DOI: 10.31038/PSC.2023311
Abstract
Background/Aim: Cremaster muscle is a specialized skeletal muscle, and its differentiation into mature skeletal muscle is normally delayed until after gubernacular migration to the scrotum. The molecular cues regulating its morphology remain elusive. We examined gene expression and immunofluorescence of known markers expressed in cremaster muscle to determine the effect of androgen blockade.
Methods: Gubernacular cells from wild-type (WT) and androgen receptor knock-out (ARKO) mice were cultured at E17, D0 and D3 (n=3 animals/group/day) and qPCR performed for b-Catenin; Desmin; Myogenin; Ki67; Pax 7; PPAR-g; Myh3; and Androgen receptor (AR). The same age groups were also processed for fluorescent immunohistochemistry, and visualized by confocal microscopy.
Results: Β-Catenin expression at D3 was increased in ARKO compared to control animals, and β-Catenin immunofluorescence demonstrated increased cytoplasmic staining in D3 ARKO animals and displaced in the banding pattern of mature skeletal muscle. Desmin, Myogenin and Ki67 expression were all increased in D3 ARKO compared to control animals.
Conclusions: Blockade of androgen in mice demonstrates increased expression of myogenic proteins at D3. This is consistent with premature maturation of cremaster muscle, which is associated with failed elongation of the gubernaculum, incomplete testicular migration to the scrotum, leading to cryptorchidism.
Keywords
Cryptorchidism, Beta-catenin, Testes, Cremaster muscle
Highlights
- What is currently known about this topic?
- What new information is contained in this article?
- Hutson JM, Balic A, Nation T, Southwell B (2010) Cryptorchidism. Seminars in Pediatric Surgery 19: 215-24.
- Hutson JM, Hasthorpe S, Heyns CF (1997) Anatomical and functional aspects of testicular descent and cryptorchidism. Endocrine Reviews 18: 259-80. [crossref]
- Hutson JM (1985) A biphasic model for the hormonal control of testicular descent. Lancet 2: 419-21. [crossref]
- Favorito LA, Costa SF, Julio-Junior HR, Sampaio FJ (2014) The importance of the gubernaculum in testicular migration during the human fetal period. International Braz J Urol: Official Journal of the Brazilian Society of Urology 40: 722-9. [crossref]
- Costa WS, Sampaio FJ, Favorito LA, Cardoso LE (2002) Testicular migration: remodeling of connective tissue and muscle cells in human gubernaculum testis. J Urol 167: 2171-6. [crossref]
- Harnaen EJ, Na AF, Shenker NS, et al. (2007) The anatomy of the cremaster muscle during inguinoscrotal testicular descent in the rat. Journal of Pediatric Surgery 42: 1982-7
- Churchill JA, Buraundi S, Farmer PJ, et al. (2011) Gubernaculum as icebreaker: do matrix metalloproteinases in rodent gubernaculum and inguinal fat pad permit testicular descent? Journal of Pediatric Surgery 46: 2353-7
- Nation TR, Buraundi S, Balic A, et al. (2011) The effect of flutamide on expression of androgen and estrogen receptors in the gubernaculum and surrounding structures during testicular descent. Journal of Pediatric Surgery 46: 2358-62. [crossref]
- Perera N, Szarek M, Vannitamby A, et al. (2018) An immunohistochemical analysis of the effects of androgen receptor knock out on gubernacular differentiation in the mouse. Journal of Pediatric Surgery 53: 1776-80. [crossref]
- Li Z, Marchand P, Humbert J, Babinet C, Paulin D (1993) Desmin sequence elements regulating skeletal muscle-specific expression in transgenic mice. Development (Cambridge, England) 117: 947-59. [crossref]
- Faralli H, Dilworth FJ (2012) Turning on myogenin in muscle: a paradigm for understanding mechanisms of tissue-specific gene expression. Comparative and Functional Genomics 2012: 836374. [crossref]
- Notini AJ, Davey RA, McManus JF, Bate KL, Zajac JD (2005) Genomic actions of the androgen receptor are required for normal male sexual differentiation in a mouse model. Journal of Molecular Endocrinology 35: 547-55.
