DOI: 10.31038/CST.2023811
Abstract
Background and aim: Colorectal Cancer (CRC) is the third leading cause of global cancer death in humans and its incidence is gradually rising in developing nations including Bangladesh. The study was carried out to unveil the demographic and clinicopathological profile of CRC cases in Bangladeshi patients.
Methods: A cross-sectional study was conducted among purposively selected 50 patients irrespective of age and sex with histologically proven colorectal cancer at a tertiary level hospital for a period of 2 years. Large bowel resection specimens of CRC made the samples. Demographic and clinical information were recorded in a pre-tested, structured case record. Relevant macroscopic and microscopic features of tumors were recorded during gross and microscopic examinations of the specimen.
Results: Mean age of the CRC patients was 48.60±14.6 years. Adult active males (35-54 years old) were significantly affected by CRCs (p=0.0001). Per rectal bleeding (38.7%) and generalized weakness and pallor (38.98%) were the most frequent findings in distal and proximal CRCs, respectively. Adenocarcinoma NOS was the most commonly observed histologic type. Occurrence of CRC and tumour grades were significantly (p=0.0001) related where Grade-II and Grade-III tumour occurred in 72% and 24% of cases, respectively. Majority of the cases were presented at stage pT3 (68%) and pN0 (48%). Tumors in adult active age showed a higher tendency to be presented at advanced stages.
Conclusions: Bangladeshi adult active males of 35-54 years old were predominantly affected by a locally advanced stage of CRC. Routine screening programs are proposed for early detection and treatment of the cases.
Keywords
Colon cancer; Bangladeshi patients; Clinical features; Demographic characteristics; Intestinal tumor
Introduction
Colorectal cancer is one of the most common cancers globally. It is the second most common malignancy among women and the third most common malignancy in men [1]. The global burden of colorectal cancer is expected to increase by 60% by 2030. Its incidence shows a 10-fold variation across the world [2]. The prevalence of colorectal cancer is lower in Asia than in Western countries. But the incidence has been alarmingly increasing in countries of the Asia-Pacific region during the last two decades due to the westernization of lifestyles [3]. In Bangladesh, 5-year prevalence of colon and rectal cancer are 3.28 and 3.1 per 100,000 population, respectively [4]. There are variations in risk factors, mode of disease presentation, sub-site distribution, tumor morphology, grade and stage at presentation. These tumors grow insidiously and remain undetected for long periods remaining potentially curable, premalignant lesions over several years. Therefore, screening procedures provide a unique opportunity for cancer prevention [5]. However, in the long run, the tumor cells metastasize to lymph nodes and in the other organs. The treatment, prognosis and survival rate largely depend on the stage of disease at diagnosis.
The development of both familial and sporadic colorectal cancers can be influenced by genetic factors [6]. Age is another major risk factor for sporadic CRC. The diagnosis is rare before the age of 40 years. The incidence starts to increase significantly between 40 to 50 years and the age-specific incidence rates raise in each succeeding decade thereafter [7]. Although patients over 50 years of age represent 90% of newly diagnosed cases [8], the trend is changing with the increasing incidence among the younger population who present in a more advanced stage [9]. In recent years, tumor location has also been suggested to be a valuable predictor, which has added to the difficulty of discussing its clinicopathological features and outcomes [10]. The majority of colorectal cancers belong to classical adenocarcinomas, with several histological variants associated with specific molecular characteristics [11,12]. Understanding these features could guide the proper management and prognosis of the patient. But unfortunately, there is a scarcity of data regarding the demographic and clinicopathological patterns of colorectal cancers in Bangladeshi patients. Therefore, the present study was undertaken to understand the demographic and clinicopathological profiles of patients with colorectal carcinoma in Bangladesh.
Methods
Ethical Approval
This study was approved by the local Ethics Committee (Institutional Review Board) at Bangabandhu Sheikh Mujib Medical University (BSMMU) and informed consent was obtained from all participants.
Study Design, Period and Sample
This cross-sectional, descriptive, hospital based, study was conducted in the Department of Pathology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh during the period of March 2019 to February 2021. Large bowel resection specimen histologically diagnosed as adenocarcinoma at the department of Pathology, BSMMU were included in this study. A total of 50 primary colorectal carcinoma cases irrespective of ages and sexes were included in the study. Clinically suspected colorectal carcinoma subsequently proved to be non-epithelial tumors of the colon were excluded from this study. Patients’ attendants were interviewed for demographic and clinical information, which were recorded in a performed questionnaire.
