Abstract
Purpose: To determine if the LifeWave X39 non-transdermal photobiomodulation active patch would show improved production of GHK-Cu over controls in a double blind randomized controlled trial.
Materials: BD Vacutainer Safety Loc Blood Collection sets with Pre-attached holder sized 21GX0.75 or 23GX0.75 and lavender top tubes. Kendro Sorvall Biofuge Centrifuge 75005184+ and AB Sciex API4000 Qtrap. Analysis software included: Qtrap Analyst software 1.6.2 and R software version 3.5.1. Statistical analyses were conducted using R software (version 3.5.1; http://www.r-project.org/).
Method: Sixty people age 40-80 were computer randomized into two groups. One lavender top tube was drawn and then spun in Kendro Sorvall centrifuge for 10 minutes at 1300 rcf. The plasma was placed in cryo tubes and flash frozen to -22C then shipped in dry ice to laboratory for analysis. The filtrate was concentrated by speed-vac and reconstituted with de-ionized water to 50 ul and analyzed with AB Sciex API4000 Qtrap. Statistical assessments were evaluated using a nonparametric Wilcoxon signed rank test, p values are two-sided and p<0.05 was used to define statistical significance.
Results: A significant increase in GHK-Cu concentration in the blood of the active group was seen comparing changes from Day 2 to Day 7 between Group A vs. Group B in GHK-Cu Concentration (ng/ml) at p<0.035 and in Total GHK-Cu (ng) at p<0.03.
Conclusion: This study showed a significant increase in the GHK-Cu concentration present in the blood as a result of wearing the LifeWave X39 patch for 1 week in individuals age 40 to 80. This is seen from Day 2 to Day 7 between Active vs. Control in GHK-Cu Concentration (ng/ml) at p<0.035 and in Total GHK-Cu (ng) at p<0.03.
Keywords
GHK-Cu, Meridian, Non-transdermal, Photobiology, Phototherapy
Introduction
This study explores the impact of wearing the LifeWave X39 non-transdermal photobiomodulation patch over the period of one week on levels of glycyl-L-histidyl-L-lysine-copper(2+) (GHK-Cu) levels in the blood in a double-blind randomized controlled trial. This particular tripeptide was first isolated by Dr. Loren Pickart in 1973. GHK-Cu is important as the “copper tripeptide-1 belongs to a group of emergency response molecules which are released during injury and come to the body’s aid…” [1] It is naturally sent by the body to any type of injury to tissue. For example: the “copper tripeptide-1 has been suggested to have a potential therapeutic role in age-related neurodegeneration and cognitive decline. It improves axon survival and maintenance of nerves” [1]. It has been implicated in the resetting of 4000 genes [2]. Blood samples to determine GHK-Cu levels were taken at baseline, 24 hours and at 7 days of wearing the patch. A sample of convenience of 60 subjects made up of both men and women aged 40-81 were selected to participate in this study. Participants were randomized into Group A or Group B by computer.
Background
The LifeWave X39 patch uses phototherapy to stimulate a rebalancing of the body. Based on data from other studies, it was felt that a possible change in the copper tripeptide GHK-Cu might be a factor in the effects produced by the patch. As a follow on to prior studies it was determined that a double blind study was an appropriate method of testing this theory. The tripeptide has been demonstrated to improve tissue remodeling in previous research. “It increases keratinocyte proliferation and normal collagen synthesis, improves skin thickness, skin elasticity and firmness, improves wrinkles, photodamage and uneven pigmentation, improves skin clarity, and tightens protective barrier proteins” [3]. Research has identified that the peptide is used to signal the beginning of the natural repair process.
The Tripeptide
“Copper tripeptide-1(GHK-Cu) is a small protein composed of the three amino acids (protein building blocks) glycine, histidine, and lysine combined in a specific geometric configuration with the physiologically beneficial mineral (copper)” [4]. Later research established the strong affinity between the GHK peptide and copper, and the two forms (GHK and GHK-Cu) it exists in, as this was not covered in the initial experiment. It should also be mentioned that GHK has never been found to cause an issue in all of the research that has been done [1].
