Abstract
The Endomembrane system is the system that deals with all tiny organelles present in the cell. The cytoskeleton and cytosol are structural elements that help provide the cell with its structure. The cytoplasm is everything in the cell except for the cytoskeleton and membrane-bound organelles. The cytoskeleton is composed of protein filaments and is found throughout the inside of a eukaryotic cell. The cytosol is the main component of the cytoplasm, it is the fluid that fills the inside of the cell. Both the structures like the cytoskeleton, and cytosol, are “filler” structures that do not contain essential biological molecules but perform structural functions within a cell. The part of the cell referred to as cytoplasm is slightly different in eukaryotes and prokaryotes. In eukaryotic cells, which have a nucleus, the cytoplasm is everything between the plasma membrane and the nuclear envelope. In prokaryotes, which lack a nucleus, cytoplasm simply means everything found inside the plasma membrane.
Cytoplasm
The cytoplasm of a eukaryotic cell consists not only of cytosol—a gel-like substance made up of water, ions, and macromolecules-but also of organelles and the structural proteins that make up the cytoskeleton, or “skeleton of the cell.” One major component of the cytoplasm in both prokaryotes and eukaryotes is the gel-like cytosol, a water-based solution that contains ions, small molecules, and macromolecules. In eukaryotes, the cytoplasm also includes membrane-bound organelles, which are suspended in the cytosol. The cytoskeleton, a network of fibers that supports the cell and gives it shape, is also part of the cytoplasm and helps to organize cellular components. The cytoplasm consists of all of the contents outside of the nucleus and is enclosed within the cell membrane of a cell. It is clear in color and has a gel-like appearance. The cytoplasm is composed mainly of water but also contains enzymes, salts, organelles, and various organic molecules.
Content and Structure
The cytoplasm can be divided into two primary parts: The endoplasm (endo-, –plasm) and ectoplasm (ecto-plasm). The endoplasm is the central area of the cytoplasm that contains the organelles. The ectoplasm is the more gel-like peripheral portion of the cytoplasm of a cell. Prokaryotic cells, such as bacteria and archaeans, do not have a membrane-bound nucleus. In these cells, the cytoplasm consists of all of the contents of the cell inside the plasma membrane. In eukaryotic cells, such as plant and animal cells, the cytoplasm consists of three main components. They are the cytosol, organelles, and various particles and granules called cytoplasmic inclusions.
- Cytosol: The cytosol is the semi-fluid component or liquid medium of a cell’s cytoplasm. It is located outside of the nucleus and within the cell membrane.
- Organelles: these are tiny cellular structures that perform specific functions within a cell. Examples of organelles include mitochondria, ribosomes, nucleus, lysosomes, chloroplasts, endoplasmic reticulum, and Golgi apparatus. Also located within the cytoplasm is the cytoskeleton, a network of fibers that help the cell maintain its shape and provide support for organelles.
Cytoplasm Functions
The cytoplasm functions to support and suspend organelles and cellular molecules. Many cellular processes also occur in the cytoplasm, such as protein synthesis, the first stage of cellular respiration (known as glycolysis), mitosis, and meiosis. The cytoplasm helps to move materials, such as hormones, around the cell and also dissolves cellular waste.
Cytoplasmic Inclusions
Cytoplasmic inclusions are particles that are temporarily suspended in the cytoplasm. They consist of macromolecules and granules. Three types of inclusions found in the cytoplasm are (1) Secretory inclusions, (2) Nutritive inclusions, and (3) Pigment granules. Examples of secretory inclusions are proteins, enzymes, and acids. Glycogen (glucose storage molecule) and lipids are examples of nutritive inclusions. Melanin found in skin cells is an example of a pigment granule inclusion.
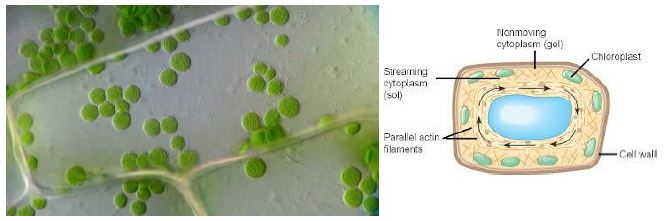
Figure 1: Cytoplasmic Streaming in plant cell
Cytoplasmic Streaming or Cyclosis
It is a process by which substances are circulated within a cell. Cytoplasmic streaming occurs in a number of cell types including plant cells, amoeba, protozoa, and fungi. Cytoplasmic movement may be influenced by several factors including the presence of certain chemicals, hormones, or changes in light or temperature.