Gross and Microscopic Evaluation of Samples
During gross examination tumor site and macroscopic features of tumors were noted. Splenic flexure was taken as demarcating point of proximal and distal lesions. After that representative tissue blocks were submitted for routine processing and paraffin embedding. Standard protocol was maintained during tissue processing and staining. Microscopic examination of tissue sections stained with hematoxylin and eosin was carried out and relevant points were recorded. The tumors were classified following World Health Organization classification of tumor and grading of tumor was done by using AJCC specified four-tiered grading system. Staging was performed following TNM Classification of Colorectal Carcinoma American Joint Committee on Cancer (AJCC).
Statistical Analysis
Data was entered in MS Excel and analyzed by using SPSS software version 21. Categorical variables were expressed as percentages and analyzed using the Chi-square test or Fisher’s exact test. Continuous variables were expressed as mean or median and analyzed using the Mann-Whitney test. Demographic factors and clinical characteristics were summarized with percentages for categorical variables and median for continuous variables.
Results
A total of 50 patients with CRC were enrolled in this study. Age of the study population varied from 19-85 years with a mean of 48.60±14.6 (SD) years. All the 50 cases were categorized into four age groups where samples from patients belonging to 15-34 years, 35-54 years, 55-74 years and 75-94 years were considered as young, adult, elderly and old age groups, respectively. Figure 1 illustrates the distribution of CRC cases in different age groups.
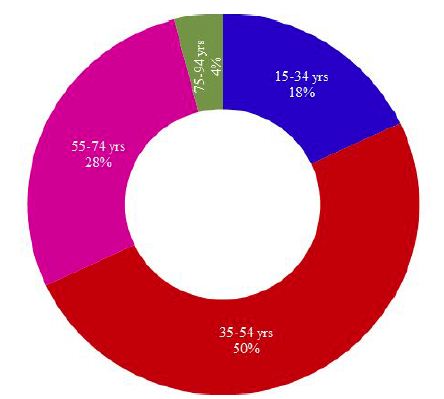
Figure 1: Distribution of CRC cases in different age groups of Bangladeshi patients
As shown in Figure 1; half (50%) of the CRC cases belonged to adult active age group (35-54 years), followed by 28% cases in elderly (55-74 years), 18% cases in young (15-34 years) and 4% cases in old age group (75-94 years). A chi-square test of independence was performed to examine the relationship between age groups and the frequency of occurrence of CRCs. The relation between these variables was significant, X2 (3, N=50)=22.48, p=.0001 at α=0.05. The adult active age group (35-54 years) was more likely to be affected by colorectal carcinoma than the other groups.
Sex-wise distribution of CRC cases of this study revealed that almost two-thirds (64%) of the study cases were male while around one-third (36%) cases were female. The occurrence of male cases was numerically high in all age groups except the old age where the percentage of both cases was equal (Figure 2). The relation between the occurrence of CRS and sexes was weakly significant, [X2 (1, N=50)=3.92, p=.048 at α=0.05]. Males were more likely to be affected by colorectal carcinoma than females (Figure 2).
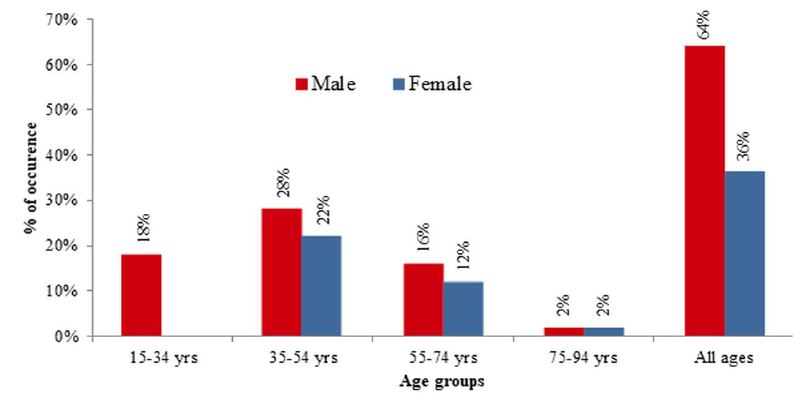
Figure 2: Sex-wise distribution of CRC cases in different age groups of Bangladeshi patients
Per rectal bleeding was the most frequent clinical presentation of CRC among the study population that was documented in 30% of cases followed by generalized weakness and pallor (26%), abdominal pain with or without features of obstruction (16%), altered bowel habit (12%), weight loss (12%) and abdominal mass (4%). Only one study case had a positive family history of colon cancer. None of the cases was presented with a history of extra-colonic malignancy. When the site of occurrence was considered, the tumor was located in the distal colon and proximal colon in 62% and 36% of cases, respectively. Only one (2%) of cases were recorded to have synchronous tumors involving both colons. However, no signification relation was observed between the tumor sites and the occurrence of CRC [X2 (1, N=50)=2.880, p=.090 at α=0.05]. The distribution of study cases based on tumor location along with presenting complaints is shown in Table 1.