- Vikraman J, Sarila G, O’Conner L, Menheniott T, Hutson JM (2022) BDNF is upregulated by androgen in the inguinal fat pad of immature mice and may regulate inguinoscrotal testicular descent. Pediatric Research 91: 846-52. [crossref]
- Kriegova E, Arakelyan A, Fillerova R, et al. (2008) PSMB2 and RPL32 are suitable denominators to normalize gene expression profiles in bronchoalveolar cells. BMC Molecular Biology 9: 69.
- Sanders N, Buraundi S, Balic A, Southwell BR, Hutson JM (2011) Cremaster muscle myogenesis in the tip of the rat gubernaculum supports active gubernacular elongation during inguinoscrotal testicular descent. J Urol 186: 1606-13. [crossref]
- Lie G, Hutson JM (2011) The role of cremaster muscle in testicular descent in humans and animal models. Pediatric Surgery International 27: 1255-65. [crossref]
- Szarek M, Li R, Vikraman J, Southwell B, Hutson JM (2014) Molecular signals governing cremaster muscle development: clues for cryptorchidism. Journal of Pediatric Surgery 49: 312-6.
- Griffiths AL, Momose Y, Hutson JM (1993) The gubernaculum in adult female, adult male and TFM male mice. International Journal of Andrology 16: 380-4. [crossref]
- Lee DK (2002) Androgen receptor enhances myogenin expression and accelerates differentiation. Biochemical and Biophysical Research Communications 294: 408-13. [crossref]
- Tanyel FC, Talim B, Atilla P, Müftüoğlu S, Kale G (2005) Myogenesis within the human gubernaculum: histological and immunohistochemical evaluation. European Journal of Pediatric Surgery: Official Journal of Austrian Association of Pediatric Surgery 15: 175-9.
- Welsh M, Saunders PT, Fisken M, et al. (2008) Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. The Journal of Clinical Investigation 118: 1479-90. [crossref]
- Schwindt B, Farmer PJ, Watts LM, Hrabovszky Z, Hutson JM (1999) Localization of calcitonin gene-related peptide within the genitofemoral nerve in immature rats. Journal of Pediatric Surgery 34: 986-91. [crossref]
- Sarila G, Hutson JM, Vikraman J (2022) Testicular descent: A review of a complex, multistaged process to identify potential hidden causes of UDT. Journal of Pediatric Surgery 57: 479-87. [crossref]
- Choi HY, Lim JE, Hong JH (2010) Curcumin interrupts the interaction between the androgen receptor and Wnt/β-catenin signaling pathway in LNCaP prostate cancer cells. Prostate Cancer and Prostatic Diseases 13: 343-9.
- Kaftanovskaya EM, Feng S, Huang Z, et al. (2011) Suppression of insulin-like3 receptor reveals the role of β-catenin and Notch signaling in gubernaculum development. Molecular Endocrinology (Baltimore, Md) 25: 170-83. [crossref]
- Hughes IA, Davies JD, Bunch TI, Pasterski V, Mastroyannopoulou K et al. (2012) Androgen insensitivity syndrome. Lancet 380: 1419-28.
- Singh J, Verma NK, Kansagra SM, Kate BN, Dey CS (2007) Altered PPARgamma expression inhibits myogenic differentiation in C2C12 skeletal muscle cells. Molecular and Cellular Biochemistry 294: 163-71. [crossref]
- Hutson JM, Baskin LS, Risbridger G, Cunha GR (2014) The power and perils of animal models with urogenital anomalies: handle with care. Journal of Pediatric Urology 10: 699-705. [crossref]
- Barthold JS, McCahan SM, Singh AV, et al. (2008) Altered expression of muscle- and cytoskeleton-related genes in a rat strain with inherited cryptorchidism. Journal of Andrology 29: 352-66. [crossref]
Androgen controls the second stage of testicular descent. As the testes descend, the cremaster muscle is formed within the gubernacular mesenchyme. Cremaster myogenesis is known to involve the expression of β-Catenin; Desmin; Myogenin; Ki67; Pax 7; PPAR-g; and Myh3.
