Abstract
Determinations were realized by the iteration method, the values of formation constants of coordination substances of their degree of accumulation were determined on a computer using the Excel program. The synthesis of complex compounds of zinc (II) with mercazolil was carried out in aqueous solutions at pH 6,0-6,5 and a molar ratio of Zn (II) components: mercazolil – 1:1; 1:2, 1:3, 1:4, 1:5. When the ratio of zinc to mercazolil is 1:1, a white precipitate is precipitated from the solution, for which, according to the data of elemental analysis, one mole of silver accounts for one mole of zinc. But when the amounts of ligand were increased i.e. with an increase in the concentration of the ligand (zinc and mercazolil in a ratio of 1:4), a white precipitate is formed, for which there are two moles of mercazolil per mole of the metal complexing agent. The individuality of the synthesized compounds was established by the data of elemental and X-ray phase analyzes, cryoscopy, as well as using modern physicochemical research methods. IR spectra of the formed coordination substances and initial ligands were recorded on “SPECORD IR-75”and “SHIMADZU” spectrometers at wavelengths from 400 to 4000 cm-1, the samples were prepared in the form of KBr tablets in vaseline oil and in the form of a suspension. The results of the studies (elemental analysis, conductometry, thermogravimetry, IR spectroscopy) revealed that at an ionic strength of 0,1 mol/L, a temperature of 308 K, concentration of zinc C Zn (II)=1 * 10-4, mol/L and mercazolil СHmerc=1*10-2 mol/L the following complex particles exist; ZnHL(H2O)3]2+(pH=4.0-,4,8), [Zn(HL)2(H2O)2]2+(pH=4,8.0-5.4), [Zn(HL)OH]+ (pH=5.4-6.0) and [ZnHL(OH)2H2O].
Conductometric studies were carried out in glass closed cells (AC bridge R-5021, frequency 1·104Hz). Antimicrobial activity and toxic properties of complex compounds were determined by the method of serial dilutions and Pershin. It has been shown that the complex compound of zinc with mercazolil belongs to the categories of low-toxic drugs for laboratory and farm animals, when administered orally in therapeutic doses.
Keywords
Zinc, Mercazolil, IR spectrum, Electrical conductivity, Antimicrobial activity
Introduction
Zinc compounds, due to their unique physicochemical properties, have found wide application in various fields of industry and the national economy. In addition, it is known [1] that for the normal development of living organisms, micro-amounts of various metals, the so-called “metals of life”, are required. In addition to widespread elements such as sodium, potassium, magnesium, calcium and iron, these include the so-called trace elements: copper, zinc, molybdenum, cobalt, manganese, chromium and some other d-elements. Zinc (II) compounds with azoles play an important role in veterinary medicine. For example, antimicrobial activity was found in zinc (II) compounds with azoles, which are used in veterinary practice as antiparasitic, anthelmintic, and antifungal drugs. However, the search for new, more effective existing anthelmintics and their improvement of a wide spectrum of action all over the world is urgent. In recent years, on a global scale, along with the search for new anthelmintics, there has been intensive work to increase the effectiveness of known therapeutic and prophylactic drugs.
Since the early 1980s, albendazole has become the most popular among benzimidazole drugs. However, in the last 2-3 years, an opinion has been increasingly expressed about the lack of effectiveness of albendazole in some trematodes of ruminants, especially in fascioliasis and dicroceliosis. One of the main reasons is the administration of a dose to the animal that is obviously lower than the therapeutic dose. In addition, a large number of drugs based on albendazole have appeared on the market, containing fillers (chalk, talc, zeolites), which reduce the bioavailability of the active substance, and without that does not exceed 50%. Among organic ligands for chemistry of zinc (II) coordination compounds, a heterocyclic compound is of particular interest. This is due to the presence of several donor atoms in their composition and their widespread use in medical practice, as pharmaceuticals, and in industry. [2]. Albendazole is structurally similar to mebendazole. The main mechanism of action of albendazole is associated with the selective suppression of β-tubulin polymerization, which leads to the destruction of cytoplasmic microtubules of cells of the intestinal tract of helminths; inhibits the utilization of glucose and inhibits the ATP synthesis, blocks the movement of secretory granules and other organelles in the muscle cells of roundworms, causing their death There is no information in the literature on coordination compounds of zinc (II) with derivative azoles, especially with albendazole. In this regard, the development of optimal conditions for the synthesis of coordination compounds of zinc (II) using organic ligands and the study of their composition and properties is an urgent task that makes it possible to develop an understanding of the nature of chemical bonds as a result of coordination of ligands to the central ion and processes of mutual substitution of ligands, as well as thermal stability of the synthesized compounds.
Materials and Method
Carrying out experimental measurements provided for the following preliminary work: preparation and testing of silver chloride and zinc electrodes; In addition, the electrode function of an amalgamated zinc electrode has been established, measuring the oxidation potential of the system depending on various concentrations of zinc (II). The starting reagent was zinc (II) sulfate of the grade “p.a.”, which was purified by recrystallization from a saturated aqueous solution. The concentration of zinc (II) in aqueous solutions was determined by titration with a 0,1 N solution of a dibasic salt of ethylenediaminetetraacetic acid (EDTA) in the presence of a black chromogen indicator.