Table 1: Distribution of study cases based on tumor location along with presenting complaints
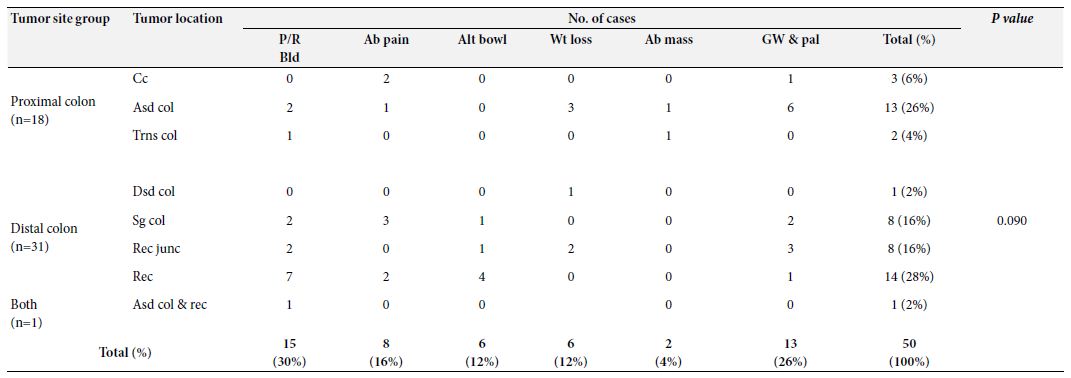
P/R Bld: Per rectal bleeding; Ab Pain: Abdominal pain; Alt bowl: Altered bowel habit; Wt loss: Weight loss; Ab mass: Abdominal mass; Gw & pal: Generalized weakness and pallor; Cc: Cecum; Asd. col: Ascending colon; Trns col: Transverse colon; Dsd col: Descending colon; Sg col: Sigmoid colon; Rec junc: Rectosigmoid junction; Rec: Rectum: Asd. col. & rec.: Ascending colon & rectum (synchronous)
The exophytic pattern was the most common gross morphology which was observed in more than half (60%) of the cases, followed by ulcerating and infiltrative patterns, seen in 30% and 10% of the cases, respectively. In our study, the frequency of the Exophytic pattern was significantly higher than the other two patterns based on the chi- square test of independence, [X2 (2, N=50)=19.00, p=.0001 at α=0.05] indicating that Exophytic pattern was more likely to occur in CRC cases (Table 2).
Table 2: Distribution of study cases based on tumor morphologies
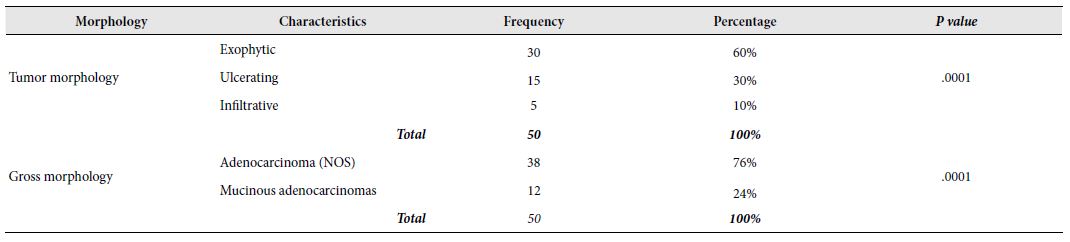
In this study, only two variants of adenocarcinoma were observed; the commonest one being adenocarcinoma (NOS) (76%) followed by mucinous adenocarcinomas (24%). The frequency of adenocarcinoma (NOS) in relation to CRC cases was highly significant [X2 (1, N=50)=13.52, p=.0001 at α=0.05] compared to mucinous adenocarcinoma. It seemed that adenocarcinoma (NOS) was the more frequent variant of CRC in Bangladeshi patients (Table 2). Among the 50 selected cases, majority (72%) of the tumours belonged to grade II (moderately differentiated) tumors. Remaining 24% and 4% cases were categorized as grade III (poorly differentiated) and grade I (well differentiated) tumors, respectively. There was a highly significant relation between the occurrence of CRC the and grade of tumours [X2 (2, N=50)=36.640, p=0.000 at α=0.05]. However, no significant relationship was seen between the tumour grades and age groups [X2 (6, N=50)=2.702, p=0.845 at α=0.05] or sex [X2 (2, N=50)=1.292, p=0.524 at α=0.05]. It seemed that Grade-II CRCs were more likely to occur in case of colorectal carcinoma in Bangladeshi patients irrespective of age and sex. Figure 3 illustrates the graphical presentation of grades of tumours according to age groups and sexes whereas Table 3 summarizes the frequency of tumours according to grades, age groups and sexes with their relationship.
