Abstract
Aims: Behavioral pattern separation is a hippocampal-dependent component of episodic memory and a sensitive marker of early cognitive decline. Here we tested whether mild traumatic injury causes loss of pattern separation in the rat and for its prevention by a novel neuroprotective peptide fragment of the human serotonin 2A receptor (SN..8).
Methods: Lateral fluid percussion was used to induce mild traumatic brain injury in male Sprague- Dawley rats. Rats were trained to distinguish between a stable vs unstable swim platform separated by increasing distances (4.5 vs 3.0 vs 1.5 feet) in a modification to the classic Morris water maze. Peptide SN..8 vs scrambled version of same amino acids (2 mg/kg) was administered via intraperitoneal route (1-, 3- and 5-days) after lateral fluid percussion or sham injury. Rats received three weeks of training and two weeks of testing before injury and were tested again at 2 and 5-weeks after injury.
Results: There was a gradient of decreasing incorrect responses to the choice between (stable vs unstable platform) as the platform separation distance was increased from 1.5 to 3.0 to 4.5 feet consistent with behavioral pattern separation. Systemic administration of SN..8 peptide (vs scrambled) peptide was associated with statistically significant lower rate of incorrect responses (at both 4.5 feet and 3.0 feet platform separation) in traumatic brain- injured rats (but not in sham-injured rats) tested at 2-weeks post-injury. Five weeks after injury, the rats had largely recovered and exhibited a much lower overall rate of incorrect responses across both drug and injury subgroups.
Conclusions: Introduction of an unstable platform (choice phase of the Morris water maze) at varying distances from the stable platform resulted in behavior having the hallmark of pattern separation. Our data are the first to suggest that systemic administration of (2 mg/kg) SN..8 peptide immediately after mild traumatic brain injury (lateral fluid percussion) appeared to protect against loss of behavioral pattern separation in the rat.
Introduction
Accelerated cognitive decline frequently complicates traumatic brain injury (TBI) [1]. Pattern separation- the ability to encode similar spatial representations as distinct objects [2] is a hippocampal- dependent component of working memory [2]. Loss of pattern separation is an early marker of cognitive decline in humans [3]. The serotonin 2A receptor (5HT2A) is expressed on neurons, and neural progenitor cells in the dentate gyrus and hippocampus [4]. Agonists of the 5HT2A receptor in this brain region were reported to impair recall of spatial memory [5]. We designed a peptide identical to a sub-region of the human 5HT2AR (SN..8) involved in long-lasting receptor activation [6,7]. Systemic administration of SN..8, in a genetic strain of rats (Zucker) harboring neurotoxic 5-HT2A receptor activating IgG plasma autoantibodies [8] enhanced acquisition and recall of spatial memory in sham, but not in traumatic brain-injured lean Zucker rats [9]. Here we tested a different strain of rat, adult male Sprague-Dawley, for neuroprotection by SN..8 when administered immediately after mild traumatic brain injury (mTBI) in a pattern separation task which is hippocampal dependent and a highly sensitive marker of early cognitive decline.
Methods
Peptides
The linear synthetic peptide, corresponding to a fragment of the serotonin 2a receptor, SCLLADDN (SN..8) and a scrambled version LASNDCLD (LD.8) were both synthesized at Lifetein, Inc. (Hillsborough, NJ). Each peptide was provided as the hydrochloride salt and had purity > 95%. The lyophilized peptides were stored (in the presence of dessicant) at −40 degrees C prior to use. Before each experiment, peptide was reconstituted fresh in sterile saline at the indicated concentration.
Animals
All procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the Veterans Affairs Medical Center (East Orange, New Jersey). Male SD rats n =38 (8-weeks-old) were obtained from Charles River Laboratories (Kingston, NY) and were individually housed with modest enrichment (wooden block). Rats were provided ad libitum access to food and water and maintained in a 12 h light/dark cycle with lights on at 0700. Training and testing were performed during the light phase of the light/ dark cycle. They underwent pattern separation pre-training training for 12 days over three weeks and testing for 6 days over two weeks. At approximately 17 weeks of age, rats underwent surgery (craniectomy) and injury (lateral fluid percussion) (See Timeline, Figure 1).
