Introduction
The risk of developing long-term diabetes related complications (retinopathy, nephropathy, neuropathy, cardiovascular disease and stroke) increases as glycated hemoglobin (A1C) levels exceed 6.5% [1,2]. All patients with diabetes should be provided with an individualized target A1C based on factors such as age, duration of disease, risk of hypoglycemia, existing comorbidities, available resources, life expectancy and cardiovascular risk [3]. A delay of therapeutic intensification of just 2 years from the time of diagnosis, can expose a patient to “glycemic burden” and a 61% increased risk of cardiovascular complications [4]. Recent data from the 2011-2014 National Health and Nutrition Examination Survey (NHANES) indicate the just 51 % of American adults with diabetes are achieving A1C levels <7%. Despite the approval and marketing of multiple new agents for diabetes (GLP-1 receptor agonists, SGLT2 inhibitors, longer acting basal insulins, and disposable insulin pumps), improvement of A1C nationally has actually declined since NHANES 2007-2010 when 52 % of patients achieved their targeted A1C [5]. Randomized clinical trials which are conducted with FDA guidance in order for a study drug to gain regulatory approval consistently demonstrate success in achieving A1C levels <7% and even 6.5 %. However, real world studies suggest that patient adherence to prescribe medications may mitigate one’s ability to achieve glycemic targets [6]. Patients in the real world may be concerned about drug side effects, complexity of treatment regimens, potential weight gain, or risk of hypoglycemia. TV advertisements which mention thyroid cancer, amputation risk and hypoglycemia may hinder one’s desire to initiate a new drug.
Former US Surgeon General, C. Everett Koop once said, “Drugs don’t work if patients who don’t take them [7]. This article will address concentrated insulin and combination fixed dose insulin + GLP-1 receptor agonists which reduce risk of hypoglycemia, weight gain, and cardiovascular disease. The use of these agents within the primary care setting may improve adherence and allow patients to safely and efficiently achieve their prescribed glycemic targets.
Insulin Action, Variability and Recommended Glycemic Targets
Insulins are formulated to bind to and activate receptors located with target organs (liver, muscles, kidneys, adipose tissue). The resultant pharmacologic action ultimately lowers plasma glucose levels to the desired range. The fact that not all insulins are created equally allows practitioners to customize their treatment protocols for each patient.
The American Association of Clinical Endocrinologists recommends the use of insulin when the endogenous insulin-secreting capacity of pancreatic beta cells has been exceeded. Insulin should be initiated in any patient with an A1C > 8.5 % who has symptoms suggestive of chronic hyperglycemia (thirst, weight loss, blurry vision, distal sensory neuropathy, weight loss and frequent urination). Patients with A1C >9% should also be considered insulin candidates [8]. Basal insulin reduces hepatic glucose production in the fasting state whereas rapid acting insulin preparations are used to minimize post prandial glucose excursions. The American Diabetes Association recommends targeting fasting glucose levels to 70-130 mg/dL and 2-hour post meal glucose of <180 mg/dL [9].
Early initiation of insulin can prove beneficial for patients with T2DM. The anti-inflammatory and antioxidant effects (ie, reduction of oxidative stress) may offer protection against vascular endothelial dysfunction and subsequent vascular disease. Insulin induces endothelial nitric oxide synthase in endothelial cells resulting in increased production of nitric oxide and the promotion of vascular dilatation [10]. Insulin is thought to preserve β-cell mass and function in patients with T2DM. Glycemic burden destroys β-cells. Reducing hyperglycemia in patients with diabetes can facilitate β-cell rest allowing for more efficient and timely production and secretion of endogenous insulin [11].
Although elevated A1C is a surrogate marker for long-term diabetes-related complications, glycemic variability (dysglycemia) can induce oxidative stress favoring the induction of complications [12]. Patients who experience dysglycemia become frustrated with their inability to efficiently regulate blood glucose levels. These patients experience wide glycemic swings throughout the day resulting in hypoglycemia and sustained hyperglycemia. Efficient pharmacotherapy for patients with type 2 diabetes must address both the effects of prolonged exposure to hyperglycemia as well as acute daily excursions of glucose levels. Elevated glucose levels promote the appearance of acute glycated end products (AGEs) which can increase one’s likelihood of developing complications. Glycemic excursions exacerbate the process of oxidative stress, a metabolic state favoring the progression of microvascular and macrovascular complications [13].