Androgen seems to delay maturation of cremaster muscle allowing elongation of the gubernaculum and migration of the testis. Androgen receptor knockout animals express markers of muscle maturation earlier than control animals, consistent with premature maturation of the cremaster muscle.
Introduction
Testicular descent is vital for fertility and prevention of testicular malignancy. Failed testicular descent is one of the most common genital anomalies, affecting 2-4% of newborn males. The gubernaculum, or genitoinguinal ligament, controls testicular descent and subsequent cremaster formation within it in both rodents and humans. Androgen is the primary hormone that regulates the second stage of testicular descent, the inguinoscrotal phase, where the gubernaculum migrates across the pubic bone to reach the scrotum. This migration phase involves gubernacular remodelling and cremaster muscle formation; however the exact molecular mechanisms controlling this remain unknown [1-5].
The gubernacular mesenchyme differentiates into the cremaster muscle, which is a specialized skeletal muscle with specialized properties that differ to other skeletal muscles. The most distal portion of the rodent gubernaculum containing more myoblasts than the proximal end, so the gubernaculum can elongate like an embryonic limb bud from a distal growth centre with cremaster development more advanced proximally [6,7]. Gubernacular eversion is an integral part of testicular descent in rodents. Rats treated with the anti-androgen (flutamide) demonstrated smaller cremaster muscle mass and a reduction in the size of the growth centre in the gubernaculum. Androgen receptor knockout (ARKO) mice demonstrate failed gubernacular eversion, and increase in gubernacular cord length with age between E17-D4 [8,9]. Androgen insensitivity in humans also leads to failed gubernacular migration and cremaster development. Although the timing of cremaster muscle development differs between rodents and humans, the basic process is similar and this makes the mouse/rat a suitable model for cremaster myogenesis in humans.
The aim of this study was to determine if androgen blockade caused altered expression of myogenic markers at e17, d0 and d3 in gubernacular fibroblasts, which form the developing cremaster muscle. In response to androgen, the Wnt pathway is known to control multiple target genes to oversee cell proliferation, differentiation and mesenchymal cell migration, so we tested a number of markers in both wild type and ARKO males, which represent different aspects of cremaster myogenesis. Firstly Ki67, which is known to label proliferating cells. β-catenin and PPAR-γ which have been linked to the canonical Wnt pathway, and Myh3 (embryonic heavy chain skeletal myosin) which is expressed in developing muscle fibres. Desmin is one of the earliest protein markers expressed in somites, initially expressed at low levels and increases in expression as cells near terminal differentiation into myoblasts [10]. Myogenin is expressed in skeletal muscle in late myogenesis. It is a skeletal muscle transcription factor, and is known to turn myoblasts into myotubes [11].
Materials and Methods
Animals
Androgen Receptor Knockout (ARKO) mice (Austin Health, Melbourne, Australia). Genetic modification of the third exon of the androgen receptor gene containing the DNA-binding domain was targeted by the Cre/loxP system to remove 1114bp, rendering the animal completely insensitive to androgens [12]. All experiments were performed with approval from Murdoch Children’s Research Institute animal ethics committee (AEC no. A854). Mice were fed normal chow and housed in an enriched, temperature-controlled environment of 23°C and 44% humidity, with a 14-hour-light and 10-hour-dark cycle. Mice were genotyped (refer to supplementary methods section 1.1) and their sex determined before experimental breeding and down-stream analysis.
Experimental ARKO Mice
Females with one copy of the mutation on the AR gene on the X chromosomes (het) were bred with wild-type (WT) males to produce litters containing ARKO males. Pregnant dams were sacrificed and fetuses collected via hysterectomy (n=3 animals/group/day litter matched) at embryonic day 17.5 (vaginal plug=day 0.5), pups at day of birth (D0) and postnatal day 3 (D3). Embryos were removed from the uterus and placed on ice for 15-20 minutes before decapitation, and then the gubernaculum was collected from the embryo and cultured in DMEM media. Gubernacular cells were isolated using trypsin for 30mins, incubated at 37°C and cultured in DMEM as single cell fibroblasts for two weeks until they were 90% confluent. These cells were used for downstream analysis. A further n=3 animals/group/day were collected at e17.5, D0 and D3 and processed for histology and immunohistochemistry.