Methods
Potentiometric
IR spectroscopy
Okislitel’nyy potentsial
Okislitel’naya fuktsiya
Metod itteratsiya
For amalgamation, the surface of the zinc electrode was ground with fine emery paper, washed with distilled water, processed for degreasing with a magnesium paste, and washed again with distilled water. The prepared in this way surface of the zinc electrode was amalgamated by immersing it for some time in a vessel with pure mercury. Carefully shaking the drops of mercury from the electrode surface, they rubbed the mercury over the entire surface of the electrode, using filtered paper. The mercury used for amalgamation was periodically purified by filtration and decantation with 0,25% nitric acid solutions and water. The amalgamated electrode was stored in a 0,1 M nitric acid solution. Evaluation of the electrode function, otherwise the calibration of the zinc electrode was to check its obedience to the Nernst equation. Since zinc (II) ions undergo hydrolysis at pH ≤ 5.5, the EMF of the system was measured at strictly fixed pH and ionic strength values using two working solutions with the same pH and ionic strength at different values of the zinc (II) concentration. Measurement of the EMF of a galvanic cell composed of zinc and silver chloride electrodes was carried out using the equation:
φ Zn (II)/Zn/Zn(II)=Е – φAg/AgCl, Cl–. (1),
where E is the electromotive force of a galvanic cell, φ Zn (0)/Zn/Zn (II) is the potential of a copper electrode, φAg/AgCl, Cl – is the potential of a chlorine-plated electrode.
The analysis of the dependence φ Zn(Hg)/Zn/Zn (II) showed that in aqueous solutions in the absence of complexing ligands within the concentration of zinc (II) from 1 х10-1 до 1 х 10-4, this electrode is an electrode of the first kind.
Results and Discussion
This work presents the results of a study of the process of complexation of zinc (II) with mercazolil in an aqueous solution at 308 K and an ionic strength of a solution of 0,1 mol/L created by sodium sulfate, by the oxredmetric method. The maximum number of coordinated mercazolil molecules attached to the zinc ion was determined from the slope of the φ versus – lgCL dependence. The shape of the curves of the dependence of ∆E on -pH indicates the stepwise nature of the complexation between zinc (II) and mercazolil. The joint analysis of the experimentally obtained dependences of the oxidation potential of the system (φ) on pH, the concentration of oxidized and reduced forms of zinc, mercazolil, the creation of a stoichiometric matrix (mathematical model) of existing equilibria in the solution showed that complexes of various compositions are formed in the system. The composition and constants of formation of coordination compounds, their degree of accumulation were refined using the Yusupov oxidation function by the iteration method. The coincidence of the experimental and theoretical oxidative functions confirmed the correctness of the definite composition of the complexes, as well as the reliability of the values of the constants of their formation. As can be seen from Figure 1, the formation of several straight sections with slopes equal to 0, ϑ/2, ϑ is observed on the curves of φ-рН dependence, which indicates the successive addition of one, two or more ligands to the central ion of the complexing agent. Moreover, the numerical values of the slope factors determine the number of attached ligands. Here it follows that the values ϑ=RT/F*2,303=63 mV. Since two electrons are involved in the transfer reaction, the contribution of one ligand to a decrease in the oxidation potential is 31,5 mV. A further decrease in the oxidative potential at pH greater than 6,0 can be associated with the formation of poorly soluble hydrolysis forms of zinc (II) compounds.
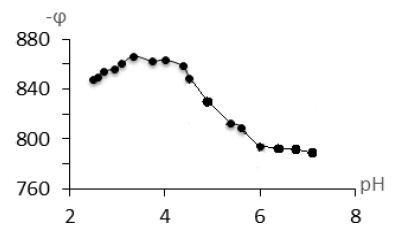
Figure 1: Dependence of oxidizing potential (j) on pН. CZn(II)=1*10-4 mol/L, I=0,1 mol/L, СHMer=1*10-2 mol/L, T=308 K
In an acidic medium up to pH <4.0 (Figure 1), complexation of zinc with mercazolil does not occur and this corresponds to the literature data. Zn (II) ions to pH <4.0 are in the form of zinc (II) aqua complexes.
From pH> 4.0, zinc complexation with mercazolil begins. In the pH range from 4,5 to 6,0, mono- and binuclear coordination compounds of the following composition are presumably formed: [ZnHmerc]2+, [Zn(Hmerc)2]2+. After pH> 6.0, zinc(II) hydroxo-complexes are presumably formed with mercazolil [Zn(Hmerc)OH]+ и [Zn(Hmerc)2OH]+. ϕTo determine the number of nuclei in coordination compounds, the experimental dependences of ϕ on рCZn2+ were taken, which are shown in Figure 2. To determine the coordinated number of mercazolil groups in complex particles, the experimental dependences of the oxidation potential on the inverse logarithm of the mercazolil concentration were taken (Figure 2) The slope values and the composition of the set coordination compounds are shown in Table 1.
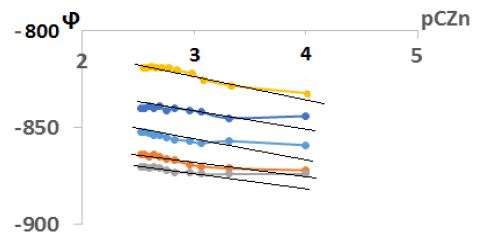
Figure 2: Dependence of the oxidation potential (j) on pCОХ. CZn(II)=1*10-4 mol/L, I=0,1 mol/L, СHmerc=1*10-2 mol/L, T=308 K
As can be seen from the dependence of (j) on pCZn (Figure 2), with an increase in the concentration of the metal of the complexing agent, the potential of the system increases, which indicates the participation of the metal of the complexing agent zinc in the complexation.