Ideal basal insulins should be simple to initiate and titrate, result in minimal glycemic variability, provide prolonged duration of action, while reducing one’s risk of hypoglycemia and weight gain. In addition, insulins should not increase one’s risk of cardiovascular disease, especially in patients who have already experienced a stroke or myocardial infarction. Table 1 lists the coefficients of variability of available basal insulins. The lower the variability, the less likelihood of developing treatment emergent hypoglycemia as noted in Figure 1 a and 1 b.
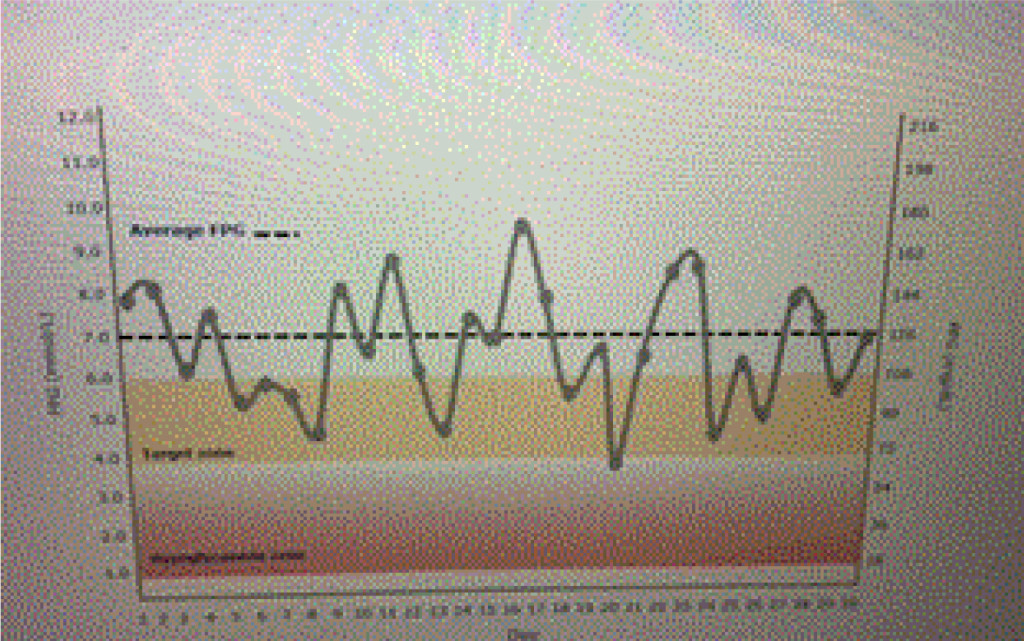
Figure 1a. Emergent hypoglycemia.
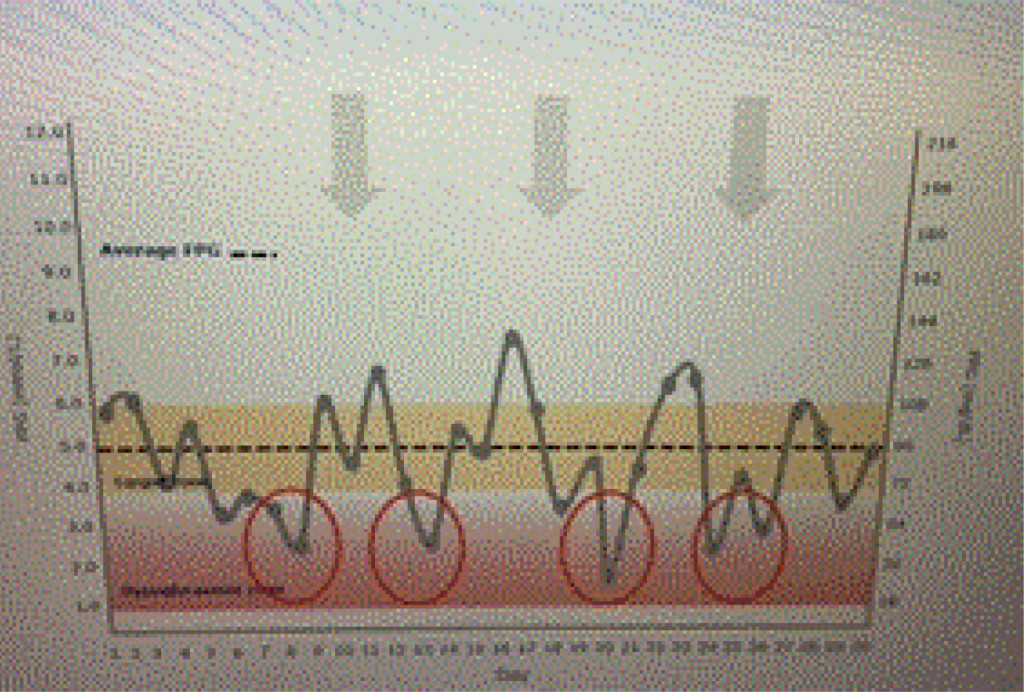
Figure 1b. Dysglycemia.
