DOI: 10.31038/CST.2024942
Abstract
Objective: Because the current treatment technology cannot really solve the problem of the loss of melanocytes in the area of vitiligo, resulting in poor curative effect and low cure rate of vitiligo, known as the cancer of immortal people; Based on this, Liu Jingwei’s team proposed “the theory of implanting melanocyte processing plant in vitiligo affected areas” to fundamentally solve the worldwide problem of melanocyte loss in vitiligo affected areas.
Methods: 50 cases of vitiligo patients who had failed various treatments were selected by homologous pairing principle, and the complete outer hair root sheath containing hair follicle melanocyte stem cells was extracted and isolated by patented technology, and the resting hair follicle melanocyte stem cells in the outer hair root sheath were activated, and the outer hair follicle root sheath was prepared into a processing plant of melanocyte and implanted in the affected area of vitiligo.
Results: The melanocyte stem cells in the outer hair root sheath could be continuously transformed into melanocytes and enter the epidermis along the outer hair root sheath, thus inducing white spots to recolor. After 1 year, the cure rate of 50 patients with vitiligo was as high as 92%. At present, this technology has obtained 1 Chinese invention patent and 11 utility model patents, and also obtained international PCT patents, and obtained patent acceptance in the EU, the United States, Japan, South Korea and Thailand through the PCT patent way.
Conclusion: “The theory of implanting melanocyte processing plant in vitiligo affected area” were successfully transplanted to the affected area of vitiligo, which breaks through the traditional vitiligo treatment thinking, creates a new theory of vitiligo treatment, completely solves the source of melanocytes in vitiligo affected area, so that it has increased its cure rate to more than 90%. This patented technology cannot only completely cure vitiligo but also is not easy.
Keywords
PCT, Vitiligo, Outer root sheath, Melanocyte stem cell, Melanocyte processing plant
As a clinical refractory disease, vitiligo has a significant impact on the physical and mental health of patients, threatening the state of their marriage, social interactions, and employment. As the pathogenesis of vitiligo remains unknown, the ineffective rate of various treatments for vitiligo patients has reached 50% [1]. Therefore, vitiligo has always been regarded as a chronic disease in dermatology. The new method for treating vitiligo invented by the team of Liu JW (Nanhai Renshu International Skin Hospital) has been granted patents by the China Patent (Invention Patent) [2] (Technical Method for Treating Leucoderma Based on Hair Follicle Melanocyte Stem Cell Transplantation, Patent No.: ZL201910769979.1) and by the Patent Cooperation Treaty (PCT) [3] (Patent No.: PCT/CN2021/072340). At present, there are many surgical methods for treating vitiligo that utilize melanocyte (MC) transplantation. However, only the hair follicle MC stem cell (McSC) transplantation technology has been used effectively, becoming a massive breakthrough in the treatment of vitiligo.
General Data
A total of 50 vitiligo patients who had been treated in Nanhai Renshu International Skin Hospital using other methods for more than 1 year between June 2020 and March 2022 with unsatisfactory outcomes were selected as the research subjects for the present study. Inclusion criteria were as follows: 1) patients meeting the diagnostic criteria for vitiligo, 2) individuals over 4 years old, 3) those with no contraindications for ultraviolet radiation and no photosensitivity, 4) patients and their guardians who were able to adhere to the medical treatment, 5) patients who had not received any other treatments within 1 week and those with more than two white patches, at least one of which had received only the 308-nm excimer laser therapy as the control group, and 6) those who signed the informed consent form. Exclusion criteria included the following: 1) patients with malignant skin tumors, 2) those with mental disorders, 3) individuals with infected lesions at the white patch site, and 4) pregnant or lactating women. The present study was a key research and development project of Hainan Province in 2021, named Clinical Research and Application of the Transplantation of the Complete Outer Root Sheath of the Hair Follicle in the Treatment of Vitiligo (Project No.: ZDYF2021SHFZ048), which was approved by the Ethics Committee of the hospital on June 1, 2020 [Approval No.: 2020 (Clinical Research) RS002].