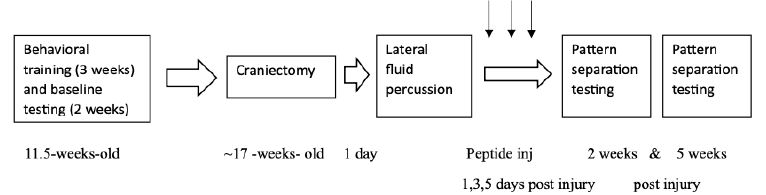
Figure 1: Timeline of experimental procedures
Injections
Peptide (SN..8 or LD.8) was dissolved in sterile saline (2 mg/kg) and administered via intraperitoneal (IP) route 1-, 3- and 5-days after mild TBI vs sham injury.
Surgery/Injuries
Craniectomy and delivery of a pressure wave (lateral fluid percussion) procedures were carried out as previously reported [10]. The procedures are briefly summarized here. Day 1: Craniectomy-A 4 mm diameter craniectomy was performed under anesthesia with isoflurane, 3mm posterior and 3.5mm lateral to the bregma was made unilaterally in either the left or right parietal bone (figure). During the craniectomy, the skull was removed but the dura mater remains intact. A luer-lock connector was glued to the skull surrounding the craniectomy. A plastic cylinder about 2 mL was placed surrounding the craniectomy to protect the luer-lock connector. Dental cement was placed inside the plastic cylinder. A small Kim wipe was inserted inside the luer-lock to keep the dura moist and clean of debris. Lateral Fluid Percussion Injury– Twenty-four hours after the surgery, rats were anesthetized with isoflurane at 5 liters/min for (1min 30sec). The Kim wipe was removed from the luer-lock and filled with sterile saline. The luer-lock was then connected to the fluid percussion device and once rats reacted to a strong toe pinch, the fluid percussion injury was delivered to the exposed dura matter dorsal to the parietal lobe, via a voice-coil piston device. The pressure sensors located at the end of the pistol records PSI waves. Acute signs (Table 1) were recorded at the time of injury, including: startle, apnea (time in seconds from the time of injury to the time the rat returns to regular breathing) and righting reflex (RR= time in seconds from the time of injury until the time the rat fully supports its weight on all four paws). Sham animals underwent all procedures except they did not receive the fluid percussion injury.
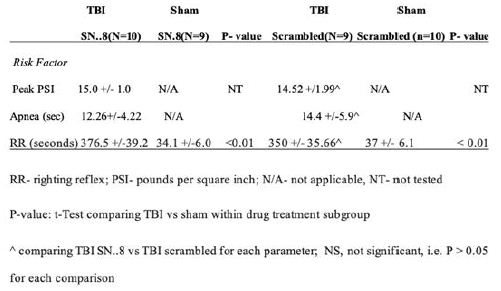
Table 1: Acute signs of injury in rats randomized to either SN..8 or scrambled LD..8 peptide injections and mild TBI vs sham injury
Behavioral Tests
Pattern Separation
A sixty-three- inch diameter metal pool with a stable and unstable platform visible above water line was used to complete the pattern separation task. The stable platform fully supports the weight of the rat and enables them to completely climb out of the water. The unstable platform appears identical to the stable platform; however, it does not support the rats’ weight and will not allow the rat to the climb out of the water.
Training
All animals underwent 3 weeks of training consisting of 4 consecutive training days per week of followed by 3 days of rest, e.g. week 1, training days 1-4; week 2 training days 5-8, week 3, training days 9-12 (Figure 1). This was followed by six days of baseline testing.
Training Day 1: Animal learns that to ‘escape’ from the pool, must be from stable platform. The maximum time for each trial is 60 seconds (60s); the rat must remain on the stable platform for 30 seconds (30s) to complete the trial. If after the 60s they can’t find the stable platform, they are guided to it and must stay on it for 30s before being removed. During trial 1, they are placed on the stable platform in the middle of the pool. In trial 2, they are placed in the middle between the platform and the edge of the pool approximately 12 inches from the platform. In trial 3, they are placed on the edge of the pool approximately 32 inches.
Training Day 2: Animal are run through a classic water maze protocol where the platform stays in the same location during all three trials. Stable platform is placed in quadrant 1 of the pool and rats undergo 3 trials with 1 hour time in between trials. The starting location of the rat changes in between trials. During trial 1, the starting location of the animals was between quadrant 1 and 2. In trial 2, the starting location was between quadrant 2 and 3. In trial 3, the starting location was between quadrant 3 and 4 (Figure 2).