Non-transdermal Patch
All X39 patches are sealed to prevent the contact of any of the substances inside to the skin. The sealing of the patches allows for consistent light flow through the patch the entire time that the patch is worn. Patches are designed to reflect wavelengths of light in the infrared, near infrared, and visible light bands. Using the same adhesives as band-aids, this limits the level of irritation which might be developed through consistent daily use of the patch.
Phototherapy
Phototherapy has been used for over 100 years in various forms. There has been little evidence of negative side effects throughout that time period. This suggests that phototherapy is a relatively untapped option for healing, and one that has relatively few risks [5].
Purpose
To determine if the LifeWave X39 non-transdermal photobiomodulation active patch would show improved production of GHK-Cu over controls in a double blind randomized controlled trial.
Procedure
Once human research studies ethics board approval was received (NFFEH 01-16-20-01) recruitment was begun. Flyers advertising for interested research participants were posted at various local sites. Participants would call into the main study phone number and were assessed for inclusion and exclusion criterion. If appropriate they were scheduled for consenting. At the time of arrival at the study site, each participant was consented and then randomized into group A or B. Individual participants were then taken into the exam room and a blood sample was taken at baseline. Additional samples were taken at 24 hours and 7 days of patch placement.
For convenience, participants were asked to use what is a recognized meridian point, GV14 or CV6 [6], for the patch placement. BD Vacutainer Safety Loc Blood Collection sets were used with Pre-attached holder sized 21GX0.75 or 23GX0.75 and placed in lavender top tubes. Each blood sample was then placed in the Kendro Sorvall Biofuge centrifuge 75005184+ HERAEUS 7591 with a 4000 RPM rotor, spun for 10 minutes at 1300 rcf to separate the plasma, which was then placed in the cryo tubes, and then flash frozen using a medical freezer at -22C. Samples were then placed in 2″ thick polystyrene containers, wrapped in thermal box liners and placed in double walled boxes with dry ice for overnight shipping. Samples were sent to HT-Labs, a division of AxisPharm in San Diego, CA.
Analysis of Blood Samples
The blood samples were processed according to the original thesis of Dr. Pickard. The filtrate was concentrated by speed-vac and reconstituted with de-ionized water to 50 ul and analyzed with AB Sciex API4000 Qtrap. The data was analyzed with Analyst software 1.6.2. Values were placed in a spread sheet and then sent for statistical analysis. Both the blood analysis and statistical analysis was done at groups independent from the principle research laboratory.
Statistical Analysis
Absolute changes in GHK and GHK-Cu levels from baseline to the 24 hours and day 7 assessments were summarized in terms of means, standard deviations, medians and ranges. Changes from baseline to the 24 hours and day 7 assessments were evaluated using a nonparametric Wilcoxon signed rank test. All reported p values are two-sided and p<0.05 was used to define statistical significance. Statistical analyses were conducted using R software (version 3.5.1; http://www.r-project.org/). Once the statistical analysis was complete, the blind was broken.
Results
A sample of convenience of individuals consisted of 60 individuals randomized into two groups (A and B) with an age range of 41-80. Significant results of the LifeWave X39 patch testing are as follows:
Discussion
This was a randomized double blind trial which used a sample of convenience recruited from the general population of the greater Tucson, AZ area. Individuals were age 40-81. It should be noted that this trial was interrupted by the COVID SARS-2 pandemic in March of 2020 and resumed in Aug of 2020. At that time special procedures were put in place to be sure of the safety and health of all participants. This included separation of times for scheduled blood draws and special cleaning procedures between each participant: UV-C wanding of all hard surfaces, Clorox wipe of draw chair, changes in gloves and gowns for all study team members and the wearing of masks for both participants and study team members. Study team members were tested weekly to confirm no contagion. No study participant developed COVID SARS-2 through participation in this study process. This study confirmed that there was a significant change in the levels of GHK-Cu in 7 days in both concentration and total amount. This confirms data from earlier studies [7,8]. The repeated trials data supports promotion of positive benefits to the body through increased production of GHK-Cu by the wearing of the LifeWave X39 non-transdermal photobiomodulation patch.