The 50 study subjects included 24 males and 26 females 4–62 years old, with an average age of (34.23±4.14) years. Vitiligo can be classified into localized type (n=29), generalized type (n=3), acrofacial type (n=5), and vulgaris type (n=14). In addition, leukoderma can be categorized into progressive (n=8) and stable (n=42) stages. In the control group, a total of 89 white patches were not surgically treated, and each patient had at least one such white patch. These white patches took up an area of 680 cm2 in total, with the largest area per patch of 89 cm2 and the smallest area per patch of 2 cm2. A total of 126 white patches were surgically treated in the treatment group, taking up a total area of 2,517 cm2, with the largest area per patch of 135 cm2 and the smallest area per patch of 1 cm2.
Instrument
The equipment used in the present study included a Peninsula 308- nm excimer laser system [model: XECL-308C; Shenzhen Peninsula Medical Co., Ltd. (Shenzhen, Guangdong, China), working medium: xenon chloride (XeCl), wavelength: 308 nm].
Therapeutic Dose
Prior to the 308-nm excimer laser therapy, the minimal erythema dose was tested in the abdomen of all patients using the instrument in the operation mode for the minimal erythema dose. The minimal erythema dose response was observed in each patient within 24–48 h after the irradiation. This dose was considered as the initial dose of the first operation.
Surgical Procedures
For the treatment group, disinfection and local anesthesia were carried out in a 10,000-level laminar flow operating room. Vitiligo was surgically treated according to the method recorded in the PCT- protected Technical Method for Treating Leucoderma Based On Hair Follicle Melanocyte Stem Cell Transplantation (hereinafter referred to as the invention patent) as follows: 1) the outer root sheath (ORS) containing hair follicle McSCs was extracted, and complete hair follicles containing McSCs were obtained using follicular unit extraction technology; 2) the complete ORS containing McSCs was obtained via the hair follicle separation method specified in the invention patent; 3) the obtained hair follicle McSCs were cultured in vitro using a special culture medium that is described in the invention patent. The stem cell activity was further generated to achieve transformation into mature MCs; 4) the obtained hair follicles containing McSCs were inactivated using the utility model patent Novel Vitiligo Hair Follicle Inactivation Needle (Patent No.: ZL201921329885.4) [4] according to the inactivation method in the invention patent, thus achieving dark pigmentation in the skin of vitiligo patients without hair growth; and 5) hair follicles containing McSCs with a complete ORS were transplanted using two utility model patents, including Planting Needle for Vitiligo Treatment (Patent No. ZL201921450324.X) [5] and A Plant Pilot Pin for Hair Follicle Transplants (Patent No.: ZL201921277579.0) [6].
In both the treatment group after the operation and control group, irradiation was conducted using a Peninsula 308-nm ultraviolet light therapy device 1–2 times/week, and the interval between the two irradiation procedures was no more than 7 days. The initial irradiation time was set up based on the minimal patient erythema dose. If erythema persisted for 12–48 h after the treatment, the irradiation dose was appropriate. Each white patch was irradiated 30 times as a course of treatment, and clinical observation of all patients lasted for more than half a year. Local patients in Hainan Province received free phototherapy once a week in the hospital. Patients outside the province underwent phototherapy using a home-use Peninsula 308- nm excimer laser therapy device as required, and the therapy status was reported at least once a week.
Evaluation Criteria
- The efficacy for the vitiligo treatment was evaluated based on the efficacy evaluation criteria formulated by the Pigmentation Disorder Group of the Dermatology and Venereal Disease Committee of the Chinese Society of Integrated Traditional Chinese and Western The therapy was regarded as effective only when patients were cured. Vitiligo was deemed to be cured after patches at the treatment site completely disappeared and the skin color basically returned to normal. The cure rate was calculated according to the following formula: cure rate = number of cured cases/total number of cases × 100%. The efficacy was also compared.