Table 1. Basal Insulin Coefficients of Variability.
|
Insulin |
Within Subject Variablity* |
| NPH |
68 |
| Glargine U-100 |
48 |
| Detemir |
27 |
| (Concentrated) Glargine U-300 |
34.8 |
| Degludec |
20 |
* Percentage within subject variability based upon glucose infusion rates and area under the curve. Patients receive 4 single subcutaneous doses of 0.4 U/kg under euglycemic glucose clamp conditions on 4 study days [14-16].
A patient is using an insulin with a high coefficient of variability. Glucose levels demonstrate “dysglycemia (Figure 1 a).” Increasing the basal insulin further is likely to increase the risk of hypoglycemia which will reduce adherence to the prescribed treatment regimen (Figure 1 b). The use of basal insulin formulations with low glycemic variability will allow patients to achieve their fasting blood glucose targets more efficiently with less fear of hypoglycemia.
New Insulin Formulations
Advances in basal insulin formulations have provided clinicians and patients with options that provide favorable pharmacokentic (insulin absorption) and pharmacodynamic (glucose lowering) properties. Newer insulins have flatter, peakless action profiles, demonstrate less variability and a longer duration of action allowing for flexible dosing. The risk of nocturnal and diurnal hypoglycemia is subsequently reduced. Insulin preparations do not appear to increase one’s cardiovascular risk [17,18]. Patients may also safely combine a GLP-1 receptor agonist as either a separate injection or as a component of a fixed-ratio drug. The use of fixed drug combinations may improve adherence and allow patients to achieve their metabolic targets [19].
Glargine U-300
Glargine U-300 (Toujeo) is a long-acting insulin containing 300 units/mL of insulin glargine. As a concentrated insulin, Glargine U-300 contains 3 times as much insulin per mL as glargine U-100 allowing for a lower volume of injected insulin. Glargine U300 was detectable at 32 hours post injection with 0.4 units/kg dosing compared with 28 hours with glargine U100 dosing [20]. At 0.4 u/kg, U300 has 14 % less variability than U100, allowing clinicians to titrate the insulin to target lower fasting glucose levels without risking hypoglycemia [21].
Degludec U100 and U300
Within the insulin pen, insulin degludec (Tresiba) is formulated as “insulin diheximers.” Once injected, the diheximers form multiheximer chains within the subcutaneous depot held together by zinc and phenol. As the zinc dissociates, the multiheximers form insulin monomers which pass into the capillaries and are carried via albumin to insulin receptors at target organ sites. Degludec U200 contains as much insulin as degludec U100 in just ½ the injection volume. The two insulins are bioequivalent and lower glucose levels at the same rate. Degludec appears to have the lowest coefficient of variability of all insulins allowing ambitious dosing to targeted fasting glucose levels with less likelihood of nocturnal and overall hypoglycemia when compared with insulin glargine [22]. Due to the prolonged duration of action (42 hours), degludec may be dosed at any time of the day, which may improve adherence for patients who are shift workers, travel frequently, or have difficulty remembering to dose their basal insulin [23].
U-500 Regular Insulin
Regular insulin U-500 (Humulin R U-500 insulin) is structurally identical to human insulin. Because the drug is formulated as 500 units/mL, this insulin is five times as potent as regular insulin U-100 [24]. U-500 is indicated for patients with type 1 or type 2 diabetes requiring more than 200 units of insulin daily. The concentrated insulin is delivered in a lower injection volume which improves the drug’s absorption from the subcutaneous depot. U-500 has a slower onset of action (60 min) when compared with rapid acting prandial insulins. The peak onset of action is 2-4 hours post injection and the duration of action is 4-6 hours [25]. U-500 can be dosed as prandial insulin using a pen injector 30-45 minutes prior to eating.
