DOI: 10.31038/CST.2020534
Abstract
Background: Cellular immunotherapy with autologous T cells genetically engineered to express chimeric antigen receptors is emerging as a promising new class of immunotherapeutic agents, however may cause unique symptoms of neuro-toxicity, such as toxic encephalopathic state with symptoms of confusion and delirium, and occasionally seizures and cerebral oedema.
Case presentation: Hereby, we report a case of a 4-year-old boy, with B-cell precursor acute lymphoblastic leukemia and refractory CNS involvement, which was treated with CAR T-cells. The patient developed severe encephalopathy, high fever and seizures, and was treated with steroids and anticonvulsants. Nevertheless, the patient rapidly deteriorated and developed diffused brain oedema and herniation of cerebellar tonsils. Unfortunately, the patient showed no neurological improvement and suffered brain death.
Conclusion: Neurotoxicity is an important and common complication of CAR-T cell therapies. Usually, severe neurological symptoms are manageable in most patients, which respond to standard interventions. Early detection of neurological deterioration is of paramount importance, and pediatric intensivists should consider pre-emptive management for brain oedema, even prior to radiological evidence. Randomized prospective studies of treatment algorithms are urgently needed to improve patient monitoring and management.
Keywords
Chimeric antigen receptors (CAR), cytokine-release syndrome (CRS), Immune effector cell-associated neurologic syndrome (ICANS), Neurotoxicity
Background
Cellular immunotherapy with autologous T cells genetically engineered to express chimeric antigen receptors (CARs) is emerging as a promising new class of immunotherapeutic agents in relapsed and refractory B-cell malignancies [1,2]. As CAR T-cell therapies become more widely used, recognition of their unique toxicities, which are distinct from those seen with traditional chemotherapies, monoclonal antibodies, and small-molecule targeted therapies, is of the utmost importance [1]. The two most commonly observed toxicities with CAR-T-cell therapies are: 1) cytokine-release syndrome (CRS), characterized by high fever, hypotension, hypoxia, and/or multiorgan toxicity; and 2) Immune effector Cell-Associated Neurologic Syndrome (ICANS), which may occur in more than 60% of patients treated with CAR T-cells [2]. ICANS is typically characterized by a toxic encephalopathic state with symptoms of confusion and delirium, and occasionally seizures and cerebral oedema, and can occur with or after CRS [1] with peak incidence occurring 4–6 days after infusion [3-5]. About 20% of patients will present severe neurotoxicity [5], and grade 5 fatal neurotoxicity has been described in clinical studies in adults treated with CD19-directed CAR T-cells, with an incidence of up to 3% [4]. Hereby, we are the first to present an extreme form of neurotoxicity in a young child, resulting in brain oedema and death.
Case Presentation
We report a case of a 4-year-old boy, with B-cell precursor acute lymphoblastic leukemia (ALL) with CNS involvement. Due to high risk relapse/refractory disease he was enrolled on a clinical trial using CD19 CAR T-cells. The patient developed CRS on day +3 (grade 1), and due to encephalopathy, high fever and seizures he was transferred to pediatric intensive care (PICU) on day 5 of CAR-T treatment. Prior to transfer to PICU, due to a clinical diagnosis of ICANS grade 3, he was commenced on dexamethasone, on top of Levetiracetam prophylaxis (started on day -3). Following this event, a brain CT was performed and was normal, showing no intracranial bleeding or oedema. EEG revealed general encephalopathy. Following repeated tonic-clonic seizures despite increase in the Levetiracetam dose and steroids treatment, he was loaded with phenytoin as well as a few midazolam boluses to stop the seizures. During the first 24 hours in PICU, the patient remained stable, encephalopathic, however maintained GCS of 8-10. The following day (+6) a brain MRI was performed under general anesthesia, showing high T2-FLAIR signal involving the hemispheral sub-cortical white matter, hippocampi and capsule externa, in addition to high signal in the thalami bilateral. Furthermore, there were cortical areas with diffusion strain which correlate with ICANS. The patient was extubated and returned to PICU drowsy but responsive. Upon returning from MRI the patient had a sudden acute deterioration, with apneic episode and GCS which dropped to 3, therefore was immediately intubated. An urgent repeat CT brain was performed revealing diffused brain oedema with developing herniation of cerebellar tonsils (Figure 1). During the next 24 hours he received mannitol, hypertonic saline, and noradrenaline to maintain proper cerebral perfusion pressure and reduction of oedema, he was started on broad spectrum antibiotics and anti-viral empiric therapy for possible meningo-encephalitis, as well as pulse methylprednisolone and tocilizumab. Unfortunately, the patient showed no neurological improvement and had absent brain stem reflexes and anisocoric pupils. SPECT was performed showing absent flows which correlates with brain death.