- Adverse reactions in all patients during the 308-nm excimer laser therapy, such as folliculitis, blisters, skin itching, burning sensation, and pain, were counted and recorded.
- Efficacy satisfaction questionnaires were distributed to all patients with a total score of 100 points on the last day of the follow-up. A score lower than 90 points was considered to indicate unsatisfactory efficacy.
Results
Therapeutic Results
One patient in the whole cohort received surgery at two different sites and was recorded as two cases. In the treatment group, 46 cases (92%) were cured, while four cases (8%) were not, resulting in the total cure rate of 92%. None of the 50 cases were cured in the control group and had a cure rate of 0%. Among the uncured patients, two suffered from hypothyroidism and took Eutyrox for a long time. Two acrofacial type patients were over 50 years old.
Adverse Reactions
During the 6-month follow-up after the treatment, the incidence rate of adverse reactions was 10% in the treatment group, with one case of skin itching, four cases of folliculitis, and zero cases of other discomforts. No obvious adverse reactions were detected in the control group.
Satisfaction Degree
Efficacy satisfaction questionnaires were distributed to all patients on the last day of the follow-up. The score was 100 points in 22 patients, 95 points in 20 patients, 90 points in five patients, and below 90 points in three patients, demonstrating an efficacy satisfaction rate of 94%.
A Typical Case
A 35-year-old male patient had multiple depigmented patches on the right side of his face for the duration of 17 years. White patches the size of a small fingernail appeared on the right side of the patient’s face for no obvious reasons 17 years prior. Various drug therapies, fire acupuncture, and laser therapies were performed in this patient with unsatisfactory results. Over the course of the past year, white patches on the right side of the patient’s face expanded, gradually affecting the forehead, eyelids, eyebrows, part of the nose, lower lip, and right side of the neck, occupying large areas. Due to the patient’s lack of confidence in stem cell transplantation, white patches in some areas (marked in Figure 1) were surgically treated for the first time. Two months after stem cell transplantation combined with 308-nm excimer laser therapy, a large amount of melanin was produced in the white patch areas. White patch areas that were previously operated on were repigmented six months after the operation. In particular, the lips and eyelid mucosa where vitiligo could not be cured in the past were repigmented with no color difference. White patches on the unoperated area only received 308-nm excimer laser therapy and did not change as a result (Figure 1).
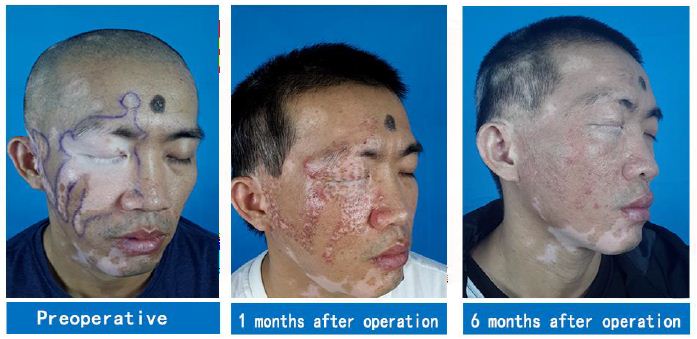
Figure 1: A case of vitiligo on the face: The first operation
The patient underwent a second operation combining stem cell transplantation with eyebrow implantation on the remaining white patch area on the face and neck six months later. Six months after the combined therapy, white patches in the operated area were completely cured, while those in the unoperated area receiving only the 308- nm excimer laser therapy remained unchanged (Figure 2). White patches on the ears and scalp of the patient have been recently treated surgically and are now recovering.