As can be seen from the dependence of (j) on pCL (Figure 3), the increases of the ligand concentration decrease the potential of the system, which indicates the participation of the ligand in the complexation process.
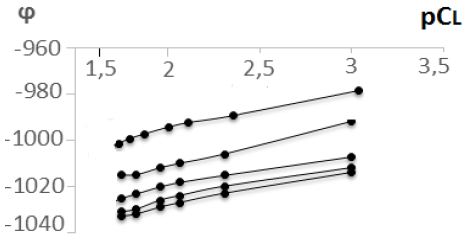
Figure 3: Dependence of oxidation potential (j) on pCL. CZn(II)=1*10-4 mol/L, I=0,1 mol/L, СHMer=1*10-2 mol/L, T=308 K
Thus, the partial derivatives of the equation: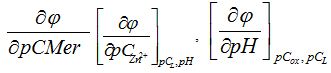 , and the results of the experiment showed that in the pH range from 4,5 to 6 in the system under study, complex particles of zinc with mercazolil are formed, the preliminary composition of which is given in Table 1.
, and the results of the experiment showed that in the pH range from 4,5 to 6 in the system under study, complex particles of zinc with mercazolil are formed, the preliminary composition of which is given in Table 1.
The theoretical oxidation function was used to calculate the equilibrium in the system and to calculate the stability constants of the complexes:
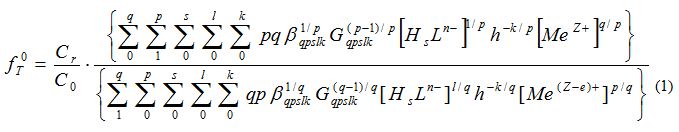
The experimental oxidation function f0e was calculated using the equation:
![]()
According to Eqs. (1) and (2), the values of the experimental and theoretical oxidation function were calculated, which made it possible to plot the dependence of the oxidation function lgfe, t on pH. Graphs of dependences lgfe, t on pH at ionic strengths and temperatures are shown in Figure 4.
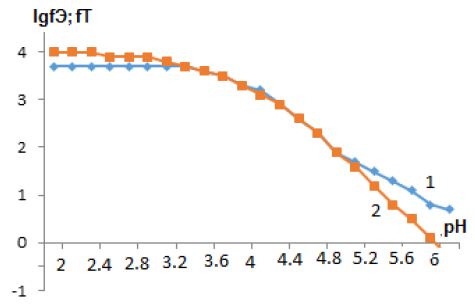
Figure 4: Dependence of the logarithms of the experimental (f0e) and theoretical (f0t) oxidation function on pH of Zn(Hg)-Zn(II)-merc-H2O solution system CZn(II)=1*10-4 mol/L, I=0,1 mol/L, Сmerc=1*10-2 mol/L, T=308 K.
The coincidence of the experimental f0e and theoretical f0t curves indicates that the composition of the formed coordination compounds has been established quite accurately. Then the calculation of the formation constants of prepared complex particles is started. In addition, when calculating the theoretical oxidation function and calculating the constants of the formation of complexes, approximations were made to the values of the equilibrium concentrations of the complex and zinc ions; therefore, we adopted the confidence probability (P) equal to 0,75. The calculated numerical values of the formation constants of the established coordination compounds (Tables 1 and 2) using equation (1,2), as well as the equilibrium concentrations of free and bound zinc ions in the binuclear complex , calculated by the method of successive approximation, made it possible to calculate the molar fractions of free and bound zinc (II) ions in the complex. Distribution diagrams are shown in Figure 5 in the form of the dependence of the degree of accumulation aqpslk on pH. The molar fractions of equilibrium particles in the investigated redox system were calculated based on the general formula Ni=ni/Snij. Based on this expression, we represent the molar fractions of the complexes in the form of the following equations:
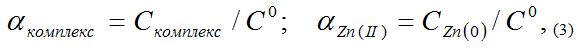
Table 1: The composition of the complex compound of zinc with mercazolil in the system Zn(Hg)- CZn(II)=1*10-4 mol / L, I=0,1 mol/L, СHmerc=1*10-2 mol/L, T=308 K.
|
рН |
Tangents of slope angles of dependencies |
Composition of the complexes |
||
|
ϕ-pH |
ϕ-pCZn |
ϕ-pCL |
||
| 3,7-4 | 0 | n/2 | n/2, -n | ZnL;ZnL |
| 4,0-4,5 | -n | n/2 | n/2, -n | ZnL;ZnL2 |
| 4,5-5,4 | -n | n/2 | n/2, -n | ZnL; ZnL2 |
| 5,4-6,0 | -n | n/2 | n/2 | ZnL |
Table 2: The chemical model of the Zn(Hg) -Zn(II) – merc- H2O system at CZn(II)=1*10-4 mol/L, I=0,1 mol/L, СHмерк=1*10-2mol/L, T=308 K.