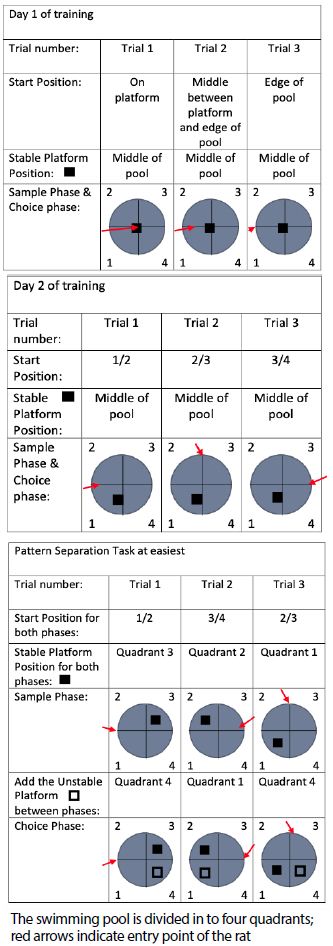
Figure 2: Training in the pattern separation task
Training Days 3-4: animal learns to search for stable platform within trials. Here, the location of the stable platform and the start position of the animal’s changes between trials. Within each trial, all animals are put in the water twice (sample phase and choice phase). During all three trials, the starting location of the animals to the platform is 4.5ft. During trial 1 the starting location of the animals was between quadrant 1 and 4. In the sample and choice phase, the stable platform is in quadrant 2. In trial 2, the starting location to the platform was between quadrant 3 and 4 and the location of the stable platform remained in quadrant 1 for both sample and choice phase. Trial 3 starting location to the platform was between quadrant 2 and 3 and the location of the stable platform remained at quadrant 4 for both the sample and choice phase.
Training Days 5-6: Introduction of unstable platform. Animal learns to search for stable platform within a trial and ignore unstable platform. The stable platform and the start point of the animals stayed the same between trials. During trial 1, animals run through easy pattern separation where the sample phase only consists of the stable platform and during the choice phase, we introduced the unstable platform. The unstable platform is placed in the water during the choice phase. For trial 2 and 3, the stable and the unstable platform are in the water at the same, at different location. The starting location of the animals during all three trials remain the same. Animal are run through “easy pattern separation” with the distance from the starting location to the platform to be 4.5ft.
Training Days 7-12: animal learns to search for stable platform within a trial and ignore unstable platform. Run animal through easy pattern separation task (at 4.5 ft from start location). Start location of animals, location of stable and unstable platform changes during each trial. After 12 days it was determined that approximately 25% of rats were correctly choosing the stable vs unstable platform, and in order to avoid ‘overtraining’ no further baseline training trials were performed.
Test Trials
Testing consists of sample phase and choice phase which begin 3 days after training and span 6 days over a two-week time period (three days per week). Results in the choice phase are indicative of pattern separation. The start location (pool quadrant) and the relative location of the unstable and stable platforms changes between individual testing trials (3 trials per day) and on each new testing day. Between the sample and choice phases, the rats are removed from the platform and given a 30- second break. There is an additional one- hour break between each successive trial.
Scoring/Data Collection
During testing, an incorrect response is when the rats touch and attempt to climb the unstable platform. The number of times each rat attempt to go to the unstable trial within each trial is recorded and percent incorrect is calculated as [incorrect responses/total responses].
Statistics
Student’s t-test was used for single comparisons. A P-value <0.05 was considered significant and values are expressed as means ± SEM. There was no correction for multiple comparisons.
Results
Acute Signs of Mild TBI (Lateral Fluid Percussion)
Mean apnea time and mean righting reflex time were significantly longer in rats subjected to mTBI vs sham-injury (Table 1). There was no statistically significant difference in mean apnea time or mean righting reflex time following lateral fluid percussion in rat subgroups randomized to treatment with SN..8 vs scrambled peptide injections on days 1, 3 and 5 after injury. The mean peak pressure (PSI, pounds per square inch) applied during the fluid percussion wave did not differ significantly between rats treated with SN..8 vs scrambled peptide following mTBI (Table 1).