Lispro U-200 Insulin
Prandial insulin lispro U-200 (Humalog U-200) contains 200 units of rapid acting insulin per mL compared with insulin lispro U-100 which contains 100 units/mL. Thus, U-200 is twice as concentrated as U-100 allowing patients requiring > 20 units of insulin per meal to inject less volume [26]. The pharmacokinetic and pharmacodynamic effects of both Lispro U-200 and Lispro U-100 are equivalent [27].
Table 2 suggests which concentrated insulin or fixed insulin combination might be appropriate for patients with type 2 diabetes.
Table 2. Rationale Use of Concentrated Insulins.
|
Condition |
Rationale |
Product of Choice |
| Nocturnal hypoglycemia | Needs peak-less (flat) basal insulin profle
Insulins with lower glycemic variability will allow safer titration to fasting glucose levels without risk of hypoglycemia |
Glargine U300
Degludec U100 or U200 |
| Severe insulin resistance requiring the use of > 200 units of insulin daily | High potency concentrated insulin can result in a low volume subcutaneous insulin depot
U500 insulin is 5 times as potent as U100 insulin with 1/5 the injection volume Insulin resistance requires high dose insulin |
Regular U500 insulin ) |
| Patient requires > 80 units of basal insulin per injection | Concentrated insulin formulations have been developed to address the need for higher dose insulin delivery of a single daily injection | Degludec U-200
Glargine U-300 |
| Patient requires flexible daily dosing due to work schedule or frequent travel | Degludec can be administered daily at any time of day, with injection timing varied without compromising glycemic control or safety | Insulin degludec U100 or U200 |
| Patient requires > 20 units of prandial insulin | Lowers cost. Low volume insulin reduces the number of pen requirements monthly | Lispro U-200 |
| Post-prandial and fasting glucose coverage is needed | Fixed-dose combination therapy with insulin +GLP-1 receptor agonist can reduce total daily dose of insulin, risk of weight gain associated with insulin use, and provide coverage for postprandial excursions | Insulin degludec + liraglutide (IDegLIra- Xultophy 100.3.6)
Insulin glargine + lixisenatide (100/33) (Soliqua) |
Fixed Dose-Combinations (Basal insulin and GLP-1 Receptor Agonists)
The combination of basal insulin analogues with a glucagon-like peptide receptor agonist (GLP-1 RA) is intriguing. Basal insulin essentially targets hepatic glucose production which is excessive in patients with type 2 diabetes However, the use of basal insulin may result in weight gain and/or hypoglycemia which affects adherence [28]. GLP-1 RAs, typically target post prandial glucose excursions by decreasing the excretion of endogenous glucagon, a counter regulatory hormone with induces hepatic glucose production [29]. Patients using GLP-1 RAs tend to lose weight. Because this class of drugs is “glucose dependent, “ the glucose lowering effect in patients with lower blood glucose levels is reduced. Thus, patients tend to experience less hypoglycemia. Liraglutide, a GLP-1 RA, has demonstrated a 22% reduction in cardiovascular mortality [30]. However, extrapolation of CV outcomes in fixed dose combinations cannot be inferred without having trials specific to the dual therapy option.
Table 3 lists the features of basal insulin plus GLP-1 RAs which makes their combination therapies attractive in patients with type 2 diabetes.
Table 3. Combination of Basal Insulin and GLP-1 Receptor Agonists.