|
№ п/п |
Fe 2+ |
H+ |
L– |
ОН– |
Composition of the complexes |
bqslk |
Equilibria of fragments in the redox system |
|
q |
s |
l |
K |
||||
|
1 |
1 |
0 |
0 |
1 |
Zn (H2O)4]2+ | b1001 | nlg(h3+b1001h2) |
|
2 |
1 |
1 |
1 |
0 |
[ZnHL(H2O)3]2+ | b1110 | nlg(h3+b1110K1Ca1h2) |
|
3 |
1 |
2 |
2 |
0 |
[Zn(HL)2(H2O)2]2+ | b1220 | nlg(h3+b1220K1Ca1h) |
|
4 |
1 |
1 |
1 |
1 |
[ZnHLOH(H2O)2]+ | b1111 | nlg(h3+b1111K1Ca1h) |
In equations (3) and (4) Сcomplex is the equilibrium concentration of the complex, Сo and aZn(II) are the mole fractions of zinc ions. The equations (3-4) made it possible to calculate the molar fractions of free and bound zinc ions in the complex at different ionic strengths and temperatures, which are presented in the form of distribution diagrams which are shown in Figure 5.
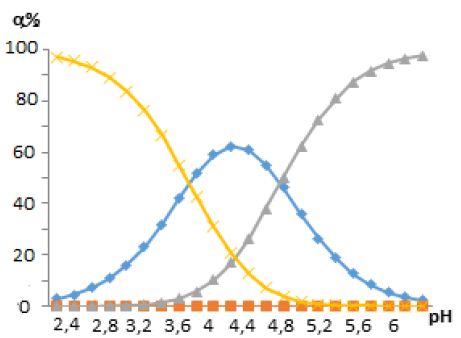
Figure 5: Diagrams of the distribution of free and bound Zn (II) ions in coordination compounds. The curves are designated as follows: 1)Zn(H2O)4]2+; 2)[ZnHL(H2O)3]2+ ; 3)[Zn(HL)2(H2O)2]2+; 4) [ZnHLOH(H2O)2]+.
The analysis of the presented distribution diagram and the results of oxredmetry show that with an increase in the pH of solutions in the system under study, coordination particles of different composition, stability and of regions of dominance are gradually formed. For example, the complex particle [Zn(HL)2(H2O)2]2+ is formed in the pH range 4,6-5,5, and its maximum content is at pH 5,0, etc. Thus, the composition of the new coordination compounds was established by the methods of oxedmetry, and the formation constants and the regions of dominance of zinc (II) complexes with mercazolil and hydroxyl ions were determined using the oxidative function, which also made it possible to reveal the thermodynamic conditions for the synthesis of the binuclear mercazolylate complex of zinc (II), which has the highest numerical value of the formation constant. It was found that at an ionic strength of 0,1 mol/L, a temperature of 308 K, zinc concentration C Zn (II)=1 * 10-4, mol/L and mercazolil С Hmerc=1 * 10-2 mol/L, the following complex particles exist; ZnHL(H2O)3]2+(pH=4.0-,4,8), [Zn(HL)2(H2O)2]2+(pH=4,8.0-5.4), [Zn(HL)OH]+ (pH=5.4-6.0) and [ZnHL(OH)2H2O].
Synthesis of New Coordinate Compounds of Zinc(II) with Mercazolil
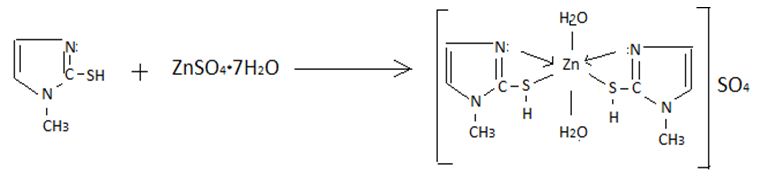
We used double recrystallized ZnSO4·7H2O as starting compounds in the synthesis of zinc (II) coordination compounds, and mercazolil (chemically pure) as ligands. Other organic solvents were purified according to the procedure. Before determining the halogen content, a weighed portion of the complex was decomposed with a nitric acid solution. The nitrogen content was determined by the Dume micromethod, carbon and hydrogen by burning a sample of the complex in a stream of purified oxygen. The sulfur content was determined gravimetrically, according to the method.
Synthesis of [Zn(HL)2]SO4 · 7H2O
1,14 g of mercazolil (0,005 mol) was dissolved in 5 ml of water, and a solution of 1,44 g (0,005 mol) of ZnSO4· 7H2O was added in 5 ml of water with vigorous stirring. The molar ratio of the reacting components of the system was 1:2. The reaction mixture was heated in a water bath for 4 hours until the color of the solution changed and a precipitate formed. The precipitated white precipitate was filtered off, washed with ethanol (25 ml), acetone (20 ml), ether (30 ml), and dried in a vacuum desiccator over solid KOH to constant weight. The resulting compound is readily soluble in water, DMSO and poorly soluble in ethanol, in DMF and insoluble in acetone,. The yield is 90%. Found,%: Zn-31,5; S-29; (N– 5,6;) H2O– 3,33; For [ZnLSO4] · 7H2O calculated,%: Zn – 13,80; S – 6,79; N- 5,940; H2O– 7,64. The formation of a zinc coordination compound in an aqueous ethanol medium is described by the following reaction:
Discussion of the Results
The synthesis of complex compounds of zinc with mercazolil was carried out in an aqueous solution at pH=5,5-6,0. Figure 6 shows the distribution diagrams of different forms of mercazolil (pKa=3,88) in a wide pH range. From the distribution diagram it follows that the neutral form of mercazolil is in the range of pH=3,88-6,0. In this form, mercazolil enters the complexation reaction with zinc.