Standard Histology
Pelvis from a fetus/pups (n=3 animals/group/day) were collected at e17.5, D0 and D3 fixed in 4% paraformaldehyde (PFA) at 4°C overnight before being processed through graded alcohols and xylene and embedded in paraffin. Samples were sectioned in the sagittal plane at 5µm and floated on silane-coated slides. Slides were stored at room temperature (RT) for minimum 24 hours before being stained.
Immunofluorescence
Specimens were sectioned (5μm) and prepared for immunohistochemistry according a previously described protocol [13]. Antibodies selecting specific stages of differentiation were used, β-Catenin; Desmin; Myogenin; Ki67. Single and double labelling of gubernacular sections occurred with the antibodies described in Table 1.
Table 1: Primary and secondary antibodies. DAPI: *4’6-Diamidino-2-phenylindole (labels nuclei of all cells)
|
Company/Catalogue number |
Raised in/clonality |
Working Con. (vol/vol) |
|
| Primary Antibody | |||
| Anti-PPARg | BioVision, 3585BP-50 | Mouse/polyclonal |
1/200 |
| Anti-bCatenin | Abcam, ab2365 | Rabbit/polyclonal |
1/200 |
| Myogenin | Abcam, ab1835 | Mouse/monoclonal |
1/200 |
| Myh3 | DSHB, BF-G6 | Mouse/monoclonal |
1/200 |
| Desmin | CST, D93F5 | Rabbit/monoclonal |
1/500 |
| Ki67 | Abcam, AB16667 | Rabbit/monoclonal |
1/300 |
| Pax 7 | Abcam, 34360 | Rabbit/polyclonal |
1/500 |
| Secondary antibody | |||
| Alexa 488 | Life Tech, A21202 Lot #898250 | Donkey/mouse |
1/1000 |
| Alexa FluorÒ 488 | Invit/a21206 Lot#93b2 | Donkey/rabbit |
1/1000 |
| Alexa 568 | Mol Prob, A-11019 | Goat/mouse |
1/1000 |
| DAPI 454 | Invitrogen, D3571 | N/A |
1/1000 |
Confocal Imaging
Sections of the mouse pelvis were imaged on the Dragonfly spinning disc confocal microscope and images acquired via the Fusion Software (version 2.0, Andor, Northern Ireland). Primary antibodies along with secondary antibodies (Table 1) and DAPI (4, 6-diamidinophenylindole, 1:5000 in PBS) were used to label all nuclei. Confocal images were captured at 40x and 60x magnification. Laser at 488mm, 637 mm excited the DAPI and Alexa 568, respectively to create merged images, which were edited with Fiji Image J software (version 1.50; LOC1, University of Wisconsin-Madison, Madison, Wisconsin, USA) for colour, brightness, contrast correction and scale-bar inserted.
Real-Time qPCR
Total RNA was extracted from the cultured gubernacular fibroblasts of 3 independent ARKO and wild-type males at E17.5, D0 and D3 using the RNeasy mini column (Qiagen, Cat:74104). RNA was treated with DNase I (Qiagen, Cat: 79254) and the concentration was determined using NanoDrop spectrophotometer. Gene expression was measured using 10ng/µl of cDNA by GoTaq qPCR (Promega, Cat: A6001) for real time quantitative polymerase chain reaction (RT-qPCR). Nucleotide sequences (Table 2) were used and the expression was normalised to Rpl32. Rpl32 is a housekeeping gene which enables normalisation for heterogeneity in clinical samples, as well as for variability introduced during RNA extraction and cDNA synthesis [14].
Statistical analysis was performed using Student’s t-test or ANOVA, as appropriate using GraphPad Prism version 7.04 for Windows (GraphPad Software, San Diego, California USA, www.graphpad.com). Error bars on the graphs are presented as standard error of the mean (SEM). A P-value of <0.05 was considered statistically significant.