Plants employ cyclosis to shuttle chloroplasts to areas receiving the most available sunlight. Chloroplasts are the plant organelles responsible for photosynthesis and require light for the process. In protists, such as amoebae and slime molds, cytoplasmic streaming is used for locomotion. Temporary extensions of the cytoplasm known as pseudopodia are generated that are valuable for movement and capturing food. Cytoplasmic streaming is also required for cell division as the cytoplasm must be distributed among daughter cells formed in mitosis and meiosis.
Cytosol
One major component of the cytoplasm in both prokaryotes and eukaryotes is the gel-like cytosol, a water-based solution that contains ions, small molecules, and macromolecules. In eukaryotes, the cytoplasm also includes membrane-bound organelles, which are suspended in the cytosol. The cytoskeleton, a network of fibers that supports the cell and gives it shape, is also part of the cytoplasm and helps to organize cellular components. The interior of a cell is composed of organelles, the cytoskeleton, and the cytosol. The cytosol often comprises more than 50% of a cell’s volume. Beyond providing structural support, the cytosol is the site wherein protein synthesis takes place, and provides a home for the centrosomes and centrioles. These organelles will be discussed more with the cytoskeleton.
Content and Structure
Even though the cytosol is mostly water, it has a semi-solid, Jello-like consistency because of the many proteins suspended in it. The cytosol contains a rich broth of macromolecules and smaller organic molecules, including glucose and other simple sugars, polysaccharides, amino acids, nucleic acids, and fatty acids. Ions of sodium, potassium, calcium, and other elements are also found in the cytosol. Many metabolic reactions, including protein synthesis, take place in this part of the cell.
Function
The jelly-like fluid that fills a cell is called cytoplasm. It is made up of mostly water and salt. The cytoplasm is present within the cell membrane of all cell types and contains all organelles and cell parts. The cytoplasm has various functions in the cell. Most of the important activities of the cell occur in the cytoplasm. The cytoplasm contains molecules such as enzymes which are responsible for breaking down waste and also aid in metabolic activity. The cytoplasm is responsible for giving a cell its shape. It helps to fill out the cell and keeps organelles in their place. Without cytoplasm, the cell would be deflated and materials would not be able to pass easily from one organelle to another. The cytosol is the part of the cytoplasm that does not contain organelles. Instead, the cytosol is confined by the boundaries of a matrix that fills the part of the cell that does not contain organelles.
Cytoskeleton
We often think about cells as soft, unstructured blobs, interestingly enough, the same is true for a cell. But in reality, they are highly structured in much the same way as our own bodies. They have a network of filaments known as the cytoskeleton (literally, “cell skeleton”), which not only supports the plasma membrane and gives the cell an overall shape, but also aids in the correct positioning of organelles, provides tracks for the transport of vesicles, and (in many cell types) allows the cell to move. Have we ever thought, what would happen if someone snuck in during the night and stole your skeleton? Just to be clear, that’s not very likely to happen, biologically speaking. But if it did somehow happen, the loss of your skeleton would cause your body to lose much of its structure. Your external shape would change, some of your internal organs might start moving out of place, and you would probably find it very difficult to walk, talk, or move. The cytoskeleton is a network of filaments and tubules that extends throughout a cell, through the cytoplasm, which is all of the material within a cell except for the nucleus. It is found in all cells, though the proteins that it is made of vary between organisms. The cytoskeleton supports the cell, gives it shape, organizes and tethers the organelles, and has roles in molecule transport, and cell signaling. All cells have a cytoskeleton, but usually, the cytoskeleton of eukaryotic cells is what is meant when discussing the cytoskeleton. Eukaryotic cells are complex cells that have a nucleus and organelles. Plants, animals, fungi, and protists have eukaryotic cells. Prokaryotic cells are less complex, with no true nucleus or organelles except ribosomes, and they are found in the single-celled organism’s bacteria and archaea. The cytoskeleton of prokaryotic cells was originally thought not to exist; it was not discovered until the early 1990s. The cytoskeleton is similar to the lipid bilayer in that it helps provide the interior structure of the cell the way the lipid bilayer provides the structure of the cell membrane. The cytoskeleton also allows the cell to adapt. Often, a cell will reorganize its intracellular components, leading to a change in its shape. The cytoskeleton is responsible for mediating these changes. By providing “tracks” with its protein filaments, the cytoskeleton allows organelles to move around within the cell. In addition to facilitating intracellular organelle movement, by moving itself the cytoskeleton can move the entire cells in multi-cellular organisms. In this way, the cytoskeleton is involved in intercellular communication.
