Abstract
The outbreak of the novel corona virus disease, COVID-19, has presented health care professionals with the unique challenges of trying to select appropriate pharmacological treatments with little time available for drug testing. Given the development times and manufacturing requirements for new products, Value Added Medicines (repurposing – reformulation of existing drugs) could be one possibility to beat the COVID-19 outbreak. This review explores reformulation alternatives which could be progressed with chloroquine and hydroxychloroquine; two antimalarial drugs, that are being tested on a global scale as a potential therapeutic option. The key areas for improvement have been reviewed and the potential solutions to the problems and limitations of current formulations are discussed. The pharmaceutical challenges discussed are those of highly soluble drugs, needed to be given at high doses and presenting a real bitter taste challenge with significant gastrointestinal side effects that could be translated and repurposed into fit for purpose reformulations.
Introduction
COVID-19 is the infectious disease caused by the most recently discovered corona virus. This newly emerged virus and disease were unknown before the outbreak began in Wuhan, China, in December 2019. COVID-19 is now a pandemic affecting many countries globally and to date no antiviral or therapeutic has been approved for treating patients. As the number of cases continues to rise, the geographic range of the virus increases, and with the development of a vaccine being at least 12 months away, there is a growing urgency/pressure on pharmaceutical industry and regulatory agencies to expedite the development and approval of both experimental drugs and repurposing of existing therapeutics that have been already approved for human use by the health agencies. Among the landscape of therapeutics being analysed as potential repurposing candidates for COVID-19, the antimalarial and immunomodulatory drugs chloroquine (CQ) and hydroxychloroquine (HCQ), both 4-aminoquinolines, are being tested on a global scale as a potential treatment and prevention of COVID-19 [1]. Recent publications have drawn attention to the possible benefit of CQ sulphate and phosphate salts (CQ diphosphate) and HCQ for the treatment of SARS-CoV-2 infected patients [2-7]. CQ phosphate or sulphate is referenced on the World Health Organisation (WHO) Model List of Essential Medicines for the treatment of Plasmodium vivax infection (malaria) [8, 9]. In addition to their antimalarial use, both CQ and HCQ are used in continuous daily dosing for rheumatoid arthritis, systemic and discoid lupus erythematosus and psoriatic arthritis. CQ and HCQ are one of four potential treatments that WHO has included in the global SOLIDARITY Clinical Trial in 90 countries to generate the robust data needed to establish efficacy and safety in COVID- 19 treatments [10]. There are several other trials ongoing in different countries [11, 12] to name a few, a UK wide randomized, controlled trial in over 130 hospitals called Randomised Evaluation of COVID Therapy (RECOVERY) [13] is underway and in Europe its Trial of Treatments for COVID-19 in Hospitalized Adults (DisCoVeRy) [14]. In US, NYU and University of Washington has fast trackeda major clinical trial to determine role of HCQ in prevention of corona virus [15]. Both CQ and HCQ are primarily available as tablets for oral administration [16-18] and have been in clinical use for decades thus their safety profile is well established [19]. However, this oral formulation presents the following problems: swallow ability difficulties for certain patients groups such as the young, older people and patients in critical care, as well as extremely poor palatability due to the bitter taste of the drugs [20, 21]. In addition, the oral administration of CQ and HCQ frequently causes gastrointestinal side (GI) effects such as nausea and vomiting [22-24]. HCQ being reported to have better safety profile than CQ, better gastrointestinal tolerability, and less retinal toxicity [25]. This review sets the scene and explores promising reformulation alternatives which could be progressed with CQ and HCQ, for adults and children. Alternatives routes of administration are also explored to address oral administration challenges. The problems and limitations with existing formulations are discussed and the key areas for improvement are reviewed. It is important to note while CQ and HCQ are under investigation in clinical trials for use on COVID-19 patients, as of the date of this publication, none of these compounds and medications have been approved for the treatment of COVID-19. Considering it is a rapidly changing area with new conflicting outcomes coming up every day, the repurposing of CQ and HCQ for COVID-19 is still questionable as only limited evidence is available at the present time. However, this review will be useful in various scenarios 1) if the trials are successful and the use of CQ in HCQ for COVID -19 is recommended 2) if no good evidence on use of CQ and HCQ for COVID-19 is generated in time, the reformulation strategies proposed in this review will be still relevant for antimalarial and rheumatic disorders treatment 3) the approaches discussed could be translated to other Active Pharmaceuticals Ingredients (APIs) presenting similar pharmaceutical challenges.
