris JT, Allred PM (1987) Photoinduced toxicity of polycyclic aromatic hydrocarbons to aquatic organisms. In: Oil in Freshwater, eds. J.H. Vandermeulen, S. E. Hrudey. New York: Pergamon Press.
- Larson RA, Berenbaum MR (1988) Environmental phototoxicity: Solar ultraviolet radiation affects the toxicity of natural and man-made chemicals. Environ Sci Technol 22: 354-360.
- Pelletier MC, Burges RM, Ho KT, Kuhn A, McKinney RA, et al. (1997) Phototoxicity of individual polycyclic aromatic hydrocarbons and petroleum to marine invertebrate larvae and juveniles. Environ Toxicol Chem 16: 2190-2199.
- Sweet LE, Magnuson J, Garner TR, Alloy MM, Stieglitz JD, et al. (2017) Exposure to ultraviolet radiation late in development increases the toxicity of oil to mahi-mahi (Coryphaena hippurus) embryos. Environ Toxicol Chem 36: 1592-1598. [crossref]
- Alloy MM, Boube I, Griffitt RJ, Oris JT, Roberts AP (2015) Photo-induced toxicity of Deepwater Horizon slick oil to blue crab (Callinectes sapidus) larvae. Environ Toxicol Chem 34: 2061-2066. [crossref]
- Alloy M, Garner TR, Bridges K, Mansfield C, Carney M, et al. (2017) Co-exposure to sunlight enhances the toxicity of naturally weathered Deepwater Horizon oil to early lifestage red drum (Sciaenops ocellatus) and speckled seatrout (Cynoscion nebulosus). Environ Toxicol Chem 36: 780-785. [crossref]
- Almeda R, Harvey TE, Connelly TL, Baca S, Buskey EJ (2016) Influence of UVB radiation on the lethal and sublethal toxicity of dispersed crude oil to planktonic copepod nauplii. Chemosphere 152: 446-458. [crossref]
- Barron MG, Carls MG, Short JW, Rice SD (2003) Photoenhanced toxicity of aqueous phase and chemically dispersed weathered Alaska North Slope crude oil to pacific herring eggs and larvae. Environ Toxicol Chem 22: 650-660. [crossref]
- Boese BL, Lamberson JO, Swartz RC, Ozretich RJ (1997) Photoinduced toxicity of fluoranthene to seven marine benthic crustaceans. Arch Environ 32: 389-393. [crossref]
- Bridges KN, Lay CR, Alloy MM, Gielazyn ML, Morris JM, et al. (2018a) Estimating incident ultraviolet radiation during the Deepwater Horizon oil spill. Environ Toxicol Chem 37: 1679-1687. [crossref]
- Cleveland L, Little EE, Calfee RD, Barron MD (2000) Photoenhanced toxicity of weathered oil to Mysidopsis bahia. Aquat Toxicol 49: 63-76. [crossref]
- Damare LM, Bridges KN, Alloy MM, Curran TE, Soulen BK, et al. (2018) Photo-induced toxicity in early life stage fiddler crab (Uca longisignalis) following exposure to Deepwater Horizon Ecotoxicology 27: 440-447. [crossref]
- Diamond SA, Milroy NJ, Mattson VR, Heinis LJ, Mount DR (2003) Photoactivated toxicity in amphipods collected from polycyclic aromatic hydrocarbon-contaminated sites. Environ Toxicol Chem 22: 2752-2760. [crossref]
- Finch BE, Stefansson ES, Langdon CJ, Pargee SM, Stubblefield WA (2018) Photo-enhanced toxicity of undispersed and dispersed weathered Macondo crude oil to Pacific (Crassostrea gigas) and eastern oyster (Crassostrea virginica) larvae. Mar Pollut Bull 133: 828-834. [crossref]
- Finch BE, Stubblefield WE (2016) Photo-enhanced toxicity of fluoranthene to Gulf of Mexico marine organisms at different larval ages and ultraviolet light intensities. Environ Toxicol Chem 35: 1113-1122. [crossref]
- Little EE, Cleveland L, Calfee R, Barron MG (2000) Assessment of the photoenhanced toxicity of a weathered oil to the tidewater silverside. Environ Toxicol Chem 19: 926-932.