Content and Structure
The eukaryotic cytoskeleton consists of three types of filaments, which are elongated chains of proteins: microfilaments, intermediate filaments, and microtubules. The microfilaments of this cell are shown in red, while microtubules are shown in green. The blue dots are nuclei. These three types of protein are distinct in their structure and specific function, but all work together to help provide intra-cellular structure. Because they are so diverse, it is very difficult to study the specific functions of the cytoskeletal components.
Microfilaments
Microfilaments are also called actin filaments because they are mostly composed of the protein actin; their structure is two strands of actin wound in a spiral. They are about 5 to 9 nanometers thick, making them the thinnest filaments and narrowest in all 3 types of protein fibers in the cytoskeleton. They are made up of many linked monomers of a protein called actin, combined in a structure that resembles a double helix. Because they are made of actin monomers, microfilaments are also known as actin filaments. Actin filaments have directionality, meaning that they have two structurally different ends.
Functions
Microfilaments have many functions. They aid in cytokinesis, which is the division of the cytoplasm of a cell when it is dividing into two daughter cells. They aid in cell motility and allow single-celled organisms like amoebas to move. They are also involved in cytoplasmic streaming, which is the flowing of cytosol (the liquid part of the cytoplasm) throughout the cell. Cytoplasmic streaming transports nutrients and cell organelles. Microfilaments are also part of muscle cells and allow these cells to contract, along with myosin. Actin and myosin are the two main components of muscle contractile elements. Actin filaments have a number of important roles in the cell. For one, they serve as tracks for the movement of a motor protein called myosin, which can also form filaments. Because of its relationship to myosin, actin is involved in many cellular events requiring motion. For instance, in animal cell division, a ring made of actin and myosin pinches the cell apart to generate two new daughter cells. Actin and myosin are also plentiful in muscle cells, where they form organized structures of overlapping filaments called sarcomeres. When the actin and myosin filaments of a sarcomere slide past each other in concert, your muscles contract. Actin filaments may also serve as highways inside the cell for the transport of cargoes, including protein-containing vesicles and even organelles. These cargoes are carried by individual myosin motors, which “walk” along actin filament bundles, like start superscript, 1, end superscript. Actin filaments can assemble and disassemble quickly, and this property allows them to play an important role in cell motility (movement), such as the crawling of a white blood cell in your immune system. Finally, actin filaments play key structural roles in the cell. In most animal cells, a network of actin filaments is found in the region of cytoplasm at the very edge of the cell. This network, which is linked to the plasma membrane by special connector proteins, gives the cell shape and structure. Actin is the most abundant protein in most eukaryotic cells. Most actin molecules work together to give support and structure to the plasma membrane and are therefore found near the cell membrane.
Intermediate Filaments
Intermediate filaments are a type of cytoskeletal element made of multiple strands of fibrous proteins wound together. These are the first class of proteins that compose the cytoskeleton. These structures are fibrous and rope-like in appearance. As their name suggests, intermediate filaments have an average diameter of 8 to 12 nm, in between that of microfilaments and microtubules.