Challenges with existing CQ and HCQ formulation for COVID-19 treatment
CQ and HCQ are both Biopharmaceutics Classification System Class 1 compound [26] and extremely bitter [20, 21]. One study found the threshold bitterness of the pure CQ to be at 40 μg/ml [27]. After oral administration both are rapidly and almost completely absorbed from the gastrointestinal tract. They have a long and variable plasma elimination half-life because of a high volume of distribution with about half the drug metabolites undergoing unmodified renal clearance. CQ has a low safety margin and is very dangerous in overdose situations or when combined with other medicines [28]. There is no firm evidence on the optimal dosing and duration treatment for CQ or HCQ, hence the range of regimens are used across trials. In general, the regimen of CQ and HCQ used is substantially more aggressive than that recommended as an antimalarial [29]. For instance, National Health Commission of the People’s Republic of China recommended dose of 500mg twice daily for seven days for oral administration in 18–65 years of infected adults [30]. In the RECOVERY Trial, the loading dose of HCQ (1860mg) is twice the normal dose for treating malaria. However, this dose has been selected based on the available data of the IC50for SARS-CoV-2 [13] Several dosing regimens are proposed based on PBPK simulation combined with known clinical exposure–response relationships [3, 31-33]. Based on PBPK model, the typical dose for HCQ for treating COVID-19 is 400 mg twice daily on the first day, followed by 200 mg twice daily for four more days [31, 33]. Although HCQ shows better safety and toxicity profiles than CQ, symptomatic effects at these high doses have not been explored in enough depth. The question remains, how will gastro-intestinal symptoms prevail, will the benefits outweigh the risks and symptomatic effects; especially for critically ill COVID-19 patients in ICU. A wide range of data from various patients groups needs to be gathered to reliably surmise this. Both CQ and HCQ are metabolised in the liver with renal excretion of some metabolites, hence should be prescribed with care in people with liver or renal failure [34-36]. Recently published Surviving Sepsis Campaign guidelines [37] on the management of critically ill patients with COVID-19 concluded that there was insufficient evidence to offer any recommendation on the routine use of these drugs in patients admitted to the intensive care unit (ICU).The ongoing trials will be able to answer whether antimalarial drugs could be effective in changing the disease course in patients with severe COVID-19—in particular, in cases requiring ICU admission. At present, the safety of CQ in the treatment of elderly patients with COVID‐19 is unclear. The rate of critical illness in this population is high and CQ could still be used as an alternative drug, although it should not be given to those elderly patients with underlying heart and other conditions [38]. Based on the published clinical guidelines and research results, Sun et.al (38) have proposed the pharmaceutical care for the elderly using CQ phosphate in the treatment of COVID‐19. This includes the administration method, dosage of CQ phosphate for elderly, adverse drug reactions and drug interactions of CQ phosphate. For elderly patients with a bodyweight of more than 50 kg, CQ phosphate 500 mg orally, bid, for 7 days is recommended. CQ and HCQ is licensed for use in children with malaria. The WHO recommended HCQ dose to treat COVID-19 infected children is 25mg/kg given over 3 days. However, this may not be optimal to treat COVID-19, as recent studies show that older infants and children may need a higher mg/kg dose to reach the similar concentrations as adults. In contrasts, it is likely that neonates and young infants will need lower doses per kg body weight. Paediatric CQ dose for COVID-19was determined by Verscheijden et.al. [39]. The study proposestotal cumulative doses: 35 mg/kg (CHQ base) for children 0-1month, 47 mg/kg for 1-6 months, 55 mg/kg for 6 months-12 years and 44 mg/kg for adolescents and adults, not to exceed 3300 mg in any patient. Currently, there is a CQ phosphate syrup [40] on the market, while no pediatric or easy to swallow formulations exist for HCQ. However, as HCQ is highly soluble compound, it is expected that manipulation of the formulation will have minimal impact on bioavailability. The European Paediatric Formulary (PaedF) Working Party at the European Directorate of the Quality of Medicines and Healthcare (EDQM) has compiled existing knowledge on pediatric formulations for active substances which are under investigation for the treatment of COVID-19 as well as known authorised medicinal products [41]. This includes the information on extemporaneous preparations of CQ and HCQ which may be suitable for treatment of pediatric patients with COVID-19. It suggests the preparation of a pediatric suspension formulation from a 200mg tablet. This instructs pharmacists to ‘strips’ the outer film coating, crush the tablet(s), and then suspend the powder in water with a flavouring agent such as Ora-plus®. In the USA, The Nationwide Children’s pharmacy [42] and Michigan Collaborative Standardization of Compounded Oral Liquids [43] have formerly investigated utilising a crushed standard 200mg tablet of HCQ in Ora-plus® to form an oral liquid suspension. The resultant suspension concentration is 25mg/ml (i.e. 800mg (32ml) of suspension). A liquid dosage form of HCQ would be applicable to both the younger and older generations to address both patient dysphagia and compliance respectively. However, Ansah EK et al. reported improved compliance in children in rural Africa who were treated with CQ tablets or segments (crushed and mixed with sugar or honey) rather than CQ syrup [44] Moreover, the preparation of this extemporaneous suspension, especially stripping or/and crushing process is cumbersome. The suspension media such as Ora-plus® is also costly and may not be easily available in some regions, and the process may result in a loss of active pharmaceutical ingredient. Batches could be prepared on a demand basis for these patient groups but it is time consuming to prepare large quantities of suspension that have a shelf life of only 30 days at 2-8°C protected from light. Besides, the same GI side effects would likely still be present with additional issues of the drug’s bitter taste [45, 46] which is unlikely to be concealed. Thus, for this suspension to obligate its benefits, taste masking seems essential [47].