Table 2: Mouse primer sequences
|
Gene |
Forward Primer Sequence 5’ |
Reverse primer Sequence 5’ |
| Rpl32 |
GAGGTGCTGCTGATGTGC |
GGCGTTGGGATTGGTGACT |
| β-catenin |
ACCTTTCAGATGCAGCGACT |
TGGCACACCATCATCTTGTT |
| Desmin |
GTGGATGCAGCCACTCTAGC |
TTAGCCGCGATGGTCTCATA |
| Mhy3 |
ATGGTGGATGTGGAAAGAGC |
CCGTTTCACGGTTTCAAGTT |
| Myogenin |
ACTCCCTTACGTCCATCGTG |
CAGGACAGCCCCACTTAAAA |
| PPAR-γ |
GTCACACTCTGACAGGAGCC |
TCACCGCTTCTTTCAAATCT |
| Ki67 |
GACAGCTTCCAAAGCTCACC |
TGTGTCCTTAGCTGCCTCCT |
| Pax 7 |
GGAAAACCAGTGTGCCATCT |
CCTTGTCTTTGGCACCATTT |
Results
β-Catenin Expression in ARKO and Control Animals
In the cultured gubernacular fibroblasts, β-catenin expression was not significantly altered in e17.5 and D0 ARKO animals compared to WT. By contrast, β-catenin expression was upregulated in D3 ARKO fibroblasts, compared to the WT male counterpart (P=0.0003) (Figure 1A).
β-Catenin Immunoreactivity in D3 ARKO and Control Animals
Immunofluorescence of the gubernaculum in D3 ARKO and control animals showed differing patterns of staining (Figure 2A). Control animals showed staining throughout the cell cytoplasm, with cell membrane staining as well. ARKO animals showed staining throughout the cytoplasm with displacement into bands, consistent with myotube formation.
Desmin, Myogenin and Ki67 Expression and Immunoreactivity in ARKO and Control Animals
In the cultured gubernaculum; Mhy3 expression was upregulated in D0 ARKO animals, compared to the WT male counterpart (P < 0.0001) (Figure 1B). Immunofluorescence at D0 confirmed this, with decreased fluorescence in the wild type animal compared to the ARKO (Figure 2B). PPAR-γ expression was upregulated in ARKO males at D0 compared to WT male counterpart (p=0.022) (Figure 1C). Immunofluorescence at D0 confirmed this (results not shown). Desmin expression was upregulated in D0 ARKO and D3 ARKO animals, compared to the WT male counterpart (P=0.0242, P=0.05) (Figure 1D). Immunofluorescence at D3 confirmed this (results not shown). Myogenin expression was upregulated in ARKO males at D3, compared to WT male (P=0.05) (Figure 1E). Immunofluorescence at D3 confirmed this, with less fluorescence in wild type animals compared to ARKO (Figure 2C). Ki67 expression was upregulated in D3 ARKO animals, compared to the WT male counterpart (P=0.05) (Figure 1F). Immunofluorescence at D3 confirmed this, with less fluorescence in wild type animals compared to ARKO animals (Figure 2D).