Functions
Intermediate filaments come in a number of different varieties, each one made up of a different type of protein. One protein that forms intermediate filaments is keratin, a fibrous protein found in hair, nails, and skin. For instance, you may have seen shampoo ads that claim to smooth the keratin in your hair! Intermediate filaments, in the form of keratins are also present in animals with scales, horns, or hooves), vimentin, desmin, and lamin. All intermediate filaments are found in the cytoplasm except for lamins, which are found in the nucleus and help support the nuclear envelope that surrounds the nucleus. The intermediate filaments in the cytoplasm maintain the cell’s shape, bear tension, and provide structural support to the cell. They are not found in all animal cells, but in those in which they are present they form a network surrounding the nucleus often called the nuclear lamina. Other types of intermediate filaments extend through the cytosol. The filaments help to resist stress and increase cellular stability. Unlike actin filaments, which can grow and disassemble quickly, intermediate filaments are more permanent and play an essentially structural role in the cell. They are specialized to bear tension, and their jobs include maintaining the shape of the cell and anchoring the nucleus and other organelles in place.
Microtubules
Microtubules are the largest of the cytoskeleton’s fibers at about 23 nm. A microtubule is made up of tubulin proteins arranged to form a hollow, straw-like tube, and each tubulin protein consists of two subunits, α-tubulin and β-tubulin. Microtubules form structures like flagella, which are “tails” that propel a cell forward. They are also found in structures like cilia, which are appendages that increase a cell’s surface area and in some cases allow the cell to move. Despite the “micro” in their name, microtubules are the largest of the three types of cytoskeletal fibers, with a diameter of about 25 nm. Microtubules, like actin filaments, are dynamic structures: they can grow and shrink quickly by the addition or removal of tubulin proteins. Also similar to actin filaments, microtubules have directionality, meaning that they have two ends that are structurally different from one another. In a cell, microtubules play an important structural role, helping the cell resist compression forces. Microtubules are relatively unstable and go through a process of continuous growth and decay.
Functions
Most of the microtubules in an animal cell come from a cell organelle called the centrosome, which is a Microtubule Organizing Center (MTOC). The centrosome is found near the middle of the cell, and microtubules radiate outward from it. Microtubules are important in forming the spindle apparatus (or mitotic spindle), which separates sister chromatids so that one copy can go to each daughter cell during cell division. They are also involved in transporting molecules within the cell and in the formation of the cell wall in plant cells. In addition to providing structural support, microtubules play a variety of more specialized roles in a cell. For instance, they provide tracks for motor proteins called kinesins and dyneins, which transport vesicles and other cargoes around the interior of the cell. During cell division, microtubules assemble into a structure called the spindle, which pulls the chromosomes apart. Certain proteins will use microtubules as tracks for laying out organelles in a cell. Basically these long, cylindrical structures composed of the protein tubulin and organized around a centrosome, an organelle usually found in the center of the cell near the cell nucleus. Unlike actin molecules, microtubules work separately to provide tracks on which organelles can travel from the center of the cell outward. Microtubules are much more rigid than actin molecules, one end of each microtubule is embedded in the centrosome; the microtubule grows outward from there.
Flagella, Cilia, and Centrosomes
Microtubules are also key components of three more specialized eukaryotic cell structures: flagella, Cilia and Centrosomes. Prokaryotes also have structures like flagella, which they use to move. The eukaryotic flagella we’re about to discuss have pretty much the same role, but a very different structure.
We know that our friends the prokaryotes also have structures like flagella, which they use to move. Don’t get confused-the eukaryotic flagella we’re about to discuss have pretty much the same role, but a very different structure.
Flagella
Flagella (singular, flagellum) are long, hair-like structures that extend from the cell surface and are used to move an entire cell, such as a sperm. If a cell has any flagella, it usually has one or just a few. Motile cilia (singular, cilium) are similar, but are shorter and usually appear in large numbers on the cell surface. When cells with motile cilia form tissues, the beating helps move materials across the surface of the tissue. For example, the cilia of cells in your upper respiratory system help move dust and particles out towards your nostrils.
Cilia
Despite their difference in length and number, flagella and motile cilia share a common structural pattern. In most flagella and motile cilia, there are 9 pairs of microtubules arranged in a circle, along with an additional two microtubules in the center of the ring. This arrangement is called a 9 + 2 array. In flagella and motile cilia, motor proteins called dyneins move along the microtubules, generating a force that causes the flagellum or cilium to beat. The structural connections between the microtubule pairs and the coordination of dynein movement allow the activity of the motors to produce a pattern of regular beating.