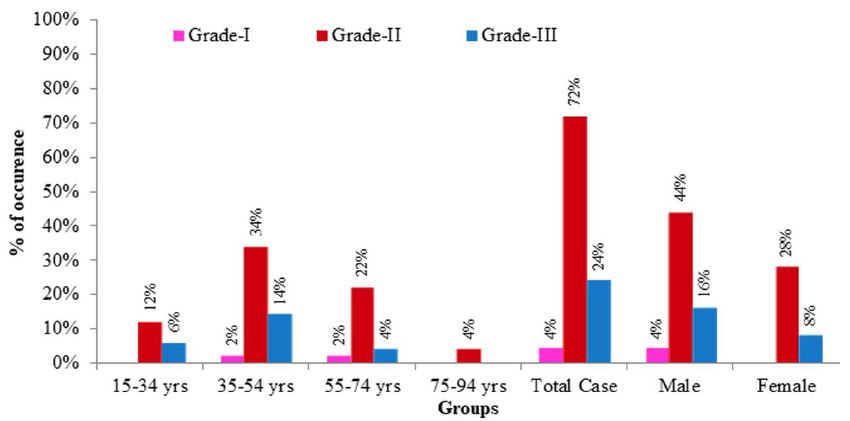
Figure 3: Graphical presentation of grades of CRC cases according to age groups and sexes. Grade-I: well differentiated tumors; Grade-II: moderately differentiated tumours; Grade-III: poorly differentiated tumours.
Table 3: Frequency of tumours according to grades, age groups and sexes with their relationship
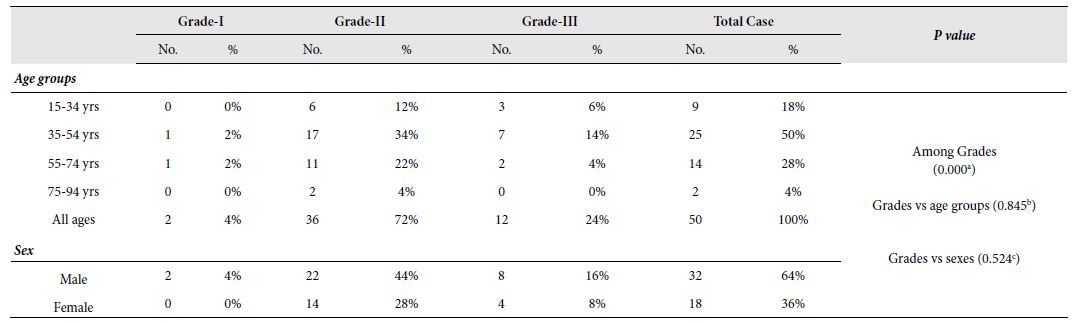
No.: Number of occurrence; %: Percentage of occurrence;
a: X2 (2, N = 50) = 36.640, p = 0.000 at α = 0.05; b: X2 (6, N = 50) = 2.702, p = 0.845 at α = 0.05; c: X2 (2, N = 50) = 1.292, p = 0.524 at α = 0.05
According to the pathological depth of invasion, studied CRC cases were ranked into 4 stages namely PT-I, PT-II, PT-III and PT- IV starting from lowest to highest invasion. Stage-3 (PT-III) was the most commonly recorded stage of tumour which was seen in 68% of the cases followed by PT-II, PT-IV and PT-I which were recorded in 22%, 8% and 2% cases, respectively. A highly significant relation was observed between the occurrence of CRC and the stage of tumours based on pathological invasion [X2 (2, N=50)=36.640, p=0.000 at α=0.05]. It seemed that PT-III is more likely to occur in Bangladeshi CRC cases. A highly significant relation was also observed between the pathological invasion of tumours and age groups [X2 (6, N=50)=2.702, p=0.845 at α=0.05] where adults are more likely to be affected by Stage-3 (PT-III) CRCs. However, no significant relationship was seen between the tumour stages and sex [X2 (2, N=50)=1.292, p=0.524 at α=0.05]. A graphical presentation of stage of tumours according to age groups and sexes has been depicted in Figure 4 and the summary of the frequency of tumours according to stages, age groups and sexes with their relationship is shown in Table 4.