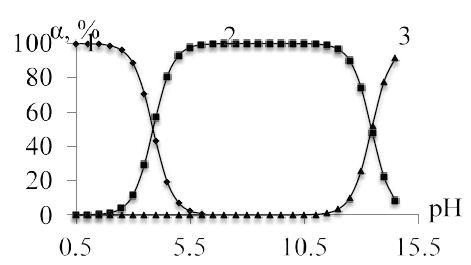
Figure 6: Particle distribution diagram of mercazolil in aqueous solution at 298 K
The synthesis of complex compounds of zinc with mercazolil was carried out depending on the ratio of the reacting components. When the zinc-mercazolil ratio is 1:2, a white precipitate is released from the solution. The reaction of the formation of this compound, based on the data of elemental analysis and the carried out physicochemical studies, can be represented by the equation:
ZnSO4+Merc+H2O↔[Zn(Merc)SO4]·H2O
If the concentration of mercazolil in the solution is increased and the ratio of the reacting components is increased to 1:2, then a cotton-like precipitate is formed, in which, according to elemental analysis, there are two moles of ligand per mole of zinc. The reaction of the formation of this complex can be represented by the equation:
ZnSO4 + 2 HMerc + 2H2O → [Zn(Merc)2]SO4 · + 2H2O
With an excess of mercazolil in the solution, when the ratio of zinc to ligand becomes 1:5, a white precipitate is first formed and then completely dissolved. The complex formation reaction can be represented by the equation:
ZnSO4 + 5 HMerc + 2H2O → [Zn(Merc)2]SO4 · + 2H2O
To determine the type of electrolyte, which includes the complex with the composition [Zn(НL)2(H2O)2]SO4, we studied its electrical conductivity in water at different temperatures and solution concentrations. Studies have shown that the electrical conductivity of [Zn(НL)2(H2O)2]SO4 in the temperature range of 20-45°С varies within 126,6-188,6 Оm-1·cm2·mol-1, which corresponds to type 1:1 electrolytes.
The study of thermal transformations of coordination compounds of zinc (II) with mercazolil showed that their character of thermal transformation is rather complicated and differs significantly from the process of thermal decomposition of uncoordinated mercazolil.
Thermal decomposition proceeds in several stages, which are characterized by weight loss, endo- and exothermic effects. Thus, thermal dehydration of the [Zn(HL2(H2O)2]SO4·H2O complex in an air atmosphere proceeds in the range of 100°С with a weight loss equal to – 10%. There is an endothermic effect on the DTA curve of the complex in this region. Theoretically, a weight loss of 4,5% corresponds to removal of one mole of water from the complex according to the equation:
[Zn(HL)2(H2O)2]SO4·H2O=[Zn(HL)2(H2O)2]SO4
The second stage of thermal decomposition of the complex occurs at 180-340°С. In this temperature range, according to the TG curve, the mass of the complex decreases by 63%. The complex heated at 340°С under isothermal conditions changes its color and becomes rustic.
The heated complex turns black and loses 63% of its mass. Taking into account the mass loss of the complex, elemental analysis data and IR spectroscopy (the absence of bands typical for mercazolil), it can be assumed that in the temperature range of 180-350°С there is a complete combustion of mercazolil molecules and formation of zinc sulfate according to the scheme:
[Zn(HL)2(H2O)2]SO4·=[Zn SO4]+2HL +2H2O
There are two exothermic effects on the DTA curve in this temperature range. In order to more accurately determine the processes occurring in the region of 500°С, 0,5 g of the complex was kept under exothermic conditions in an oven for 2,5-3 hours up to constant weight. An exoeffect is observed on the DTA curve in this temperature range. The reaction product, according to elemental analysis data, consists of metallic zinc. At the third stage of thermal decomposition, metallic zinc is formed according to the equation:
Zn SO4→ Zn +SO2+O2.
The thermogram of the complex [Zn(HL)2(H2O)2]SO4·H2O (Figure 7) in the temperature range of 100 -110°С is characterized by an endothermic effect, which corresponds to a weight loss of 4,5% on the TG curve.