Basal Body
The cilium or flagellum has a basal body located at its base. The basal body is made of microtubules and plays a key role in assembly of the cilium or flagellum. Once the structure has been assembled, it also regulates which proteins can enter or exit.
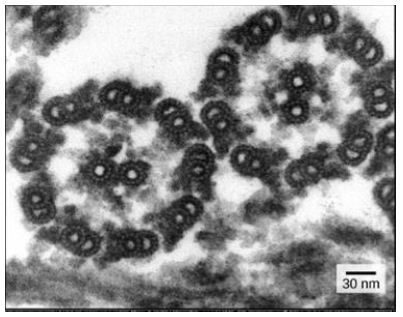
Figure 2: 9 + 2 array in the electron microscopy
Centrosome
The basal body is actually just a modified centriole. A centriole is a cylinder of nine triplets of microtubules, held together by supporting proteins. Centrioles are best known for their role in centrosomes, structures that act as microtubule organizing centers in animal cells, they are small arrays of microtubules that are found in the center of a centrosome. A centrosome consists of two centrioles oriented at right angles to each other, surrounded by a mass of “pericentriolar material,” which provides anchoring sites for microtubules. The centrosome is duplicated before a cell divides, and the paired centrosomes seem to play a role in organizing the microtubules that separate chromosomes during cell division. However, the exact function of the centrioles in this process still isn’t clear. Cells with their centrosome removed can still divide, and plant cells, which lack centrosomes, divide just fine.
Other Structure
A number of motor proteins are found in the cytoskeleton. As their name suggests, these proteins actively move cytoskeleton fibers. As a result, molecules and organelles are transported around the cell. Motor proteins are powered by ATP, which is generated through cellular respiration. There are three types of motor proteins involved in cell movement.
Motor Proteins
Kinesins move along microtubules carrying cellular components along the way. They are typically used to pull organelles toward the cell membrane.
Dyneins are similar to kinesins and are used to pull cellular components inward toward the nucleus. Dyneins also work to slide microtubules relative to one another as observed in the movement of cilia and flagella.
Myosins interact with actin in order to perform muscle contractions. They are also involved in cytokinesis, endocytosis, and exocytosis.
Cytoskeleton Functions
As described above, the cytoskeleton extends throughout the cell’s cytoplasm and directs a several number of important functions. First, it gives the support and shape to the cell. This is especially important in cells without cell walls, such as animal cells, that do not get their shape from a thick outer layer. It can also give the cell movement. The microfilaments and microtubules can disassemble, reassemble, and contract, allowing cells to crawl and migrate, and microtubules help form structures like cilia and flagella that allow for cell movement. The cytoskeleton organizes the cell and keeps the cell’s organelles in place, but it also aids in the movement of organelles throughout the cell. It assists in the formation of vacuoles. For example, during endocytosis when a cell engulfs a molecule, microfilaments pull the vesicle containing the engulfed particles into the cell. Similarly, the cytoskeleton helps move chromosomes during cell division. One analogy for the cytoskeleton is the frame of a building. Like a building’s frame, the cytoskeleton is the “frame” of the cell, keeping structures in place, providing support, and giving the cell a definite shape. The cytoskeleton is not a static structure but is able to disassemble and reassemble its parts in order to enable internal and overall cell mobility. Types of intracellular movement supported by the cytoskeleton include transportation of vesicles into and out of a cell, chromosome manipulation during mitosis and meiosis, and organelle migration. The cytoskeleton makes cell migration possible as cell motility is needed for tissue construction and repair, cytokinesis (the division of the cytoplasm) in the formation of daughter cells, and in immune cell responses to germs. The cytoskeleton assists in the transportation of communication signals between cells. It forms cellular appendage-like protrusions, such as cilia and flagella, in some cells [1-69].
References
- Analysis of the role of astral rays in pronuclear migration in sand dollar eggs by the colcemid−UV method. Acad. Sci. U.S.A. 94: 6228-6231.