Conclusion
This study explored the changes in amounts of GHK-Cu present in the blood as a result of wearing the LifeWave X39 patch 8-12 hours per day for 1 week. A significant increase in GHK-Cu concentration in the blood of the active group was seen in the p-value for comparing changes from Day 2 to Day 7 between Group A vs. Group B in GHK-Cu Concentration (ng/ml) at p<0.035 and in Total GHK-Cu (ng) at p<0.03 (Tables 1 and 2).
Table 1: A significant increase in GHK-CU concentration in the blood of the active group was seen comparing changes from Day 2 to Day 7 between Active vs. Control in GHK-Cu Concentration (ng/ml) at p< 0.035 and in Total GHK-Cu (ng) at p<0.03.
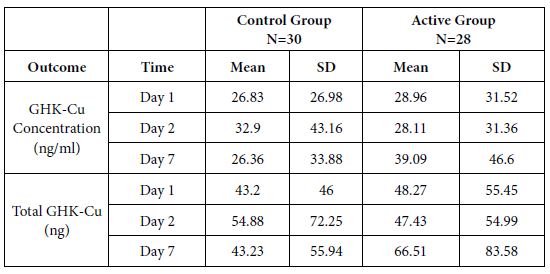
Table 2: Study X39 – Evaluation of Changes in Outcomes from Day 1 to Day 2, Day 1 to Day 7, and Day 2 to Day 7 within Groups and Comparisons of Changes between Groups.
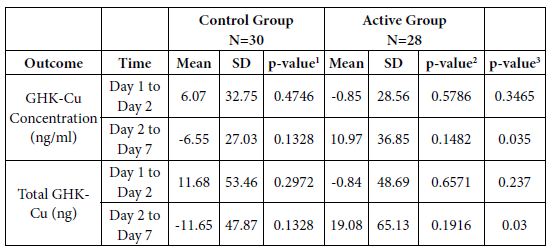
1p-value for evaluating changes from Day 1 to Day 2, and Day 2 to Day 7 within Control Group.
2p-value for evaluating changes from Day 1 to Day 2, and Day 2 to Day 7 within Active Group.
3p-value for comparing changes from Day 1 to Day 2, and Day 2 to Day 7 between Control
Group vs. Active Group.
References
- DeHaven C (2014) Copper Tripeptide-1. Science of Skincare.
- Pickart L, Vasquez-Soltero J, Margolina A (2014) GHK and DNA: resetting the human genome to health. BioMed Research International 2014: 151479. [crossref]
- Pickart L, Vasquez-Soltero J, Margolina A (2015) GHK Peptide as a Natural Modulator of Multiple Cellular Pathways in Skin Regeneration. Hindawi Publishing Corporation BioMed Research International 2015: 648108.
- Geo Peptides Staff (2015) What are Copper Peptides? https://www.geopeptides.com/copperpep.html
- Kakimoto C (2017) What is phototherapy, and how does it work? https://www.dermatologistoncall.com/blog/what-is-phototherapy-and-how-does-it-work/
- Deadman P, Al-Khafaji M, Baker K (2001) A Manual of Acupuncture. Eastland Press.
- Connor M, Connor C, Gombosuran N, Eickhoff J, et al. (2020) LifeWave X39 Pilot Demonstrates Light Triggered Changes. International Journal of Healing and Careing ijhc.org
- Connor C, Connor M, Yue D, Chang C, Eickhoff J, et al. (2020) Changes in Tripeptides Produced By the LifeWave X39 Patch. International Journal of Healing and Careing www.ijhc.org