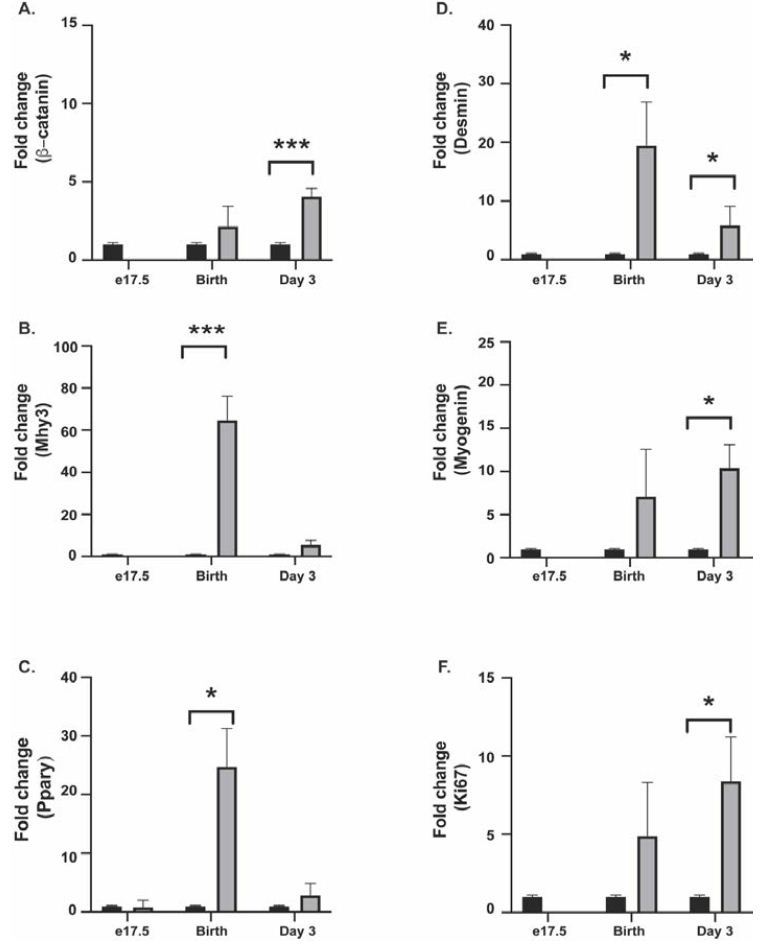
Figure 1: Real-Time qPCR was used to demonstrate gene expression changes in WT (black bar) and ARKO (grey bar) animals at e17.5, birth (day 0), and day 3 postnatal. (A) βCatenin expression (B) Mhy3 expression (C) Ppary expression (D) Desmin expression (E) Myogenin expression (F) Ki67 expression.
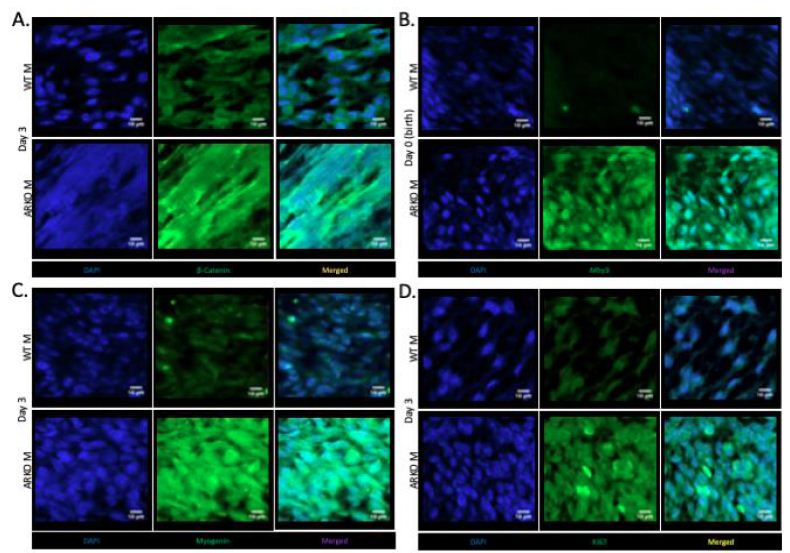
Figure 2: Immunofluorescent labelling in the gubernaculum of wild-type and ARKO animals, blue is DAPI. Scale bar equals 100 mm (imaged with 60x oil magnification). (A) β-Catenin (green) at day 3 in the wild type and ARKO animals. (B) Mhy3 (green) at day 0 wild type and ARKO. (C) Myogenin (green) at day 3 (D) Ki67 (green) at day 3 in wild-type and ARKO animals.
Discussion
The results of this study indicate that fibroblasts cultured from the developing mouse cremaster muscle express more β-catenin at D3 in androgen receptor blockaded animals, and the pattern of immunoreactivity in ARKO animals is different from WT. ARKO animals demonstrated ‘banding’, presumably from myotubes developing skeletal muscle striation and displacing cytoplasm. ARKO animals also demonstrate increased expression of Mhy3 and PPAR-γ at D0, and Desmin, Myogenin and Ki67 at D3 compared to WT animals.