- Howard J (ed.) (2001) Mechanics of Motor Proteins and the Cytoskeleton.
- Ausmees N, Kuhn JR, Jacobs-Wagner C (2003) The bacterial cytoskeleton: an intermediate filament-like function in cell shape. Cell 115: 705-713. [crossref]
- Azoury J, Lee KW, Georget V, Rassinier P, Leader B, et al. (2008) Spindle positioning in mouse oocytes relies on a dynamic meshwork of actin filaments. Curr Biol 18: 1514-1519. [crossref]
- Berdyyeva TK, Woodworth CD, Sokolov I (2005) Human epithelial cells increase their rigidity with ageing in vitro: direct measurements. Phys Med Biol 50: 81-92. [crossref]
- Brangwynne CP, MacKintosh FC, Kumar S, Geisse NA, Talbot J, et al. (2006) Microtubules can bear enhanced compressive loads in living cells because of lateral reinforcement. J Cell Biol 173: 733-741. [crossref]
- Burns JM, Cuschieri A, Campbell PA (2006) Optimisation of fixation period on biological cells via time- lapse elasticity mapping. J. Appl. Phys 45: 2341-2344.
- Bursac P, et al. (2005) Cytoskeletal remodelling and slow dynamics in the living cell. Nature Mater 4: 557-561.
- Haupt A, Minc N (2018) How cells sense their own shape – mechanisms to probe cell geometry and their implications in cellular organization and function. J Cell Sci 131: jcs214015. [crossref]
- Chaudhuri O, Parekh SH, Fletcher DA (2007) Reversible stress softening of actin networks. Nature 445: 295-298. [crossref]
- Chaudhuri O, Parekh SH, Lam WA, Fletcher DA (2009) Combined atomic force microscopy and side-view optical imaging for mechanical studies of cells. Nature Methods 6: 383-387. [crossref]
- Cheng G, Tse J, Jain RK, Munn LL (2009) Micro-environmental mechanical stress controls tumor spheroid size and morphology by suppressing proliferation and inducing apoptosis in cancer cells. PLoS ONE 4: e4632. [crossref]
- Cheng X, Ferrell JE (2019) Spontaneous emergence of cell-like organization in Xenopus egg extracts. Science 366: 631-637. [crossref]
- Chugh P, Paluch EK (2018) The actin cortex at a glance. J Cell Sci 131: jcs186254. [crossref]
- Pizarro-Cerda J, Cossart P (2006) Confinement induces actin flow in a meiotic cytoplasm. Natl. Acad. Sci. U.S.A 109: 11705-11710.
- Cooper JA, Sept D (2008) New insights into mechanism and regulation of actin capping protein. Int Rev Cell Mol Biol 267: 183-206. [crossref]
- Field CM, Wühr, M, Anderson GA, Kueh HY, Strickland D, et al. (2011) Bulk cytoplasmic actin and its functions in meiosis and mitosis. Curr Biol 21: R825-R830. [crossref]
- De Simone A, Spahr A, Busso C, Gönczy P (2018) Uncovering the balance of forces driving microtubule aster migration in C. elegans zygotes. Nat Commun 9: 938.
- Discher DE, Janmey P, Wang YL (2005) Tissue cells feel and respond to the stiffness of their substrate. Science 310: 1139-1143. [crossref]
- Discher DE, Mooney DJ, Zandstra PW (2009) Growth factors, matrices, and forces combine and control stem cells. Science 324: 1673-1677. [crossref]
- Dmitrieff S, Minc N (2019) Scaling properties of centering forces. Europhys Lett 125: 48001.