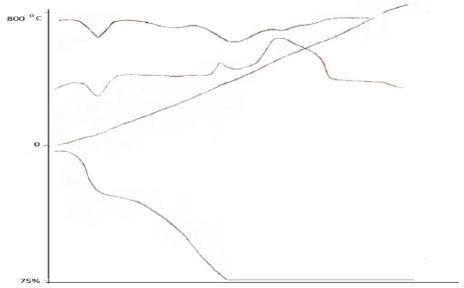
Figure 7: Thermogravigram of the complex with the composition [Zn(HL)2(H2O)2]SO4·H2O
The studies have shown that the thermal decomposition of mercazolil zinc complexes in air proceeds in three stages and covers the process of dehydration, thermal decomposition of mercazolil molecules in the complexes, decomposition of sulfate and the formation of metallic zinc. The comparison of the IR spectra of zinc sulfate, mercazolil, and the resulting complex indicates the formation of a new compound, in which characteristic bands related to stretching and bending vibrations of the starting substances appear with some changes. Infrared spectra of mercazolil, mercozincate in the frequency range from 4000 до 400 cm-1 were obtained to determine the functional groups of the studied ligands involved in complexation with zinc ions. The IR spectra (Figures 8 and 9) of the studied compounds revealed absorption bands typical for the monosubstituted benzene ring, methylene group and heterocyclic system. The shift or disappearance of characteristic absorption bands in the IR spectra of mercozincate in comparison with the spectra of zinc sulfate and mercazolil indicates the participation of specific functional groups in the formation of coordination compounds. For example, absorption bands in the range of 1619 – 1624 cm-1 characterize the stretching vibrations of the C=N bond of the conjugated aromatic system. After the formation of coordination compounds, it shifts from 1624 cm-1 in the IR spectrum of mercazolil to 1523 cm-1 in the IR spectra of mercozincate. Signs of coordination of mercazolil to zinc through a sulfur atom is proved by the fact that in the IR spectra of [Zn(HL)2(H2O)2]SO4·H2O there is a high-frequency shift of the band responsible for vibrations of C=S by 12-15 cm-1. The bands related to ν (-N (H) -C=S) in the spectra of the complex also undergo a change. The band of mercazolil at 1246 cm-1 in the spectra of the complexes completely disappears, and the band at 1276 cm-1 undergoes an insignificant high-frequency shift.
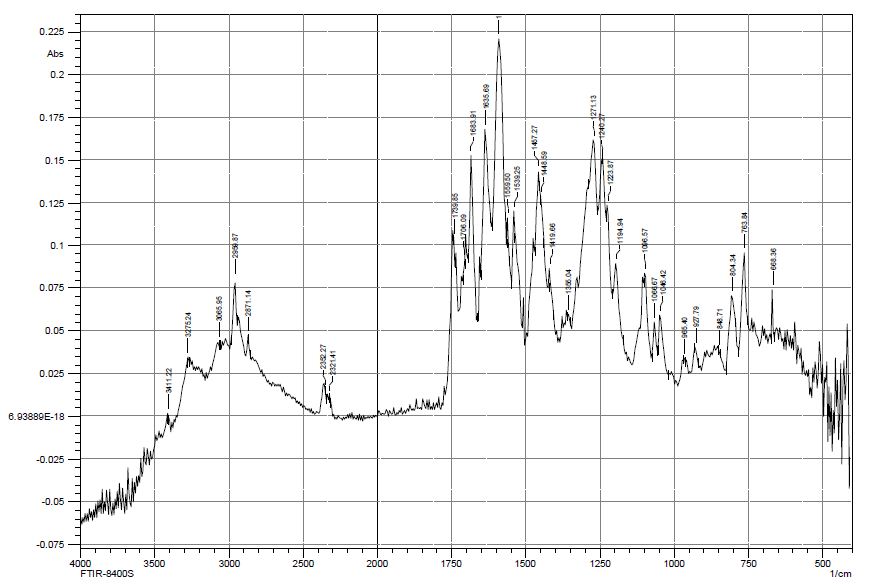
Figure 8: IR spectrum of mercazolil at 4000 cm-1-650 cm-1
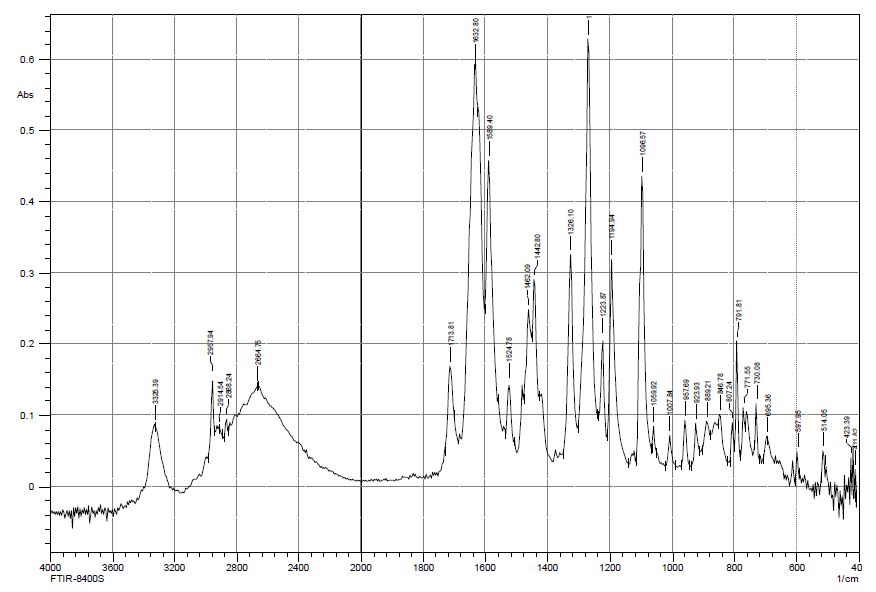
Figure 9: IR spectrum at 4000 cm-1-650 cm-1 synthesis of zinc with mercazolil
Thus, it has been shown that the coordination bond between the zinc ion with mercazolil occurs due to the thiol group of the sulfur atom.