- Sweet LE, Revill AT, Strzelecki J, Hook SE, Norris JM, et al. (2018) Photo-induced toxicity following exposure to crude oil and ultraviolet radiation in two Australian fishes. Environ Toxicol 37: 1359-1366.
- Vaca CE, Wilhelm EJ, Harms-Ringdahl M (1988) Interaction of lipid peroxidation products with DNA: A review. Mutat Res 195: 137-149. [crossref]
- Frederick PC, Loftus WF (1993) Responses of marsh fishes and breeding wading birds to low temperatures: A possible behavioral link between predator and prey. Estuaries 16: 216-222.
- Kushlan J (1980) Prey choice by tactile-foraging wading birds. Colon Waterbird 3: 133-142.
- Atlantic States Marine Fisheries Commission (2020) Red Drum. Accessed 28 January 2020.
- NOAA Fisheries (2020) Science & Data. Accessed 28 January 2020.
- Swingle WE (1990) Status of the commercial and recreational fishery. In: Red Drum Aquaculture. College Station, Texas: Texas A&M Sea Grant Program.
- Hunter JR, Kaupp SE, Taylor JH (1980) Assessment of effects of UV radiation on marine fish larvae. In: The Role of Solar Radiation in Marine Ecosystems, ed. J. Calkins. New York: Plenum Press.
- Roberts AP, Allo MM, Oris JT (2017) Review of the photo-induced toxicity of environmental contaminants. Comp Biochem Physiol 191: 160-167. [crossref]
- Ringwood AH, Houget J, Keppler CJ, Gielazyn ML, Ward BP, et al. (2003) Cellular Biomarkers (lipid destabilization, glutathione and lipid peroxidation) in three common estuarine species: A methods handbook. Marine Resource Institute, South Carolina Department of Natural Resources: Charleston, SC.
- May LA, Burnett AR, Miller CV, Pisarski E, Webster LF, et al. (2020) Effect of Louisiana sweet crude oil on a Pacific coral, Pocillopora damicornis. Aquat Toxicol 200: 105454.
- Newman MC (1995) Quantitative methods in aquatic ecotoxicology. In: Advances in Trace Substances Research. Boca Raton, Florida: Lewis Publishers.
- Hamilton MA, Russo RC, Thurston RV (1978) Trimmed Spearman-Karber method for estimating median lethal concentrations in bioassays. Environ Sci Technol 12: 417.
- Belsley DA, Kuh E, Welsch RE (1980) Regression Diagnostics: Identifying Influential Data and Sources of Collinearity. Hoboken, New Jersey: John Wiley & Sons.
- Cook R (1977) Detection of influential observations in linear regression. Technometrics 19: 15-18.
- Sea Grant Louisiana (2019) Louisiana Fisheries. Biological Info: Red Drum. Accessed 24 February 2020.
- NOAA Data Integration and Visualization Exploration and Reporting (DIVER). 2020. Deepwater Horizon DRDA Data, NOAA’s Deepwater Horizon Trustee Toxicity Testing Program Results.
- Bennett WA, Beitinger TL (1997) Temperature tolerance of the sheepshead minnows, Cyprinodon variegatus. Copeia 1: 77-87.
- Diamond SA, Mount DR, Burkhard LP, Ankley GT, Makynen EA, et al. (2000) Effect of irradiance spectra on the photoinduced toxicity of three polycyclic aromatic hydrocarbons. Environ Toxicol Chem 19: 1389-1396.
- Hodson PV (2017) The toxicity of fish embryos of PAH in crude and refined oils. Arch Environ Contam Toxicol 73: 12-18. [crossref]
- Pasparakis C, Esbaugh AJ, Burggren W, Grosell M (2019) Physiological impacts of Deepwater Horizon oil on fish. Comp Biochem Physiol Part-C: Toxicol 224: 1-29.