- Dogterom M, Yurke B (1997) Measurement of the force-velocity relation for growing microtubules. Science 278: 856-860. [crossref]
- Dumont S, Mitchison TJ (2009) Force and length in the mitotic spindle. Curr Biol 19: R749-761. [crossref]
- Dos Remedios CG, et al. (2003) Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol Rev 83: 433-473. [crossref]
- Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126: 677-689. [crossref]
- Flitney EW, Kuczmarski ER, Adam SA, Goldman RD (2009) Insights into the mechanical properties of epithelial cells: the effects of shear stress on the assembly and remodeling of keratin intermediate filaments. FASEB J 23: 2110-2119. [crossref]
- Grishchuk EL, Molodtsov MI, Ataullakhanov FI, McIntosh JR (2005) Force production by disassembling microtubules. Nature 438: 384-388. [crossref]
- Foster PJ, Furthauer S, Shelley MJ, Needleman DJ (2015) Active contraction of microtubule networks. elife 4: e10837. [crossref]
- Kimura A, Onami S (2005) Formin mDia1 senses and generates mechanical forces on actin filaments. Commun 4: 1883.
- Gardel ML, Shin JH, MacKintosh FC, Mahadevan L, Matsudaira P, et al. (2004) Elastic behavior of cross-linked and bundled actin networks. Science 304: 1301-1305. [crossref]
- Garner EC, Campbell CS, Weibel DB, Mullins RD (2007) Reconstitution of DNA segregation driven by assembly of a prokaryotic actin homolog. Science 315: 1270-1274. [crossref]
- Garzon-Coral C, Fantana HA, Howard J (2016) A force-generating machinery maintains the spindle at the cell center during mitosis. Science 352: 1124-1127. [crossref]
- Giardini PA, Theriot JA (2001) Effects of intermediate filaments on actin-based motility of Listeria monocytogenes. Biophys J 81: 3193-3203. [crossref]
- Green RA, Paluch E, Oegema K (2012) Cytokinesis in animal cells. Rev. Cell Dev. Biol 28: 29-58. [crossref]
- Grill SW, Gönczy P, Stelzer EH, Hyman AA (2001) Polarity controls forces governing asymmetric spindle positioning in the Caenorhabditis elegans embryo. Nature 409: 630-633. [crossref]
- Morin X, Bellaich Y (2011) Growth, interaction, and positioning of microtubule asters in extremely large vertebrate embryo cells. Cytoskeleton (Hoboken) 69: 738-750.
- Janmey PA, McCulloch CA (2007) Cell mechanics: integrating cell responses to mechanical stimuli. Annu Rev Biomed Eng 9: 1-34. [crossref]
- Janmey PA, Winer JP, Murray ME, Wen Q (2009) The hard life of soft cells. Cell Motil Cytoskeleton 66: 597-605. [crossref]
- Kaksonen M, Toret CP, Drubin DG (2005) A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell 123: 305-320. [crossref]
- Kato S, Espinoza N, Lange S, Villalón M, Cuello M, et al. (2008) Characterization and phenotypic variation with passage number of cultured human endometrial adenocarcinoma cells. Tissue Cell 40: 95-102. [crossref]
- Keren K, Pincus Z, Allen GM, Barnhart EL, Marriott G, et al. (2008) Mechanism of shape determination in motile cells. Nature 453: 475-480. [crossref]
- Kimura K, Kimura A (2011) Intracellular organelles mediate cytoplasmic pulling force for centrosome centration in the Caenorhabditis elegans early embryo. Proc Natl Acad Sci. U.S.A 108: 137-142. [crossref]
- Koenderink GH, Dogic Z, Nakamura F, Bendix PM, MacKintosh FC, et al. (2009) An active biopolymer network controlled by molecular motors. Proc Natl Acad Sci USA 106: 15192-15197. [crossref]
- Liu AP, Richmond DL, Maibaum L, Pronk S, Geissler PL, et al. (2008) Membrane-induced bundling of actin filaments. Nature Phys 4: 789-793. [crossref]
- Liu YJ, Le Berre M, Lautenschlaeger F, Maiuri P, Callan-Jones A, et al. (2015) Confinement and low adhesion induce fast amoeboid migration of slow mesenchymal cells. Cell 160: 659-672. [crossref]
- Malik-Garbi M, Ierushalmi N, Jansen S, Abu-Shah E, Goode BL, et al (2019) Scaling behaviour in steady-state contracting actomyosin networks. Nat Phys 15: 509-516. [crossref]
- Marcy Y, Prost J, Carlier MF, Sykes C (2004) Forces generated during actin-based propulsion: a direct measurement by micromanipulation. Nat. Acad. Sci. U.S.A 101: 5992-5997. [crossref]
- Mayer M, Depken M, Bois JS, Julicher F, Grill SW (2010) Anisotropies in cortical tension reveal the physical basis of polarizing cortical flows. Nature 467: 617-621. [crossref]
- McGrath JL, Eungdamrong NJ, Fisher CI, Peng F, Mahadevan L, et al. (2003) The force-velocity relationship for the actin-based motility of Listeria monocytogenes. Curr Biol 13: 329-332. [crossref]
- McNally FJ (2013) Mechanisms of spindle positioning. J Cell Biol 200: 131-140. [crossref]
- Minc N, Burgess D, Chang F (2011) Influence of cell geometry on division-plane positioning. Cell 144: 414-426. [crossref]
- Minc N, Piel M (2012) Predicting division plane position and orientation. Trends Cell Biol 22: 193-200. [crossref]
- Morin X, Bellaïche Y (2011) Mitotic spindle orientation in asymmetric and symmetric cell divisions during animal development. Dev Cell 21: 102-119. [crossref]
- Mogilner A, et al. (2019) Centering and symmetry breaking in confined contracting actomyosin networks. arXiv 1907: 10642.