Behavioral Pattern Separation (BPS)
Baseline
In baseline pre-injury testing, rats made fewer errors (14.2 vs 26.7 vs 35.8%) in behavioral pattern separation (Figure 3) at greater distance(s) between the (stable and unstable) platforms i.e. 4.5 vs 3.0 vs 1.5 feet. The observed gradient of increasing error rate as the spatial representations become less dissimilar is consistent with pattern separation. The difference in baseline error rate at platform separation distance of 4.5 vs 1.5 feet was statistically significant (N=39; P< 0.01). Because of the much higher baseline error rate at 1.5 foot platform separation distance, post-injury data was only analyzed and reported for the 3.0 and 4.5 foot platform separation distances.
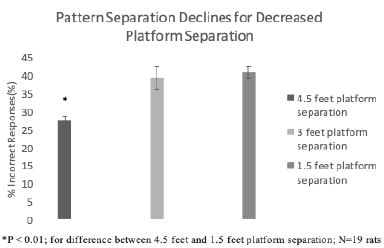
Figure 3: Baseline pattern separation declines by decreasing distance between platforms
Post-Injury
Rats treated with SN..8 vs scrambled peptide displayed significantly lower error rates (two weeks post-injury): (6.7 vs 25.9 %; N=19, P< 0.01) at both 4.5 feet and (20.0 vs 42.7% (N=19; P= 0.039); at 3.0 feet platform separation (Figure 4). There was no significant difference in BPS performance between SN..8 vs scrambled peptide- treated sham-injured rats two weeks’ post-injury (Figure 5). Across all drug and injury subgroups, the composite error rate was significantly lower at five- vs. two- weeks’ post-injury (7.75 +/- 4.4 vs 17.09 +/9%; P = 0.009) (Figure 6). This may be consistent (in part) with spontaneous recovery from injury after 5 weeks and increased experience with the task. In summary, systemic SN..8 (2 mg/kg) administered in three successive alternate daily doses (starting 1 day after mTBI) appeared to have a neuroprotective effect on early loss of behavioral pattern separation in adult male SD rats.
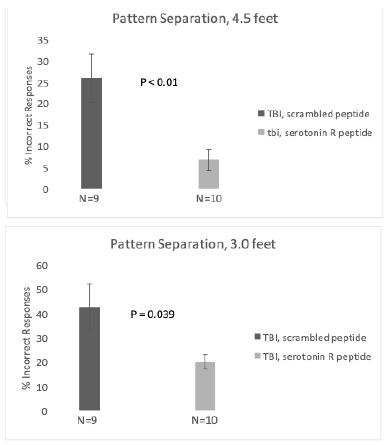
Figure 4: Treatment with SN..8 vs scrambled peptide after mTBI is associated with significantly improved behavioral pattern separation at A) 4.5 feet and B) 3.0 feet platform distances.
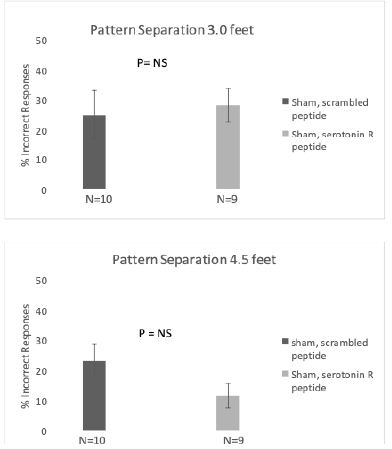
Figure 5: Treatment with SN..8 vs scrambled peptide sham injury is associated with no significant differences in behavioral pattern separation at A) 4.5 feet and B) 3.0 feet platform distance.
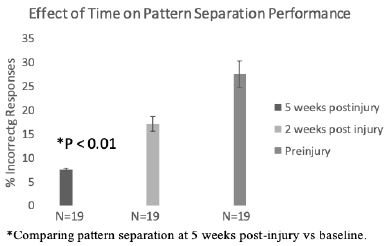
Figure 6: Substantial improvement in pattern separation performance 5 weeks post-injury
Discussion
Behavioral pattern separation is thought to be a component of working memory which has an underlying neural circuitry that largely resides in the dentate gyrus and hippocampus [11]. Transient impairment in behavioral pattern separation reported here is consistent with a prior report that spatial memory was impaired (early 1-7 days) but recovered spontaneously 21 days following mild TBI (lateral fluid percussion) in Sprague-Dawley rats [10]. The mean peak pressure, apnea period, and righting reflex times experienced (by SD rats) in the present study were slightly lower than reported in the prior study [10], but apnea and righting reflex times are consistent with mild traumatic brain injury. Our findings suggest that introducing an unstable platform during the choice phase of the classic Morris water maze test is a useful method to model behavioral pattern separation in rats.