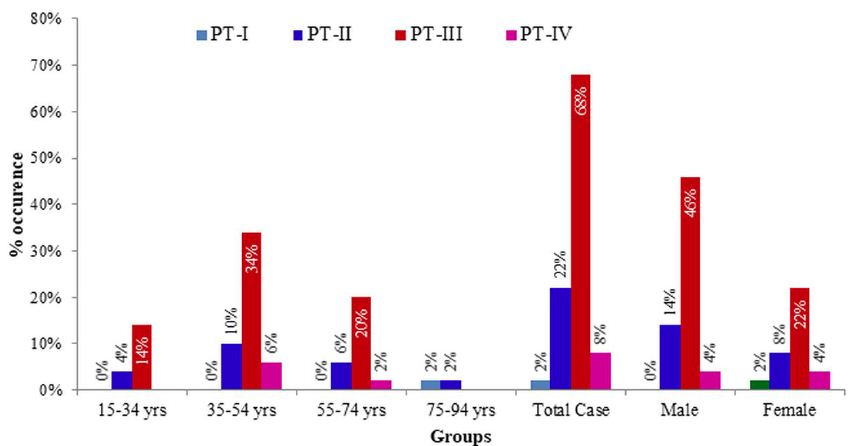
Figure 4: Graphical presentation of stages of tumours according to age groups and sexes. PT-I: low pathological invasion; PT-II: moderate pathological invasion, PT-III: fair pathological invasion; PT-IV: high pathological invasion.
Table 4: Frequency of tumours according to stage of tumor based on the pathological depth of invasion. age groups and sexes with their relationship.
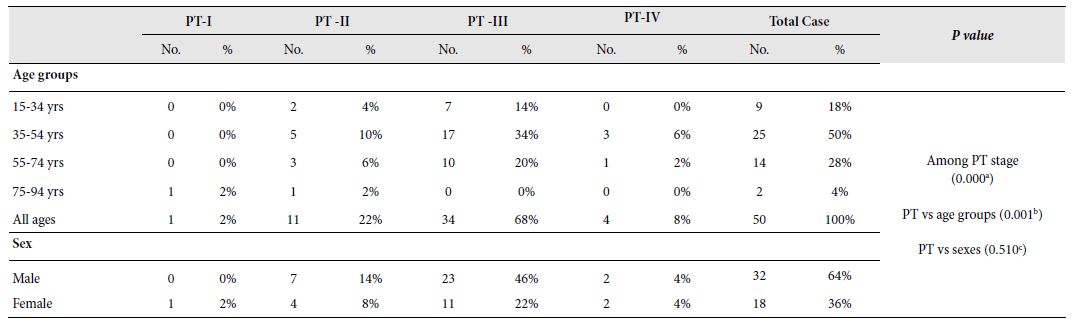
PT-I; PT-II; PT-III; PT-IV; No.: Number of occurrence; %: Percentage of occurrence;
a: X2 (2, N = 50) = 36.640, p = 0.000 at α = 0.05; b: X2 (6, N = 50) = 2.702, p = 0.845 at α = 0.05; c: X2 (2, N = 50) = 1.292, p = 0.524 at α = 0.05
Studied CRC cases were further classified based on the involvement of regional lymph nodes by the tumor (PN) where cases with no lymph node involvement was grouped in PN0, metastasis in one to three regional lymph nodes in PN1, metastasis in four or more regional lymph nodes in PN2 and the cases where Lymph nodes could not be assessed was grouped in PNx. Almost half (48%) of the study cases was categorized in PN0 followed by PN2, PN1 and PNx in 26%, 22% and 4% cases, respectively. The involvement of regional lymph nodes was highly related to the occurrence of CRCs [X2 (3, N=50)=19.600, p=0.000 at α=0.05]. It is more likely that regional lymph nodes were not involved in CRC cases. However, no significant relationship was seen between the lymph node invasion and age groups [X2 (9, N=50)=6.915, p=0.646 at α=0.05] or sex [X2 (3, N=50)=2.620, p=0.454 at α=0.05]. Figure 5 illustrates the graphical presentation of different classes of CRC cases based on the involvement of regional lymph nodes according to age groups and sexes whereas Table 5 summarizes the frequency of different classes of CRC cases based on the involvement of regional lymph nodes according to stages, age groups and sexes with their relationship.