- Ishihara K, Nguyen PA, Groen AC, Field CM, Mitchison TJ (2014) Microtubule nucleation remote from centrosomes may explain how asters span large cells. Natl. Acad. Sci 111: 17715-17722. [crossref]
- Munjal A, Lecuit T (2014) Actomyosin networks and tissue morphogenesis. Development 141: 1789-1793. [crossref]
- Naumanen P, Lappalainen P, Hotulainen P (2008) Mechanisms of actin stress fibre assembly. J Microsc 231: 446-454. [crossref]
- Parekh SH, Chaudhuri O, Theriot JA, Fletcher DA (2005) Loading history determines the velocity of actin-network growth. Nat Cell Biol 7: 1219-1223.
- Pontani LL, van der Gucht J, Salbreux G, Heuvingh J, Joanny JF, et al. (2009) Reconstitution of an actin cortex inside a liposome. Biophys J 96: 192-198. [crossref]
- Prass M, Jacobson K, Mogilner A, Radmacher M (2006) Direct measurement of the lamellipodial protrusive force in a migrating cell. J Cell Biol 174: 767-772. [crossref]
- Vallee RB, Stehman SA (2005) How dynein helps the cell find its center: a servomechanical model. Trends Cell Biol 15: 288-294. [crossref]
- Rai AK, Rai A, Ramaiya AJ, Jha R, Mallik R (2013) Molecular adaptations allow dynein to generate large collective forces inside cells. Cell 152: 172-182. [crossref]
- Ruprecht V, Wieser S, Callan-Jones A, Smutny M, Morita H, et al. (2015) Cortical contractility triggers a stochastic switch to fast amoeboid cell motility. Cell 160: 673-685. [crossref]
- Schaller V, Weber C, Semmrich C, Frey E, Bausch AR (2010) Polar patterns of driven filaments. Nature 467: 73-77. [crossref]
- Schuh M, Ellenberg J (2008) A new model for asymmetric spindle positioning in mouse oocytes. Curr Biol 18: 1986-1992. [crossref]
- Taunton J, Rowning BA, Coughlin ML, Wu M, Moon RT, et al. (2000) Actin-dependent propulsion of endosomes and lysosomes by recruitment of N-WASP. J Cell Biol 148: 519-530. [crossref]
- Tsai MY, Wang S, Heidinger JM, Shumaker DK, Adam SA, et al. (2006) A mitotic lamin B matrix induced by RanGTP required for spindle assembly. Science 311: 1887-1893. [crossref]
- Wagner B, Tharmann R, Haase I, Fischer M, Bausch AR (2006) Cytoskeletal polymer networks: the molecular structure of cross-linkers determines macroscopic properties. Natl Acad. Sci 103: 13974-13978. [crossref]
- Wu H-Y, Nazockdast E, Shelley MJ, Needleman DJ (2016) Forces positioning the mitotic spindle: theories, and now experiments. BioEssays 39: 1600212. [crossref]