Due to polycrystallinity of the obtained coordination compounds of zinc (II), X-ray phase studies of a number of synthesized coordination compounds of zinc (II) with the indicated organic ligands were carried out. The value of the molar electrical conductivity of dimethylformamide solutions of oxalate complexes with the composition [Zn(НL)2 (H2O)4] SO4 obtained in water-ethanol solutions at 25°С is anomalously high and amounts to 110-180 Om-1 cm2 mol-1, which is not typical for electrolytes. The study of the concentration dependence of the molar electrical conductivity of aqueous and dimethylformamide solutions of the studied zinc (II) compounds showed that their molar electrical conductivity is inversely related to the concentration of all coordination compounds. The obtained experimental data indicate that upon dilution of the solutions of the complexes, water or DMF molecules with donor abilities enter the inner sphere of the complexes by displacing acidoligands due to which the value of the molar electrical conductivity of the corresponding complexes increases.
The joint consideration of the results of quantitative analysis, given on the determination of water content by Fisher method and thermogravimetry and IR spectroscopy allowed us to assume the structure of the obtained coordination compounds.
The chemical name of the new chemical compound we have received is merzincate.
Recently, medical microelementology has begun to develop significantly. The correction of microelement status improves the condition of patients with various diseases. The synthesized coordination compound of zinc with mercazolil can be used as an antibacterial, antifungal, agent for the treatment of endocrine diseases of the thyroid gland. In this direction, we have investigated the harmlessness, toxicity and therapeutic activity of the compounds. The harmlessness of the complex compound of zinc sulfate and mercazolil was studied in accordance with the “Methodological guidelines for the determination of the toxic properties of drugs used in veterinary medicine and animal husbandry. “The experiment was carried out on 10 heads of rabbits and 50 heads of white mice. In order to assess the harmlessness of the preparation in an approximate therapeutic dose of 0,05 g/kg of body weight with water orally (in the form of a 10% suspension in saline solution) white mice were administered in a volume of 0,1-1,6 ml (weighing 18-20 g, n=6), rabbits of the chinchilla breed – 10 ml (weighing 2,5-2,7 kg, n=5) 2 times a day for 7 days.
The laboratory animals were observed for 14 days, taking into account the general condition, appearance, behavioral reactions, food and water intake, rhythm and heart rate, and the number of respiratory movements. The harmlessness of the approximate therapeutic dose of the preparation obtained from zinc and mercazolil is evidenced by the results of observation of animals for 14 days: there was not a single case of death of animals. The acute toxicity of a complex compound of zinc with mercazolil was studied in experiments on rabbits (weighing 1,6-2,2 kg, n=12) of which, according to the principle of paired analogs, 4 groups were formed. Before the start of the study, laboratory animals that were kept under normal conditions were observed for 14 days. The last time food was given in the evening on the eve of the experiment, water intake was not limited. The substance was administered to rabbits in the form of a 10% suspension in saline solution once orally in a volume of 0.5 ml at doses of 0,05 g/kg of body weight (group 1), 0,1 (2nd), 0,5 (3rd), 1,2 (4th), 2,5 (5th), 3,5 g/kg of body weight (6th). Control animals were administered with physiological saline in appropriate volumes.6 hours after the administration of the preparation, the rabbits were given food again, which were subsequently transferred to the usual mode (Table 3).
Table 3: Acute toxicity test results for zinc with mercazolil
|
Substance dose, g/kg of weight of lab. animals |
Actual effect |
LD (%) |
| 0,05 | 0/6 | 00 |
| 0,1 | 0/6 | 00 |
| 0,4 | 1/6 | 14,6 |
| 0,6 | 2/6 | 30,3 |
| 1,2 | 3/6 | 50 |
| 1,6 | 6/6 | 100 |
During the observation (14 days) of laboratory animals, the general condition, appearance, behavioral reactions, food and water intake, rhythm and heart rate, and the number of respiratory movements were taken into account. On the 2nd-3rd day, all animals of the 6th group died, from the animals of the 3rd group, 1 rabbit died on the 6th day, on the 5th and 7th days, 2 rabbits from the 4th group, and also during the experimental period, 3 animals from the 5th group. On examination of the internal organs of the dead animals, the gastric mucosa was hyperemic and filled with fodder, the liver was unchanged, and there was pinpoint hemorrhage in the tips of the lungs. The death of the rest of the experimental animals was not observed, the clinical state of the 1st and 2nd experimental groups and the control animals did not differ, the pathological changes in acute poisoning were absent in the animals.
Thus, according to the results of toxicological studies, it was determined that a complex compound of zinc with mercazolil 1,6 g/kg of body weight causes death of all experimental animals (LD100-1,6 g/kg) and at a dose of 1,2 g/kg causes death of 50 % of animals.
Effects on the Skin and Mucous Membranes
A single application was made of a 10% suspension of zinc with mercazolil on the skin of mice (weighing 18–20 g, n=8). The study of the repeated local irritating effect of this synthesized substance was carried out on mice (females, weighing 18 – 20 g, n=8), which daily on a clipped skin area in the interscapular region were applied 1 drop of a 10% zinc suspension with mercazolil for 14 days, and animals of the control group (n=8) – one drop of sunflower oil. The animals of both groups were observed for 30 days.