The mechanism of transient impairment of pattern separation following mTBI (lateral fluid percussion) in the SD rat is unknown. Cortical expression of both 5HT2A and a related catecholamine receptor, the alpha 1 adrenergic receptor was reported to increase (in rodents) following different forms of TBI [12, 13]. Much less is known about possible catecholamine receptor changes in the hippocampus following TBI. The hippocampus receives a dense projection of serotonergic fibers from the dorsal raphe [14]. The 5HT2AR was reported to mediate in part changes in synaptic input to hippocampal granule cells [15] which could result in impaired development of newly-born neurons derived from dentate gyrus neural progenitor cells. Reduced dentate gyrus neurogenesis is one of the mechanisms thought to underly impaired pattern separation [11]. Dentate gyrus neurogenesis plays an important role not only in pattern separation but also mood regulation, and in a prior study we found that human depression patients harbored plasma 5-HT2AR activating IgG autoantibodies [16] which impaired the survival and differentiation of rat DG neural progenitor cells [17,18] in vitro.
SN..8 is a small peptide having an amino acid sequence identical to that of a subregion of the second extracellular loop of the human 5HT2AR involved in mediating long-lasting receptor activation [6]. Although the SN..8 mechanism of action is not completely understood, it prevented neurotoxicity (in vitro) mediated by Ig isolated from plasma of patients with neurodegenerative disorders including Parkinson’ disease, dementia [18] and major depressive disorder [6]. Immunoglobulin G from a subset of patients with TBI displayed increased binding to the human 5HT2A receptor second extracellular loop peptide [6] (which includes SN..8). Baseline presence of 5HT2AR peptide binding in plasma human TBI IgG predicted accelerated (two-year) prospective decline in cognitive function in thirty-five older adult TBI patients [19].
Our underlying hypothesis is that long-lasting 5HT2AR agonist Ig may mediate in part cognitive decline following TBI. It is not clear to what extent Ig may have been a contributory factor in Sprague-Dawley rat since in our preliminary experiments (not shown here) the titer and potency of SD plasma Ig was significantly lower than what we had previously reported in the Zucker rat [8]. Still SN..8 may serve either as a ‘decoy receptor’ to prevent neurotoxicity from 5-HT2AR- targeting agonist Ig and/or stabilize an inactive conformation of the 5HT2AR. It is not known whether dysregulated serotonergic input to the hippocampus (following mTBI) might alter synaptic input to developing neurons in the dentate gyrus [15,20] which could result in reduced neurogenesis [20,21] which is a hallmark of reduced pattern separation [11].
In a prior study, systemic (IP) administration of SN..8 (vs. scrambled peptide) strengthened both recall and acquisition of spatial learning after sham injury (but not after mild traumatic brain injury) in Zucker lean rats. Genetic strain differences between Sprague- Dawley and Zucker rats might account in part for a neuroprotective effect (following mTBI) by SN..8 in SD but not in Zucker rats. It is also possible that pattern separation is a more sensitive method for detecting the earliest cognitive impairment changes following mTBI. More study using pattern separation in different genetic strains of rat can help clarify the differences.
Acknowledgments
Supported in part by a grant from the New Jersey Commission on Brain Injury Research NJCBIR PIL022 to MBZ; and a grant from the Department of Veterans Affairs, Office of Research and Development, Technology Transfer Program (Wash, DC) to MBZ.