Current approaches for taste masking of CQ and HCQ
Sensory based taste masking approaches in which sweeteners and flavours are added to obscure taste, have been commonly used for decades, but this approach does not work well for highly soluble, highly aversive APIs and/or for APIs with an intense lingering aftertaste, as any compounds dissolved in the saliva will interact with the taste receptors and elicit a response [48]. Alternatively, taste masking techniques are utilised to improve the palatability of formulations. The different complexation approaches that have been used to taste mask by creating ‘molecular’ barrier around the API CQ and HCQ were scoped as part of this review and are discussed below.
Ion pairing
Ion-pairing is a process that involves stoichiometric replacement of polar counter-ions (i.e., chloride, acetate, nitrate, etc.) in the drug with an ionic excipient of similar charge. Ion-pairing has been used in the pharmaceutical industry, mainly as an additional drug-delivery method, and has proven to be a very effective mean for controlled drug release and taste masking [49]. Pauli et al [47] created a prototype formulation using a Coni-Snap Sprinkle Capsule containing sodium carboxymethyl cellulose (Na-CMC) as the ion-pairing agent to investigate if the addition of ion-pairing excipients and the incorporation of a buffered system into the conventional tablet could overcome some of the bitter-taste issues of HCQ. They hypothesised that this would enable a dual use formulation: adults can swallow the capsule whole whereas children (or indeed any patients with swallowing difficulties) could be administered the capsule content in water. They compared the dissolution profile of this capsule to another prototype formulation in the same capsule but without any ion-pairing agent and concluded that both profiles were comparable to that of a commercially available tablet of HCQ. This a bridged research also presented how the ion-paring system as provided by Na-CMC, and by another ion-pairing agent; sodium citrate, buffered to pH 8, taste-masked the drug in vitro with an Astreeel ectronic (e-tongue) assay. However, no in vivo assessment has been made. Moreover, the concentrations tested with the e-tongue were not reflective of the clinical situation for adults with COVID-19 as it was to treat children with uncomplicated malaria, lupus erythematosus, and rheumatoid arthritis at much lower doses. This integrated system could still have promise with higher drug loads, but it is likely the ion-pairing agents would need to be present in much higher concentrations in order to match the 1:1 ratio used in the study. Independent of this, buffering the system alone to pH 8 seems to have a significant effect on taste-intensity so this could be a quicker avenue to explore, given the current urgent need. It would likely be time-consuming to re-test alternative amounts of ion-pairing agents and then carry out further compatibility testing alongside the other components of the formulation.
Ion – exchange resins
Taste masking by drug–resin complexation is achieved when an ionizable drug reacts with a suitable ion exchange resin to form a drug–resinate complex [48]. Ion exchange resins (IERs) are insoluble, pharmacologically inert, high molecular weight cross linked polymers with cationic and anionic functional groups. They bind to compounds that exchange mobile ions and ultimately form of a tasteless drug- resin complex or resinate [50]. Drugs are attached to the oppositely charged resin substrate, forming insoluble adsorbates or resonates through weak ionic bonding. The resinate needs to be stable in the drug formulation e.g. a suspension or a tablet formulation and the dissociation of the drug–resin complex should not occur under the salivary pH conditions (pH 6-7). However, at enteric pH conditions (pH<5), the drug should be rapidly and almost entirely released in order to prevent reduced bioavailability. This can suitably mask the unpleasant taste and odour of drugs [51]. Characterization of drug–resin complexes and taste masking of CQ phosphate by complexation using weak cation exchange resin have been described in the literature [27, 50, 52]. All studies showed some in vivo taste improvement. No taste masking study of HCQ using ion exchange resins was identified.