|
Metabolic Target |
Basal Insulin |
GLP-1 RA |
Combination Therapy |
| Beta Cell Function | Rests beta-cells, relieves glucotoxicity | Improves beta-cell function. May restore beta-cell mass | Additive improvement in prandial and post prandial glucose levels with lower total daily dose of insulin required |
| Alpha cell function | Reduces glucagon | Reduces glucagon | Additive improvement in glucagon secretion results in lower fasting and postprandial glucose levels. Diabetes is a bi-hormonal disorder…too much glucagon produced by the alpha cell and too little insulin secreted by the beta cell. |
| Glucose control | Targets fasting blood glucose | Targets primarily postprandial glucose | Lower fasting and postprandial glucose levels. Improved A1C |
| Weight | Tends to increase | Tends to decrease | Less weight gain noted with combination |
| Hypoglycemia risk | Increase risk | Lower risk | Lower risk due to reduced insulin dose requirements when combined with a GLP-1 RA |
| Cardiovascular risk | Insulin does NOT increase CV risk, nor is the risk reduced | Liraglutide* and Semaglutide** reduce CV risk. Other GLP-1 RAs are neutral at reducing risk | Studies have not been performed assessing the CV risk in fixed dose combination therapies, only as individual interventions |
* = Marso et al. [31]; ** Marso et al., [32]
Summary
The treatment of type 2 diabetes is complicated by not only the chronic progressive nature of the disease, but the multiple “core” defects which much be addressed. Patients with diabetes experience beta cell failure, increased insulin resistance due to hepatic glucose production and a reduced glucose uptake in the muscle and fat cells. The kidneys absorb excessive amounts of glucose in the face of hyperglycemia, and even produce glucose in the form of gluconeogenesis. Due to the reduction in circulating insulin, fat cells produce excessive amounts of free fatty acid which further increases hepatic glucose production. Feelings of satiety are reduced in patients with type 2 diabetes resulting in over-consumption of nutrients and weight gain. Native GLP-1 (a gut hormone released in response to a carbohydrate load) is either reduced or its action compromised at the receptor site resulting in a reduction in prandial insulin production and excess glucagon production from the pancreatic alpha cells. The use of concentrated insulins as well as new insulins with more favorable pharmacokinetic and pharmacodynamic profiles may profoundly improve glycemic control in patients with diabetes. Fixed dose combinations which employ basal insulin plus a GLP-1 RA appears to be a rationale choice for patients who require better postprandial glucose coverage.
Patients who are non-adherent to a treatment regimen, may benefit from concentrated or combination therapies. Adherence is likely to improve fasting and postprandial glucose control allowing patients to successfully achieve their targeted A1C and reduce their “glycemic burden.” Using insulins with less variability and risk of hypoglycemia will also improve adherence. Because 90 % of all patients with diabetes are managed within the primary care setting, the treatment of diabetes must be intensified within our environment. Early and successful treatment of these complex individuals will likely improve long-term outcomes and the quality of life of our patients.
The author thanks the staff at Vindico Medical Education for their assistance in the preparation of this manuscript.
Acknowledgements: Dr. Unger would like to thank Vindico CME for providing assistance with the editing of this manuscript.
References
- Selvin E, Ning Y, Steffes MW, Bash LD, Klein R, et al. (2011) Glycated hemoglobin and the risk of kidney disease and retinopathy in adults with and without diabetes. Diabetes 60: 298–305. [Crossref]
- Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, et al. (2010) Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 362: 800–811. [Crossref]
- Inzucchi SE, Bergenstal RM, Buse JB (2015) Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 38: 140–149. [Crossref]
- Paul SK, Klein K, Thorsted BL, et al. (2015) Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovascular Diabetology 14: 100.
- Edelman SV, Polonsky WH (2017) Type 2 Diabetes in the Real World: The Elusive Nature of Glycemic Control. Diabetes Care 40: 1425–1432. [Crossref]
- Rothwell PM (2005) External validity of randomised controlled trials: “to whom do the results of this trial apply?”. Lancet 365: 82–93. [Crossref]
- Lindenfeld J, Jessup M (2017) ‘Drugs don’t work in patients who don’t take them’ (C. Everett Koop, MD, US Surgeon General, 1985). Eur J Heart Fail 19: 1412–1413. [Crossref]
- Rodbard HW, Jellinger PS, Davidson JA (2009) AACE/ACE Consensus Statement. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 15 (1): 540–559. [Crossref]
- American Diabetes Association (2016) 5. Glycemic Targets. Diabetes Care 39 Suppl 1: S39–46. [Crossref]
- Owens DR (2013) Clinical evidence for the earlier initiation of insulin therapy in type 2 diabetes. Diabetes Technol Ther 15: 776–785. [Crossref]
- Turner RC, McCarthy ST, Holman RR, Harris E (1976) Beta-cell function improved by supplementing basal insulin secretion in mild diabetes. Br Med J 1: 1252–1254. [Crossref]
- Unger J (2008) Reducing oxidative stress in patients with type 2 diabetes mellitus: A Primary Care call to action. Insulin 3: 176–184.