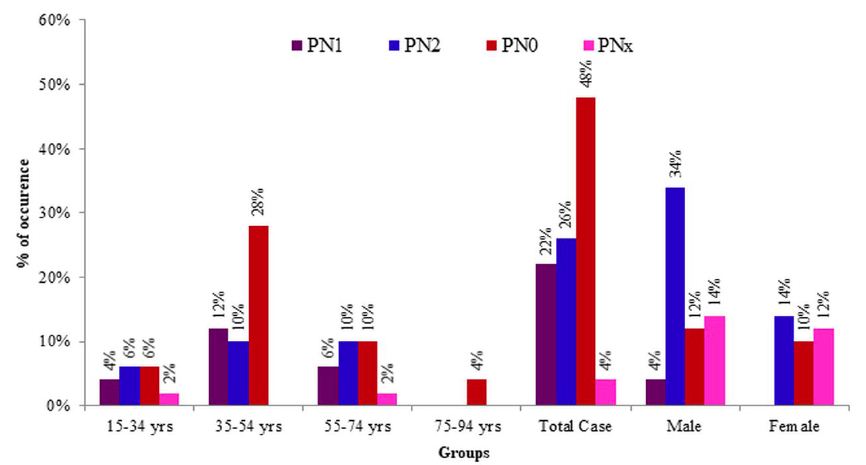
Figure 5: Graphical presentation of different classes of tumours based on involvement of regional lymph nodes according to age groups and sexes. PNO: no lymph node involvement; PN1: metastasis in one to three regional lymph nodes; PN2: metastasis in four or more regional lymph nodes; PNx: lymph nodes could not be assessed.
Table 5: Frequency of tumours according to involvement of regional lymph nodes by the tumor, age groups and sexes with their relationship
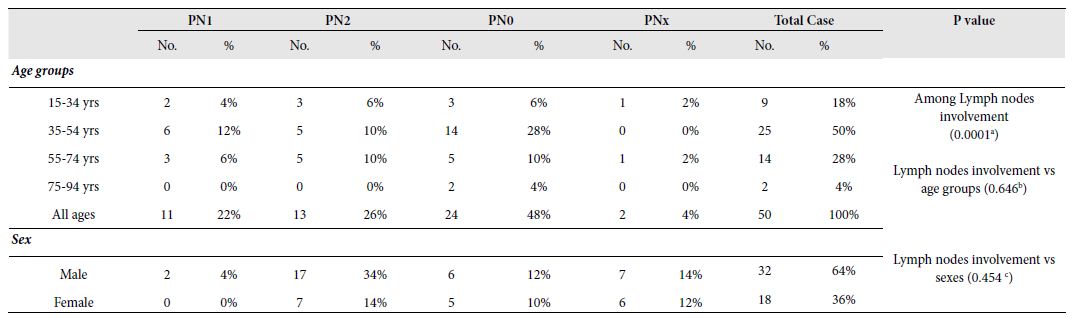
N1: ; N2: ; N3: ; N0: ; No.: Number of occurrence; %: Percentage of occurrence;
a: X2 (3, N = 50) = 19.600, p = 0.0001 at α = 0.05; b: X2 (9, N = 50) = 6.915, p = 0.646 at α = 0.05; c: X2 (3, N = 50) = 2.620, p = 0.454 at α = 0.05
Discussion
Although CRC is more likely to occur at old age, nowadays, younger patients are increasingly affected by different types of CRCs [13]. In this study, half (50%) of the cases belonged to the 35-54-years age group, with a mean of 48.60 ± 14.6 years which is lower than that reported age from the Western world [13-15]. Salminen, et al., 2005 recorded 59 years as the mean age of CRC in their study in the Finnish population and Turner, 2014 observed only 20% cases to occur below the age of 50 years [14,15]. However, Saha et al., 2016 and Raza et al., 2016 recorded the mean age as 50.77 years and 47 ± 14.8 years, respectively in Bangladeshi colorectal cancer patients [16,17], which are in good agreement with this study. The occurrence of CRCs specially in relatively young age group may be attributed to the recent change in food habits of Bangladeshi active-aged people (35-54 years) who have shifted to western diets that ae well documented as a contributor to colon cancer. It has been reported that consumption of red or processed meats is associated with an increased risk of colon cancer [18]. Consumption of high fat containing foods like red or processed meats induces increased bile acids secretion that in turn results in altered microbial composition in gut [19, 20]. Such alternation in microbial community contributes to the production of different metabolites that might influence the development of colon cancer [20]. Moreover, geographical and environmental factors of the study population may have influenced the findings as well. However, the higher incidence of CRCs in the younger age group of Bangladeshi patients in comparison to western studies is alarming. This interesting finding necessitates the importance of cancer screening program and this report may be considered in future studies to evaluate predisposing and genetic factors for CRCs in this region.