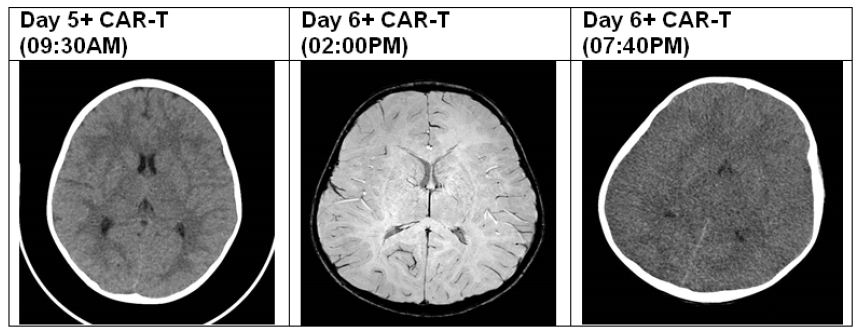
Figure 1: CT/MRI findings.
Discussion
We describe a young child with relapse ALL that was commenced on CAR-T therapy and very rapidly, after 5 days of treatment, developed severe ICANS presenting as encephalopathy and seizures. He received the acceptable treatment with anti-epileptic drugs and steroids, unfortunately suffered from very extreme and rare complication of CAR-T treatment as of brain oedema, followed by tonsillar herniation and death. The oedema itself may have risen as the sequelae of some other underlying process, and in our patient might have been main cause of the neurological deterioration. Neurotoxicity is an important and common complication of CAR-T cell therapies. Acute neurologic signs and/or symptoms occur in a significant proportion of patients with clinical manifestations that include headache, confusion, delirium, language disturbance, seizures and rarely, acute cerebral oedema. The mechanisms that lead to neurotoxicity remain unknown, but data from patients and animal models suggest there is compromise of the blood-brain barrier, associated with high levels of cytokines in the blood and cerebrospinal fluid, as well as endothelial activation [6]. This cytokine production is correlated to early onset of severe CRS, or may be associated with expansion and activation of CAR T-cells that lead to a direct parenchymal CAR T-cell infiltration [5]. Such toxicities have also been observed in patients treated with other redirected-T-cell therapies and bispecific T-cell-engaging antibodies [1]. Gust et al., [4] described a potential mechanism for the cases of diffuse and often fatal cerebral oedema, with findings of widespread endothelial activation as well as findings of meningeal inflammation from a mouse model of CAR T-cell neurotoxicity [7]. Toxicity may also be primarily mediated by the inflammatory cytokine surge that accompanies CAR-T cell expansion in the marrow, rather than the CAR-T cells themselves [6].