At the onset of rodent inguinoscrotal testicular descent, the gubernaculum is a solid pyramidal structure of mesenchyme which everts from the abdominal cavity through the nascent inguinal canal to form a hollow cone lined by the future processus vaginalis mesothelium [6]. The solid mesenchymatous gubernacular tip is an undifferentiated ‘growth centre’ which has similar properties to an embryonic limb bud [15]. As the gubernaculum elongates to the scrotum the primitive fibroblasts differentiate into myoblasts and then eventually to mature muscle [16,17].
These results suggest that androgen signalling is critical in allowing the cremaster differentiation to be delayed long enough to allow gubernacular eversion, as mature skeletal muscle may be too stiff to permit the radical remodelling required. The increased β-catenin expression by D3 is consistent with more mature muscle formation in the ARKO gubernaculum, and the situation seen in the cytoplasm shows what appears to be much more advanced development of skeletal cremaster muscle. By contrast, in the WT gubernaculum, there is no striated pattern of β-catenin, consistent with less advanced cremaster skeletal muscle differentiation, until migration is complete.
The increased expression of Myh3 at D0, as well as PPAR-γ and desmin on D0, also suggest early differentiation into skeletal in the ARKO gubernaculum when in WT animals expression of these early markers of muscle development are much lower. The significant increase in myogenin expression at D3, a marker of later muscle development, is consistent with rapid differentiation of fibroblasts into mature cremaster muscle without androgen signalling, which is prevented in the WT mouse. The increased expression of Ki67 in D3 ARKO gubernacular fibroblasts is of uncertain significance, as in vivo the gubernaculum fails to migrate and remains bulky with persistence of hydrophilic extracellular matrix, and eventually undergoes metaplasia into adipose tissue [18]. These findings indicate the cremaster muscle behaves differently from other sexually dimorphic skeletal muscle in which AR signalling accelerates differentiation [19].
The cremaster is a specialised skeletal muscle that has some properties alike cardiac and smooth muscle. The cremaster is not under conscious control, and responds to temperature and touch, as in the cremaster reflex. It’s different response to androgen stimulation is consistent with its different formation and physiological properties. Cremaster muscle myogenesis has been examined in the human fetus and is thought to have some smooth muscle characteristics [20].
Historically, the cremaster muscle was thought to form passively by the gubernaculum collecting fibres from the internal oblique muscle as the testis descended past the internal inguinal ring. We now know androgen actively governs gubernacular growth and cremasteric development, during the window of androgen sensitivity, between E15-E19 of the rat embryo [21]. Androgen acts both directly and indirectly on the gubernaculum [22]; indirectly by causing the genitofemoral nerve (GFN) to release calcitonin gene-related peptide (CGRP), a known neuropeptide, which regulates gubernacular migration; and later directly by androgen receptors in the gubernaculum itself. We know that gubernacular eversion is a key aspect of testicular descent in rodents, and gubernacular eversion fails in androgen blockaded/ or knock out models. Despite the relative anatomical simplicity which occurs during gubernacular eversion, the effect of androgen on the linked molecular pathways remains complex.
Androgen stimulation during inguinoscrotal descent has been linked to the canonical Wnt pathway and beta-catenin (β-catenin). β-catenin either binds to the androgen receptor and moves into the cell nucleus where it acts as a potent co-activator of the androgen receptor/ androgen receptor-positive genes; or it binds to the T-cell factor to promote activation of the Wnt-responsive genes [23]. In rats, it has been hypothesized that the interaction between androgen and β-catenin may be a necessary step in the digestion of collagen by androgen receptor-positive cells, assisting with cell migration from the core of the gubernaculum and enabling gubernacular eversion [24]. With androgen interacting with Wnt proteins, allowing nuclear translocation of β-catenin, androgen blockade leads to β-catenin accumulating in the cytoplasm and reduced myogenic proteins, thus myogenic gene transcription is necessary before gubernacular eversion and migration towards the scrotum. A conditional knockdown of β-catenin caused failure of CM myogenesis resulting in an intrabdominal testis [25]. Research using microarray analysis has suggested that Wnt signalling, working in conjunction with androgen receptor, may contribute to CM formation and testicular descent, as a defect in the Wnt signalling pathway and AR both produced intra-abdominal testis, which is a phenotype commonly associated with patients presenting with complete androgen insensitivity syndrome [26].