References
- Walker KR, Tesco G (2013) Molecular mechanisms of cognitive dysfunction following traumatic brain Front Aging Neurosci 5: 29. [crossref]
- Yassa MA, Stark CE (2011) Pattern separation in the hippocampus. Trends Neurosci. 34 (10): 515-25
- Stark SM, Yassa MA, Stark CE (2010) Individual differences in spatial pattern separation performance associated with healthy aging in Learn Mem. May 21;17 (6): 284-8 [crossref]
- Xu T and Pandey S C (2000) Cellular localization of serotonin (2A) (5HT (2A)) receptors in the rat brain. Brain Res. Bull. 51, 499-505. [crossref]
- Zhang G, Stackman RW Jr (2015) The role of serotonin 5-HT2A receptors in memory and cognition. Front Pharmacol 6: 225 [crossref]
- Zimering MB (2019) Autoantibodies in Type-2 Diabetes having Neurovascular Complications Bind to the Second Extracellular Loop of the 5-Hydroxytryptamine 2A Endocrinol Diabetes Metab J 3: 118. [crossref]
- Zimering MB (2021) A serotonin 2A receptor decoy peptide potently lowers blood pressure in male Zucker diabetic fatty rats. Endo Diab Metab J 5: 1-13. [crossref]
- Zimering MB, Grinberg M, Burton J, Pang K (2020) Circulating Agonist Autoantibody to 5-Hydroxytryptamine 2A Receptor in Lean and Diabetic Fatty Zucker Rat Endocrinol Diabetes Metab J 4: 413. [crossref]
- Grinberg M, Burton J, Pang KCH, Zimering MB (2023) Neuroprotective Effects of a Serotonin Receptor Peptide Following Sham Mild Traumatic Brain Injury in the Zucker Rat. Endocrinol Diabetes Metab J Volume 7 (3): 1-9. [crossref]
- Pang KC (2015), et al., Long-lasting suppression of acoustic startle response after mild traumatic brain injury. J Neurotrauma, 32 (11): p. 801-10. [crossref]
- Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, et al (2008) Spatial relational memory requires hippocampal adult neurogenesis. PLoS One 3: e1959. [crossref]
- Collins SM, O’Connell CJ, Reeder EL, Norman SV, Lungani K, Gopalan P, Gudelsky GA, Robson MJ (2022) Altered Serotonin 2A (5-HT2A) Receptor Signaling Underlies Mild TBI-Elicited Deficits in Social Dominance. Front Pharmacol. 15;13: 930346. [crossref]
- Kobori N, B Hu and PK Dash (2011) Altered adrenergic receptor signaling following traumatic brain injury contributes to working memory dysfunction. Neuroscience, 172: p. 293-302. [crossref]
- Kohler C, Steinbusch H (1982) Identification of serotonin and non-serotonin- containing neurons of the mid-brain raphe projecting to theentorhinal area and the hippocampal formation: a combined immunohistochemical and fluorescent retrograde tracing study in the rat brain. Neuroscience 7: 951-975. [crossref]
- Nozaki K, Kubo R, Furukawa Y (2016) Serotonin modulates the excitatory synaptic transmission in the dentate granule J Neurophysiol. 115 (6): 2997-3007 [crossref]
- Zimering MB (2017) Diabetes Autoantibodies Mediate Neural- and Endothelial Cell- Inhibitory Effects Via 5-Hydroxytryptamine- 2 Receptor Coupled to Phospholipase C/Inositol Triphosphate/Ca2+ J Endocrinol Diab. 4 (4): 1-10 [crossref]
- Zimering MB, Behnke JA, Thakker-Varia S, Alder J (2015) Autoantibodies in Human Diabetic Depression Inhibit Adult Neural Progenitor Cells In vitro and Induce Depressive-Like Behavior in Rodents. J Endocrinol Diabetes. [crossref]
- Zimering MB (2018) Circulating Neurotoxic 5-HT2A Receptor Agonist Autoantibodies in Adult Type 2 Diabetes with Parkinson’s Disease. J Endocrinol Diabetes. [crossref]
- Zimering MB, Grinberg M, Myers CE, Bahn G (2022) Plasma Serotonin 2A Receptor Autoantibodies Predict Rapid, Substantial Decline in Neurocognitive Performance in Older Adult Veterans with Endocrinol Diabetes Metab J. 6 (1): 614 [crossref]
- Zimering MB, Mirkovic N, Pandya M, Zimering JH, Behnke JA, Thakker-Varia S, Alder J, Donnelly RJ (2016) Toxic Immunoglobulin Light Chain Autoantibodies are Associated with a Cluster of Severe Complications in Older Adult Type 2 J Endocrinol Diabetes. [crossref]
- Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T (2005) GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. [crossref]