The management of ICANS remains an area of active investigation. Therapy rests upon symptomatic management, seizure control, and corticosteroids. Despite the widespread use of corticosteroids, it is unknown to what degree they influence CAR T cell–mediated anticancer effects [2]. Presently, corticosteroids and tocilizumab are the mainstays of treatment for both CRS and neurotoxicity [1]. However, treatment with tocilizumab for CRS causes serum IL-6 to rise, which may predispose to more severe neurotoxicity [6]. In sicker patients with depressed level of consciousness, dexamethasone should be added and seizures need to be ruled out and controlled. In the sickest patients who are unarousable, with status epilepticus, motor weakness or diffuse cerebral oedema, or when brain MRI identifies focal or diffuse oedema, high dose methylprednisolone should be started. Anakinra (anti-interleukin-1 receptor antagonist) has been anecdotically proposed [5,6]. Although symptoms could present at virtually any time within the first few weeks after CAR T-cell infusion, patients who developed early CRS are more likely to develop severe neurotoxicity. Severe neurotoxicity represents a negative prognostic factor for overall survival with potential therapy-related mortality and underline the importance of rigorous monitoring of these patients [2]. Usually, ICANS is manageable in most patients, although some require monitoring and treatment in the intensive-care setting. It is thus imperative that clinicians remain vigilant in their workup and management of all neurological symptoms, especially those that deviate from the expected course of recovery and responsiveness to standard interventions. The role of intensivists is crucial and PICU specialists may help anticipate the risk for developing organ dysfunction or sepsis, based on patient’s frailty, immunity and comorbid conditions. After CAR-T infusion, when patients develop subacute fever and mild organ derangement, early PICU admission is recommenced. PICU intensivists should consider early management for brain oedema with possible intubation and secure airway, hyperosmolar therapy, and raising the cerebral perfusion pressure by vasoactive support. All of these measures should be considered at a very early stage of ICANS, even prior to radiological evidence, as most if not all patients will have brain oedema to some degree at presentation with encephalopathy. Diabetes ketoacidosis is a similar example where an inflammatory state associated with an immune and systemic inflammatory response results in disruption in the integrity of brain capillaries tight junctions which causes capillary permeability and brain oedema [8]. Our problem in clinical practice is that we are unable to quantify BBB function in real time during the acute course of ICANS treatment. Hence, from a pragmatic perspective, recognizing and providing preemptive treatment is paramount for pediatric intensivists.
Conclusion
Early detection of neurological deterioration is of paramount importance after CAR-T cell treatment, and PICU intensivists should consider early management for brain oedema, even prior to radiological evidence. Randomized prospective studies of treatment algorithms are urgently needed to improve patient monitoring and management.
List of Abbreviations
CAR: Chimeric antigen receptors
CRS: Cytokine-Release Syndrome
ICANS: Immune Effector Cell-Associated Neurologic Syndrome
ALL: Acute Lymphoblastic Leukemia
PICU: Pediatric Intensive Care
Declarations
- Ethics approval and consent to participate.
- Consent for publication – there is an ethical approval and consent to participate by the local IRB committee.
- Availability of data and materials – all data was described in references.
- Competing interests – no competing interests.
- Funding – no funding.
- Authors’ contributions – RKL wrote the manuscript with the help of EJ. Initiated, supervised and finally edited and approved by GP. All authors read and approved the final manuscript.
- Acknowledgements – not applicable.
References
- Neelapu SS, Tummala S, Kebriaei P, William Wierda, Cristina Gutierrez, et al. (2017) Chimeric antigen receptor T-cell therapy—assessment and management of toxicities. Nat Rev Clin Oncol 15: 47-62.
- Philipp Karschnia, Justin T. Jordan, Deborah A. Forst, Isabel C. Arrillaga-Romany, Tracy T. Batchelor, et al. (2019) Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CART cells. Blood:
- Makita S, Yoshimura K, Tobinai K (2017) Clinical development of anti- CD19 chimeric antigen receptor T-cell therapy for B-cell non-Hodgkin lymphoma. Cancer Sci 108:1109-111.
- Juliane Gust, Kevin A Hay, Laïla-Aïcha Hanafi, Daniel Li, David Myerson, et al. (2017) Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov Dec 7: 1404-1419.
- Elie Azoulay, Michael Darmon, Sandrine Valade (2020) Acute life‑threatening toxicity from CAR T‑cell therapy. Intensive Care Med 46:1723-1726.
- Daniel B. Rubin, Husain H. Danish, Ali Basil Ali, Karen Li, Sarah LaRose, et al. (2019) Neurological toxicities associated with chimeric antigen receptor T-cell therapy.
- Margherita Norelli, Barbara Camisa, Giulia Barbiera, Laura Falcone, Ayurzana Purevdorj, et al. (2018) Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nature Medicine 24: 739-748.
- Robert CT, Carlo LA (2014) Cerebral edema in children with diabetic ketoacidosis: vasogenic rather than cellular? Pediatric Diabetes 15: 261-270.