Peroxisome proliferator-activated receptors (PPAR) also have been linked to the canonical Wnt pathway. PPAR’s are nuclear receptors that belong to the nuclear hormone superfamily and they share similar structural features with other nuclear receptors, such as androgen receptors (AR). PPAR-γ expression is critical for differentiation of rat skeletal muscle in vivo [27], and has also been linked to slow twitch skeletal muscle fibres known to be present in cremaster muscle. PPARs act as ligand-activated transcription factors which regulate gene expression by binding onto the Retinoid X Receptor (RXR), and specific regions of DNA sequence elements termed PPAR Elements (PPARE) in promoter regions of target genes, to modulate transcription. While the full range of PPAR ligands is not yet known, they are usually fatty acids or their derivatives. PPAR-γ may also interact with Beta (β)-catenin, a cytokine which has already been linked to testicular descent and cremaster development. b-catenin is thought to enhance PPAR-γ activity, leading to specific target gene activity. PPAR-γ has not been linked to cremaster myogenesis and the gubernaculum previously.
The appropriateness of the mouse gubernaculum model to investigate cremaster development in humans is debated. Certainly, the anatomy of the cremaster muscle is different, with the rodent muscle forming a bi-laminar sac around the process vaginalis, while in the human it is just a strip [28]. However in both rodents and humans remodelling of the gubernacular mesenchyme is similar, except for timing, and cremaster develops within the gubernaculum itself. In both species androgen resistance prevents gubernacular migration and normal cremaster muscle development. We propose that once the difference in timing and cremaster development are taken into account, we can then extrapolate the results of this to suggest that, not only in the rodent but also in the human, androgen blockade may trigger premature differentiation of embryonic myoblasts in the gubernaculum, which may interfere with gubernacular eversion and/or elongation. Maintaining the cremaster myoblasts in a less differentiated state may allow the gubernaculum to elongate and migrate from the external inguinal ring to the scrotum in both species. Once terminal differentiation into mature muscle fibres has occurred, further elongation of the cremaster muscle within the gubernaculum is likely to be impaired. These alterations in muscle-related genes have also been detected in rat strains with inherited cryptorchidism and in anti-androgen-treated rats [29]. This is consistent with morphological changes showing disorganised abnormal striated cremaster muscle in cryptorchid rodents.
The main limitation of this work is the small number of animals used, and that only 3 time points were studied. However, the consistency between the immunofluorescence and the gene expression results suggest that these limitations were not critical.
Conclusion
Testicular descent requires delayed development of cremasteric muscle; allowing migration of the testis into the scrotum. In the absence of AR, gubernacular mesenchymal cells prematurely develop into myoblasts prior to cremaster muscle differentiation.
This study suggests that in the wild-type mouse, cremaster muscle maturation is usually delayed at the myoblast stage allowing eversion of the gubernaculum around D0 and then elongation to the scrotum. This study is consistent with AR blockade producing premature maturation which would inhibit eversion and gubernacular migration. We propose that undescended testes could be due to the premature maturation of cremaster muscle in AR-blockaded animals which prematurely halts the eversion and movement of the gubernaculum towards the scrotum. It is possible that defects in the response to androgen stimulation in the Wnt, b-catenin and PPAR signalling pathways may lead to cryptorchidism in boys without androgen resistance. These intracellular signalling systems should be included in future searches for the causes of cryptorchidism using modern genetic analysis.
Funding
NHMRC grant APP1144752.
Disclosures and Declaration of Interests
None
Human Ethical Approval
Murdoch Children’s Research Institute Animal Ethics Committee AEC no. A854.
References