In the current study, there was male preponderance (64%) with a male to female ratio 1.8:1. Studies conducted elsewhere also showed higher incidence of CRC among male patients [14,16,17]. Urbanization and changes in lifestyles might have contributed to the transformation in food habit from native to western diets rich in high fat which are known to trigger colon cancer. In association with altered food habit, higher incidence of smoking, alcohol consumption or genetic factors might be responsible for this male predominance.
Clinical presentations are different for proximal and distal CRC. In our study, per rectal bleeding was the most frequent finding (38.7%) in distal CRCs whereas, generalized weakness and pallor were the chief complaints of the patients with CRCs in proximal colon (38.98%). These features are consistent with the findings of reported elsewhere [5].
The most commonly observed location of CRC in this study is distal colon (62%). Rectum was most frequently observed site (28%). Other Bangladeshi studies conducted also found similar findings [17,21,22]. Occurrence of CRC in Bangladeshi population is higher in distal colon due to some unknown etiology which may be related to some genetic factors.
The exophytic pattern was the most common gross morphology observed in 60% cases followed by ulcerating (30%) and infiltrative pattern (10%). In this study, only two histological types of colorectal adenocarcinoma: adenocarcinoma (NOS) and mucinous adenocarcinoma were observed. Adenocarcinoma (NOS) comprises the majority (76%) of the total cases. These findings were consistent with the findings of other studies indicating common gross and histopathological feature of CRCs [17,21-23].
Among the 50 cases, 72% cases were Grade II tumours, 24% were Grade III and 4% were Grade I tumours. All of the mucinous adenocarcinoma was considered poorly differentiated (grade III). Several studies suggest that CRCs occuring in younger patients (less than 50 years of age) are less differentiated [24]. In the present study, 34% of Grade-II and 14% of Grade III tumours were observed in 35- 54 years of age whereas 22% Grade-II and 4% Grade III tumours were recorded in 55-74 years of age. Present study findings are in close agreement with previous findings that documented less differentiated CRCs are likely to occur within 50 years of age [23]. However, a statistically significant association between age group and tumor grade was not observed possibly due to a smaller sample size.
We also categorized study cases according to the PT and PN stage of the tumor. Our study revealed that PT3 (68%) and PN0 (48%) were the most commonly reported stages of tumor in CRC cases based on the depth of invasion and lymph node metastasis, respectively. Studies conducted elsewhere reported that colorectal cancers in young patients (<50 years of age) are biologically aggressive and present at advanced stage [9,25]. In our study, PT3 and PT4 stages were recorded in 34% and 6% cases, respectively in 35-54 years age group in comparison to 20% PT3 and 2% PT4 stages in 55-74 years age group. In the case of nodal staging, metastasis in one to three regional lymph nodes (PN1) was observed in 12% of cases in 35-54 years age group which was double of the observed cases (6%) in the 55-74 years age group. Our study findings for pathological invasion and metastasis in lymph nodes in CRC cases clearly support previous study reports that documented biological aggressiveness and advanced stage in colorectal cancers in patients under 50 years of age. All these studies suggest that patients with colorectal adenocarcinomas usually present with advanced morphology in histopathology.
Conclusion
Present study findings on colon cancer were different from that described in the western countries. Active aged people (35-54 years old) were found to be significantly affected by CRC in our study. In majority of the cases, the tumour presented at a locally advanced stage. Quick urbanization, fast shifting to western food, lack of proper knowledge on CRC might account for some of these differences. Routine screening program is proposed for early detection and treatment of the cases to reduce the burden of morbidity and mortality. Genetic tests should also be carried out to unveil the cause of young age presentation of CRCs. However, this study had its own limitations. The study reflects to the findings of a specific geographical area with a small sample size. Therefore, further studies should be conducted all over the country with a larger sample size for a more comprehensive understanding on demographic and clinicopathological profiles of CRCs in Bangladesh.
Acknowledgements
The authors gratefully acknowledge the logistics and grant support to conduct the research activities from Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh.
Availability of Data and Materials
Raw data, supplemental data and materials are available on request.
Conflict of Interests
The authors declare that they have no conflict of interests in publishing the article.
Ethical Consideration
Written consent from individual patient/representative of patients was obtained for using the samples for research purposes. No personal data/information of patients was shared in public.
Author’s Contributions
SSUM planned, designed and performed the study. She also wrote the manuscript (MS). FB, MMR & AZ helped in planning & designing of the study and developing the research question. PR, TI, USS, UTN, SA and NA helped in data collection and processing. KBMS helped in study design, data screening and performed data analyses, interpretation and MS writing. All authors read and approved the final manuscript.