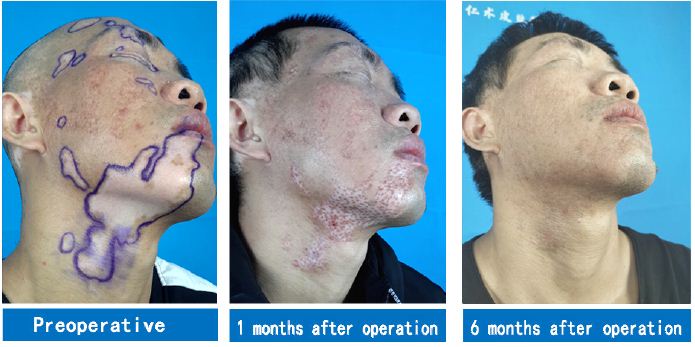
Figure 2: A case of facial vitiligo: The second operation
Discussion
Theoretical Basis and Research Progress for Hair Follicle McSCs in Vitiligo Treatment
Because mature MCs in the basal layer of the white patch area are partially or completely deficient, repigmentation of the white patch area is often achieved by the production of melanin granules by MCs migrating from outside this region. In 1959, Staricco et al. [7] have confirmed the existence of a large number of immature MCs containing no melanin in the ORS of hair follicles, which cannot synthesize melanin, are negative to dihydroxyphenylalanine (DOPA), and are thus regarded as amelanotic melanocytes (AMMCs). In 1979, Ortonne et al. [8] have found that after the psoralen plus long- wave ultraviolet therapy for vitiligo lesions, DOPA-negative and non-dendritic MCs in hair follicles migrate to the epidermis along the ORS of hair follicles and differentiate into mature MCs. On this basis, the hypothesis for the MC reservoir existence in hair follicles was put forward for the first time. In 1991, Cui et al. [9] have found that the inactivated MCs in the middle or lower part of the skin lesion hair follicle are activated and proliferate after the vitiligo treatment, changing from a non-functional to a functional state, and then migrate to the epidermis along the ORS of the hair follicle, forming pigmented spots at the hair follicular orifice. Dong et al. [10] have discovered that neural crest-derived McSCs located on the hair follicle bulge can effectively differentiate into mature MCs under the irradiation from narrow-band ultraviolet B (NB-UVB) rays and gradually migrate along the ORS to be repigmented at the hair follicular orifice of the vitiligo epidermis. Hair follicle AMMCs can serve as a reservoir for skin MCs in the treatment of vitiligo [11-13]. MCs are derived from the embryonic neural crest and begin to migrate to the epidermis and hair follicles 2–5 weeks after embryonic development. MCs migrating to hair follicles can be divided into two types: one type with melanin synthesis activity located in the hair matrix and infundibulum of the hair follicle in the anagen period, and the other type is inactivated AMMCs located in the ORS in the anagen period showing no melanin synthesis activity. In recent years, it has been shown that AMMCs can be activated by some specific factors, proliferate, migrate, and produce melanin, manifesting some characteristics of stem cells [14,15].
The McSCs and pre-MCs have been classified into AMMCs in numerous studies [16]. Hair follicle McSCs are located in the bulge area at the bottom of the hair follicle (upper 1/3), mostly in a resting state, with slow periodicity and ability to maintain self-renewal. They are typical representatives of regenerative stem cells [17]. However, as research progresses, it has been confirmed that stem cells in a transitional state, namely, pre-MCs, are present in the ORS of hair follicles. These cells do not synthesize melanin but are active in the pigment production cycle. As the direct source of MCs, pre-MCs are the earliest initiator of each pigmented hair cycle [18]. Pre-MCs are transitional cells between McSCs and MCs, which are formed by the proliferation and differentiation of McSCs in the previous hair growth cycle. They are essentially McSCs. As mature MCs in the basal layer of the white patch area are partially or completely deficient, the repigmentation of the white patch area is often achieved through the production of melanin granules by MCs migrating from outside this area. MCs migrating to the epidermis eventually settle on the basement membrane, forming mature MCs that continuously produce melanin [19]. McSCs serve as a melanocyte reservoir for the repigmentation of the affected skin in vitiligo patients. McSCs proliferate and migrate upwards to the nearby epidermis upon activation, forming pigment islands around hair follicles (Figure 3) [20].