- Monnier L, Colette C, Owens DR (2008) Glycemic Variability: The Third Component of the Dysglycemia in Diabetes. Is It Important? How to Measure It? J. Diabetes Sci Technol (6): 1094–1100. [Crossref]
- Rosseti P, Ampudia-Blasco FJ, Ascaso JF (2014) Old and new basal insulin formulations: understanding pharmacodynamics is still relevant in clinical practice. Diabetes Obes Metab 16: 695–706. [Crossref]
- Becker RHA (2015) Low within- and between-day variability in exposure to new insulin glargine 300 U/ml. Diabetes Obes Metab 17: 261–267. [Crossref]
- Jeff Unger (2012) Insulin initiation and intensification for patients with T2DM. In: Unger Jeff (Ed.) Diabetes Management in Primary Care- Second Edition. Lippincott, Williams and Wilkins. 2012. Philadelphia, PA. 453–490. [Crossref]
- ORIGIN Trial Investigators, Gerstein HC, Bosch J, Dagenais GR, Díaz R, et al. (2012) Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 367: 319–328. [Crossref]
- Marso SP, McGuire DK, Zinman B, Poulter NR, Emerson SS, et al. (2017) Efficacy and Safety of Degludec versus Glargine in Type 2 Diabetes. N Engl J Med 377: 723–732. [Crossref]
- Ferdinand KC, Senatore FF, Clayton-Jeter H, et al. (2017) Improving medication adherence in cardiometabolic disease: practical and regulatory implications. J Am Coll Cardiiol 69 (4): 437–451. [Crossref]
- Becker RH, Dahmen R, Bergmann K, Lehmann A, Jax T, et al. (2015) New insulin glargine 300 Units. mL-1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 Units. mL-1. Diabetes Care 38(4): 637–643. [Crossref]
- Becker RH, Nowotny I, Teichert L, Bergmann K, Kapitza C (2015) Low within- and between-day variability in exposure to new insulin glargine 300 U/ml. Diabetes Obes Metab 17: 261–267. [Crossref]
- Wysham C, Bhargava A, Chaykin L, de la Rosa R, Handelsman Y, et al. (2017) Effect of Insulin Degludec vs Insulin Glargine U100 on Hypoglycemia in Patients With Type 2 Diabetes: The SWITCH 2 Randomized Clinical Trial. JAMA 318: 45–56. [Crossref]
- Meneghini L, Atkin SL, Gough SC, et al. (2013) The efficacy and safety of insulin degludec given in variable once-daily dosing intervals compared with insulin glargine and insulin degludec dosed at the same time daily; a 26-week, randomized, open-label, parallel-group, treat-to-target trial in individuals with type 2 diabetes. Diabetes Care 36 (4): 858–864. [Crossref]
- Humulin [package insert] Indianapolis, Ind., Eli Lilly and Co., 2016. Available from pi.lilly.com/us/humulin-r-u500-pi.pdf. Accessed 11/10/17.
- Insulin initiation and intensification for patients with T2DM (2012) In Unger Jeff. Diabetes Management in Primary Care- Second Edition. Lippincott, Williams and Wilkins. Philadelphia, PA. 453–490.
- Humalog [package insert] Indianapolis, Ind., Eli Lilly and Co., 2015. Available from pi.lilly.com/us/humalog-pen-pi.pdf. Accessed Nov. 10, 2017
- Humalog [package insert] Indianapolis, Ind., Eli Lilly and Co., 2015. Available from pi.lilly.com/us/humalog-pen-pi.pdf. Accessed Nov. 10, 2017
- Pi-Sunyer FX (2009) The Impact of Weight Gain on Motivation, Compliance, and Metabolic Control in Patients with Type 2 Diabetes Mellitus. Postgrad Med 121 (5): 94–107. [Crossref]
- Unger J, Parkin C (2011) Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists: Differentiating the new medications. Diabetes Therapy. [Crossref]
- Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, et al. (2016) Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 375: 311–322. [Crossref]
- Marso SP, Daniesl GH, Brown-Frandsen K (2016) Liraglutide and cardiovascular outcomes in type 2 Diabetes. N Engl J Med 375: 311–322. [Crossref]
- Marso SP, Bain SC, Consoli A (2016) Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 375: 1834–1844. [Crossref]