The repeated local action of zinc with mercazolil was also studied on rabbits (females, weighing 2,5-2,7 kg, n=8), which were daily applied to the skin with 2 drops of a 10% suspension of zinc with mercazolil for 21 days.
Animals of the control group (n=8) were given 2 drops of sunflower oil by the same method. Rabbits of both groups were observed for 60 days. As a result of the experiments, it was found that the substance obtained by us does not cause even minor phenomena of hyperemia, edema, scratching at the site of application. The animals did not show signs of toxicosis during the cutaneous application of the preparation.
Thus, no skin irritant and skin resorptive action was revealed in the complex compound of zinc with mercazolil. The effect of a complex compound of zinc with mercazolil on the mucous membrane of the eye was studied on rabbits (females, weighing 2,1-2,6 kg), which were divided into two groups (n=8). Animals of the first group in the conjunctival sac were once instilled with 10% suspension of the resulting complex compound in the amount of one drop, the second (control) group – instilled water in the same amount. It has been set that the local irritating effect of a complex compound of zinc with mercazolil on the mucous membranes of the eyes with a single administration is weak [3-25].
Conclusion
Based on the foregoing, it can be concluded that the complex compound of zinc with mercazolil belongs to the categories of low-toxic compounds for laboratory and farm animals, when administered orally in therapeutic doses.
References
- Ivansky VI (1978) Chemistry of heterocyclic compounds. p. 202.
- Berezina GR (2003) Complexes of cobalt (II) and zinc (II) with heterocyclic compounds based on 1,3-indandione. Journal of General Chemistry 73: 1567-1569.
- Rajabov UR, Yormamadova SG, Rakhimova RN, Kozikhonov AU (20-14) Complexation of copper with azoles derivatives at 298K/Collection of abstracts of the VIII International Scientific Conference “Kinetics and mechanism of crystallization. Crystallization as a form of self-organization of matter “, Ivanovo, Russia p. 51-52.
- Berezin BD, Nurmatov AA, Semeykina AS, Berezin MB (1994) Replacement of acetylacetonate ligands in the coordination sphere of copper and zinc with porphyrins. Coordination Chemistry. 20: 391-396.
- Olekhnovichi RY, Korobov MS, Lyubchenko SN, Suukholenko EV, Raskina PA, et al. (1992) Degenerate configurational r↔E isolating sterically hindered orta-indoaniline ligands in tetrahedral metal chelates Zn (II) and Cd (II). Journal of General Chemistry 62: 901-902.
- Tsapkov VI, Tarkogiel Mianperen, Samus NM (1993) Intra-complex compounds of cobalt (II), nickel (II), copper (II) and zinc with 5-nitrofuryl-2-methylene hydrazole isoamine. Journal of General Chemistry. 63: 1165-1166.
- Ilyasov SG, Lobanova AA, Popov NI, Kazantsev IV (2006) Synthesis and properties of intra-complex compounds of 4-nitrosemicarbazide with Fe (II), Ni (II), Co (II) and Zn (II). Journal of General Chemistry 76: 1795-1796.
- Pridchin SN, Kochergina AA (2006) Complex formation of zinc, cadmium and manganese (II) with 2-hydroxypropylene-1,3-diamine-N, N, N, N-tetraacetic acid. Journal of General Chemistry 76: 600-601.
- Skopenko VV (2007) Coordination chemistry. Akademkniga 487 p.
- Remy G. Course of inorganic chemistry/G. Remy. – M: Mir, 1966. V. 2. – p. 836.
- Dyuga G, Penny K (1983) Chemical approaches to the mechanism of action of enzymes. Bioorganic Chemistry. 512 p.
- Knorre DG, Myzina SD (2000) Biological chemistry: a textbook for chem. biol. and med. special universities. p. 8-26.
- Shabaldova AD, Bolshinkova TA, Kornienko GK, Trushina EK (1989) Complexes of copper with N-O-containing ligands, new reagents and biologically active substances. Abstracts of the report. III All-Union Conference. on chemical reagents. Ashgabat p. 87.
- Vasilyev VP, Zaytseva GA (1979) Protonation of imidazole in aqueous solution Problems of solvation and complexation. – Ivanovo p. 94-97.
- Perekalin VV, Zonis SA (1982) Prosveshenie Organic chemistry 575 p.
- Traven VF (2006) Organic chemistry. Akademkniga 2: 85-95.
- Beloborodov VL (2002) Organic chemistry: Basic course. Book. 1. p. 31-46.
- Cotton F, Wilkinson J (1969) Modern inorganic chemistry. Chemistry of transition elements. 592 p.
- Akhmetov NS (2001) General and inorganic chemistry. 743 p.
- Glebov AE, Dyachkova TA, Tarasov OY (1994) Chemistry of heterovalent complexes 120 p.
- Lumme P, Virtanen P (1969) Thermodynamics of the protolysis of imidazole in aqueous Solutions. Suomen Kemistilehti 42: 333-338.
- Greenberg AA (1966) Introduction to the chemistry of complex compounds “Chemistry”631 p.
- Chemistry of coordination compounds. Ed. J. Baylar, D. Bush. Trans. from English Ed. I.I. Chernyaev. M., IL, 1969.695 p.
- Belikov VG (1991) Pharmaceutical chemistry. 768 p.
- Zinc in pediatric practice (textbook) 2001, 84 p.