Figure 3: A case of oral vitiligo
Clinical Research on McSC Transplantation for Vitiligo Treatment
In 2002, Nishimura et al. [21] investigated the proliferation of melanoblasts and found that stem cell factors expressed in the epidermis form a channel between the ORS and the epidermis, along which MCs migrate from the hair follicle to the epidermis. If the ORS containing McSCs is directly transplanted under the epidermis, the McSCs in the ORS can be activated by a 308-nm excimer laser, while those transported along the ORS can be processed into mature MCs.
Among all laser wavelengths, 308 nm is the laser wavelength where the absorption values of human DNA and proteins almost peak. This contributes to the production of pyrimidine dimers, purine dimers, and other substances, thus triggering the corresponding biological photoimmune response and repigmentation [22]. It has been pointed out that the 308-nm laser changed the microenvironment of hair follicles, facilitated the maturation and differentiation of McSCs, and stimulated the migration of MCs to the epidermis (Figure 4) [23].
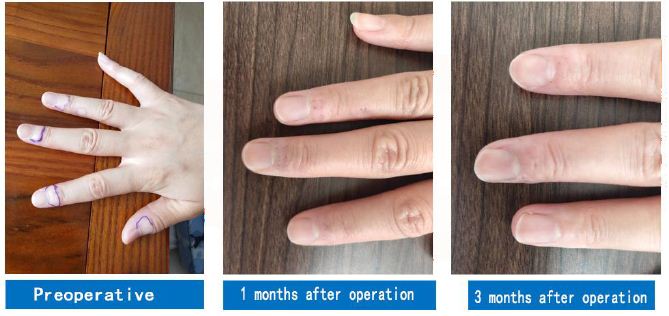
Figure 4: A case of vitiligo at the end of the finger
The transplantation of McSCs for treating vitiligo is a technological invention in the implantation of an MC processing plant, which provides a basis for a new theory of vitiligo treatment. The PCT- protected technical method used in the present study employed the following processes: extraction of autologous hair follicles, inactivation of hair follicles, separation of complete ORS, culture and activation of McSCs in the ORS, and harvesting and transplantation of functional McSCs. The complete hair follicle ORS supplies melanoblasts for McSCs. After the ORS containing functional McSCs was transplanted to an area under the epidermis, the 308-nm excimer laser activated the McSCs in the ORS to produce MCs in vitro, thereby continuously producing mature MCs and successfully establishing an MC processing plant in the affected skin of vitiligo patients.
Clinical Research and Theoretical Innovation in McSC Transplantation for Vitiligo Treatment
Although there are presently many surgical methods for treating vitiligo, including epidermal transplantation, MC transplantation, skin tissue engineering, ORS suspension transplantation [24], and single hair follicle transplantation [25], the actual transplants are MCs, which will be inactivated or become apoptotic after completing a life cycle, leading to re-whitening of the skin in vitiligo patients. In particular, surgical methods other than single hair follicle transplantation require microdermabrasion for the transplantation of MCs, resulting in significant damage, uneven repigmentation, and proneness to scarring. Single hair follicle transplantation has been adopted to treat vitiligo more than 20 years ago, but the essence of this method is to implant hair follicles into the dermis and subcutaneous tissues, through which only MCs at the junction of the basement membrane zone and the ORS can enter the epidermis. As only a small segment of the ORS has been transplanted, it is necessary to transplant a large number of hair follicles to achieve repigmentation of whole white patches. This method requires a large number of hair follicles for the treatment of hairless white patches, after which the hair becomes unmanageable. In addition, this method utilizes punctiform repigmentation in most cases and is thus ineffective for white patches in the mucosa. Therefore, it is only suitable for the treatment of vitiligo on skin portions with hair. Hair follicle McSC transplantation method in the present study was adopted to transplant the complete ORS of hair follicles to an area between the epidermis and dermis. After the operation, new MCs were continuously generated via in vitro activation of McSCs in the ORS, thus achieving patchy repigmentation. Since hair follicles were inactivated before the operation, they fell out naturally post-operation after one hair cycle (Figure 5).