References
- GLOBOCAN 2020: New Global Cancer Data. [Online]; 2020. [crossref]
- Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray 2017 Global patterns and trends in colorectal cancer incidence and mortality. Gut. 66(4): 683-691. [crossref]
- Wong MC, Ding H, Wang J, Chan PS, Huang J (2019) Prevalence and risk factors of colorectal cancer in Intestinal Research 17: 317-329. [crossref]
- Global Cancer Observatory. [Online].; 2020.
- Turner JR (2014) The Gastrointestinal In: Robbins and Cotran Pathologic Basis of Disease. 9th ed. Elsevier 749-819. [crossref]
- Valle L (2014) Valle Genetic predisposition to colorectal cancer: where we stand and future World Journal of Gastroenterology 20: 9828-9849. [crossref]
- Eddy DM. 1990 Screening for colorectal cancer. Annals of Internal Medicine 113: 373-84. [crossref]
- Paymaster JC (1964) Cancer and its distribution in India. Cancer 17: 1026-1034. [crossref]
- Mohandas M, Desai DC (1999) Epidemiology of digestive tract cancers in India large and small bowel. Indian Journal of Gastroenterology 18: 118-121. [crossref]
- Salem ME, Weinberg BA, Xiu J, El-Deiry WS, Hwang JJ, et (2017) Comparative molecular analyses of left-sided colon, right-sided colon, and rectal cancers. Oncotarget 8: 86356-86368. [crossref]
- Reynolds IS, Furney SJ, Kay EW, McNamara DA, Prehn JH, et al. (2019) Meta- analysis of the molecular associations of mucinous colorectal British Journal of Surgery. 106: 682-691. [crossref]
- Nguyen LH, Goel A, Chung DC (2020) Pathways of colorectal Gastroenterology 158: 291-302. [crossref]
- Nelson RL, Dollear T, Freels S, Persky 1997 The relation of age, race, and gender to the subsite location of colorectal carcinoma. Cancer 80: 193-197. [crossref]
- Salminen E, Palmu S, Vahlberg T, Roberts PJ, Söderström KO (2005) Increased proliferation activity measured by immunoreactive Ki67 is associated with survival improvement in rectal/recto sigmoid cancer. World Journal Gastroenterology 11: 3245-3249. [crossref]
- Turner The Gastrointestinal Tract. In: Kumar V, Abbas AK, Aster JC (2014) Robbins and Cotran Pathologic Basis of Disease. 9th ed. New Delhi: Reed Elsevier India Private Limited 810-814. [crossref]
- Saha M, Shil BC, Saha SK, Banik RK, Perveen I, et al. (2016) Study of Clinicopathological Profile of Sporadic Cases of Colorectal Euroasian Journal of Hepato-Gastroenterology 6: 134-136. [crossref]
- Raza AM, Kamal M, Begum F, Yusuf MA, Mohammad D, Begum M, et al. 2016 Clinico-demographic Characteristics of Colorectal Carcinoma in Bangladeshi Patients. Journal of Current and Advance Medical Research 3: 22-25. [crossref]
- Magalhaes B, Peleteiro B, Lunet N (2012) Dietary patterns and colorectal cancer: systematic review and meta-analysis. European Journal of Cancer Prevention 21: 15-23. [crossref]
- Islam B, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, et (2011) Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology. 141: 1773-81. [crossref]
- Yokota A, Fukiya S, Islam KB, Ooka T, Ogura Y (2012) Is bile acid a determinant of the gut microbiota on a high-fat diet?. Gut Microbes 3: 455-459. [crossref]
- Rahman MM. Expression pattern of the proliferative marker Ki-67 and status of microsatellite instability in different histomorphological patterns of colorectal carcinoma. MD Thesis, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh 2014.
- Hossain Clinicopathologic study of colorectal adenocarcinoma, MD Thesis, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh 2014.
- Hashmi AA, Ali R, Hussain ZF, FN, Khan EY, Bakar SMA, et al. (2017) Mismatch repair deficiency screening in colorectal carcinoma by a four-antibody immunohistochemical panel in Pakistani population and its correlation with histopathological World Journal of Surgical Oncology 15: 116. [crossref]
- Chou CL, Chang SC, Lin TC, Chen WS, Jiang JK, et al. (2011) Differences in clinicopathological characteristics of colorectal cancer between younger and elderly patients: an analysis of 322 patients from a single institution. American Journal of Surgical Pathology 202: 574-582. [crossref]
- Mueller M, Schneider MA, Deplazes B, Cabalzar-Wond D, Rickenbacher A, et al. (2021) Colorectal cancer of the young displays distinct features of aggressive tumor biology: A single-center cohort World Journal of Gastrointestinal Surgery 13: 164-175. [crossref]