- Duffy TA, Childress W, Portier R, Chesney EJ (2016) Responses of bay anchovy (Anchoa mitchilli) larvae under lethal and sublethal scenarios of crude oil exposure. Ecotoxicol Environ Saf 134: 264-272. [crossref]
- Johansen JL, Allan BJM, Rummer JL, Esbaugh AJ (2017) Oil exposure disrupts early life-history stages of coral reef fishes via behavioural impairments. Nat Ecol Evol 1: 1146-1152. [crossref]
- Heintz RA, Short JW, Rice SD (1999) Sensitivity of fish embryos to weathered crude oil: Part 2. Increased mortality of pink salmon (Oncorhynchus gorbuscha) embryos incubating downstream from weathered Exxon Valdez crude oil. Environ Toxicol Chem 18: 494-503.
- Raimondo S, Hemmer BL, Lilavois CR, Krzykwa J, Almario A, et al. (2015) Effects of Louisiana crude oil on the sheepshead minnow (Cyprinodon variegatus) during a life-cycle exposure to laboratory oiled sediment. Environ Toxicol 31: 1627-1639. [crossref]
- Echols B, Smith A, Gardinali PR, Rand GM (2016) Chronic toxicity of unweathered and weathered Macondo oils to mysid shrimp (Americamysis bahia) and inland silversides (Menidia beryllina). Arch Environ Contam Toxicol 71: 78-86.
- de Soysa TY, Ulrich A, Friedrich T, Pite D, Compton S, et al. (2012) Macondo crude oil from the Deepwater Horizon oil spill disrupts specific developmental processes during zebrafish embryogenesis. BMC Biol 10: 40. [crossref]
- Overton EB, Wade TL, Radovic JR, Meyer BM, Miles MS, et al. (2016) Chemical composition of Macondo and other crude oils and compositional alterations during oil spills. Oceanography 29: 50-63.
- Brette F, Shiels HA, Galli GLJ, Cros C, Incardona JP, et al. (2017) A novel cardiotoxic mechanism for a pervasive global pollutant. Sci Rep 7: 41476. [crossref]
- Barron MG, Carls MG, Short JW, Rice SD, Heintz RA, (2005) Assessment of the phototoxicity of weathered Alaska North Slope crude oil to juvenile pink salmon. Chemosphere 60: 105-110. [crossref]
- Rice SD, Short JW, Brodersen CC, Mecklenburg TA, Moles DA, et al. (1976) Acute toxicity and uptake-depuration studies with Cook Inlet crude oil, Prudhoe Bay crude oil, No. 2 fuel oil, and several subarctic marine organisms. Processed Report. Northwest Fisheries Center Auke Bay Fisheries Laboratory, Juneau, AK.
- Choi J, Oris JT (2000) Evidence of oxidative stress in bluegill sunfish (Lepomis macrochirus) liver microsomes simultaneously exposed to solar ultraviolet radiation and anthracene. Environ Toxicol Chem 9: 1795-1799.
- McCloskey JT, Oris JT (1993) Effect of anthracene and solar ultraviolet radiation exposure on gill ATPase and selected hematologic measurements in the bluegill sunfish (Lepomis macrochirus). Aquat Toxicol 24: 207-218.
- Oris JT, Giesy JPJr (1986) Photoinduced toxicity of anthracene to juvenile bluegill sunfish (Lepomis macrochirus Rafinesque): Photoperiod effects and predictive hazard evaluation. Environ Toxicol Chem 5: 761-768.
- Oris JT, Giesy JP Jr (1987) The photo-induced toxicity of polycyclic aromatic hydrocarbons to larvae of the fathead minnow (Pimephales promelas). Chemosphere 16: 1395-1404.
- Weinstein JE, Oris JT, Taylor DH (1997) An ultrastructural examination of the mode of UV-induced toxic action of fluoranthene in the fathead minnow, Pimephales promelas. Aquat Toxicol 39: 1-22.
- Diercks AR, Highsmith RC, Asper VL, Joung D, Zhou Z, et al. (2010) Characterization of subsurface polycyclic aromatic hydrocarbons at the Deepwater Horizon Geophys Res Lett 37: L20602.
- Bridges KN, Krasnec MO, Magnuson JT, Morris JM, Gielazyn ML, et al. (2018b) Influence of variable ultraviolet radiation and oil exposure duration on survival of red drum (Sciaenops ocellatus) larvae. Environ Toxicol 37: 2372-2379. [crossref]