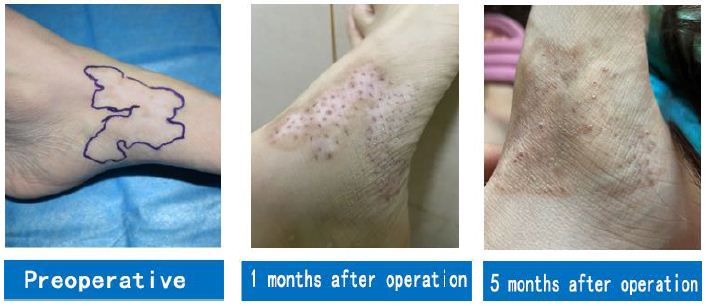
Figure 5: A case of vitiligo on the feet.
The PCT-protected Technical Method for Treating Leucoderma Based on Hair Follicle Melanocyte Stem Cell Transplantation is the first in the world to propose a technique for transplanting hair follicle McSCs to treat vitiligo based on the complete ORS. Using this method, McSCs can be directly transplanted to the epidermis of vitiligo patients, thereby treating large-area vitiligo via extraction of small quantities of hair follicles. In addition, the present invention also shows marked efficacy in hairless areas, which indicates that stem cell transplantation is also applicable for the treatment of white patches on the mucous skin membrane.
This is the first time that transplantation of the skin environment containing McSCs with complete hair follicle ORS has been proposed for treating vitiligo. Additionally, this invention patent method provides a basis for a new theory of vitiligo treatment by implanting an MC processing plant, which provides a source of MCs for the treatment of vitiligo and lays a foundation for repigmentation of white patches (Figure 6).
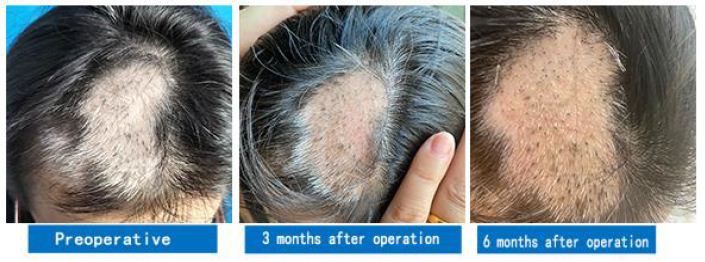
Figure 6: A case of vitiligo on the head
This invention patent introduces a new method of transplanting McSCs for vitiligo treatment without dermabrasion. Vitiligo patients were surgically treated without dermabrasion, and repigmentation with no color difference after the vitiligo operation was achieved via minimally invasion transplantation using the self-developed plant pilot pin for hair follicle transplants and planting a needle for vitiligo treatment.
Conclusion
Liu et al. have obtained McSCs in a functional state using a PCT- protected technical method and implanted them and melanoblasts to an area under the epidermis. Continuously activated by a 308- nm excimer laser in vitro, McSCs in the ORS were transformed into mature MCs and migrated along the ORS to multiple hair follicle orifices in the vitiligo area or sebaceous gland openings in the hairless area to achieve central-type repigmentation with no color difference. McSC transplantation addresses the issue of MC sources for patients with vitiligo and provides a new solution for its treatment. With a cure rate of 92%, this method brings new hope for recovery to 70 million patients with vitiligo worldwide.
References
- Patel NS, Paghdal KV Cohen GF (2012) Advanced treatment modalities for vitiligo [J]. Dermatol Surg [crossref]
- Liu J Technical method for treating leucoderma based on hair follicle melanocyte stem cell transplantation. China. ZL201910769979.1 2021.01.12, Publication Number: CN110339214A.
- LiuJing-wei. Technical method for treating vitiligo through hair follicle melanocyte stem cell transplantation. China. PCT/CN2021/072340, WO2022/151450.
- Liu J W. Novel vitiligo hair follicle inactivation needle. China. ZL201921329885.4, 2020.07.14. Publication Number: CN210990699U.
- Liu J W. Planting needle for vitiligo treatment. China. ZL201921450324.X, 2020.09.08. Publication Number: CN211434526U.
- Liu J W. A plant pilot pin for hair follicle transplants. China. ZL201921277579.0, 09.08. Publication Number: CN211433043U.
- Staricco RG. Amelanotic melanocytes in the outer sheath of the human hair follicle[J]. J Invest Dermatol 1959 [crossref]
- Tobin DJ, Bystryn JC (1996) Different populations of melanocytes are present in hair follicles and epidermis [J]. Pigment Cell Res [crossref]
- Cui J, Shen LY, Wang GC (1991) Role of hair follicles in the repigmentation of J Invest Dermatol 97(3): 410-416. [crossref]
- Dong D, Jiang M, Xu X, et al (2012) The effects of NB-UVB on the hair follicle derived neural crest stem cells differentiating into melanocyte lineage in vitro[J] J Dermatol Sci [crossref]
- Yu HS (2002) Melanocyte destruction and repigmentation in vitiligo: a model for nerve cell damage and regrowth[J]. J Biomed Sci [crossref]
- Slominski A, Wortsman J, Plonka P M, et Hair follicle pigmentation [J]. J Invest Dermatol 2005. [crossref]
- Bernard Hair cycle dynamics: the case of the human hair follicle [J]. J Soc Biol 2003 [crossref]
- Ma HJ, Zhu WY, Wang DG, et Endothelin-1 combined with extracellular matrix proteins promotes the adhesion and chemotaxis of amelanotic melanocytes from human hair follicles in vitro[J]. Cell Biol Int 2006 [crossref]
- Lei TC, Vieira WD, Hearing In vitro migration of melanoblasts requires matrix metalloproteinase-2: Implications to vitiligo therapy by photochemotherapy [J]. Pigment Cell Res 2002 [crossref]
- Takada, K, Sugiyama, K, Yamamoto, I, et al. Presence of amelanotic melanocytes within the outer root sheath in senile white hair[J]. J Invest Dermatol 1992 [crossref]
- Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: Incomplete melanocyte stem cell maintenance in the niche [J]. Science, 2005. [crossref]
- Hsu YC, Li L, Fuchs E. Transist amplifying cells orchestrate stem cell activity and tissue regeneration [J]. Cell. 2014 [crossref]
- Slominski A, Wortsman J, Plonka PM, et (2005) Hair follicle pigmentation [J]. J Invest Dermatol 124(1): 13-21. [crossref]
- Matz H, Tur Vitiligo[J] (2007) [title] Curr Probl Dermatol 35: 78-102.
- Nishimura EK, Jordan SA, Oshima H, et al. Dominant role of the niche in melanocyte stem-cell fate determination[J]. Nature 2002 [crossref]
- Jmb A, Shk B, Hjj A, et al. Suberythemic and erythemic doses of a 308-nm excimer laser treatment of stable vitiligo in combination with topical tacrolimus: A randomized controlled trial – Science Direct[J]. Journal of the American Academy of Dermatology 2020. [crossref]
- Noborio R, Nomura Y, Nakamura M, et al. Efficacy of 308-nm excimer laser treatment for refractory vitiligo: A case series of treatment based on the minimal blistering dose[J]. Journal of the European Academy of Dermatology and Venereology 2020. [crossref]
- Vinay K, DograS, ParsadD, et Clinical and treatment characteristics determining therapeutic outcome in patients undergoing autologous noncultured outer root sheath hair follicle ce11 suspension for treatment of stable vitiligo [J]. J Eur Acad Dermatol 2014. [crossref]
- Na GY, Seo SK, Choi SK. Single hair grafting for the treatment of vitiligo[J]. J Am Aead Dermatol 1998. [crossref]