Simple and Supra molecular Complexation
The host-guest complexation is a common taste masking technology [53]. By embedding the drug molecule (guest) into the cavity of a host molecule, a stable complex is generated. Generally, there are 2 mechanisms for explaining the taste-masking effect of complexation. The first mechanism is that the complexing agent will hinder interactions between drug molecules and taste cells through forming the strong binding with drugs [54]. Secondly, the complexing agent may directly bind to taste cells to mask the unpleasant. Among all the complexing agents, cyclodextrin (CD) group is a typical example [53]. Derived from the starch, CDs are cyclic polymers composed of glucopyranoside units (n) that are linked by α-1,4-glycosidic bonds. With their doughnut-shaped structures, CDs are capable of fitting lipophilic drugs or lipophilic moieties of drugs inside their hydrophobic central cavities (Figure 1). The natural and well-known CDs are α- (n=6), β- (n=7), and γ- (n=8)CD, respectively (Figure 1).
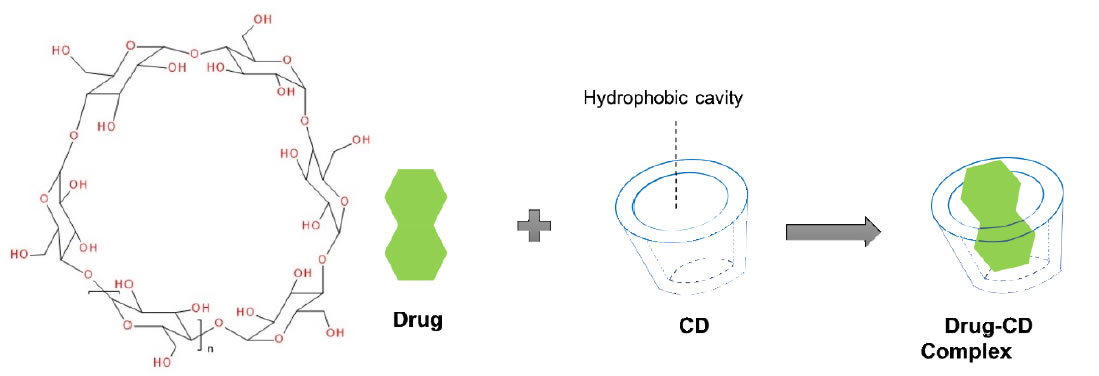
Figure 1. Cyclodextrin structure and representation of an inclusion complex of a drug residing in the cavity formed by the cyclodextrins
There is limited literature regarding the complexation between CDs and HCQ (or CQ). Guo, Wu et al [55] built a 3D model to predict the taste masking effect of CDs in different drug-CD complexes, where the Euclidean distance using an α-Astree e-Tongue was adopted to quantitate a taste masking effect (100 being the smallest euclidean distance to show taste masking efficiency). The study concluded that taste masking of HCQ was not achieved as the Euclidian distance was only 91 and proposed that CDs were ineffective because the halogen group and the chlorobenzene of the4-aminoquinolines significantly increased their molecular size and hampered the complete encapsulation of the drugs inside CDs cavities or that the alkylamino side chain is not part of the inclusion complex, allowing the tertiary amine to participate in bitter taste. Therefore, to date, no published paper indicated that natural CDs were effective in masking the bitter taste of HCQ or CQ. Woertz et al (56)conducted an INSENT e-tongue experiment to investigate taste masking effects of CDs (α-CD, β-CD, γ-CD, hydroxypropyl-β-CD, maltodextrin as well as sulfobutyl ether-β-CD (SBE-β-CD) on quinine. All CDs failed to mask the bitter taste of quinine, except for the modified CD, SBE-β-CD. It was thought that the aliphatic ring of quinine was embedded inside the CD cavity, whereas the quinoline ring of quinine was left outside the cavity, generating an incomplete inclusion complex with CDs, thereby the quinoline part could still interact with taste receptors and cause the bad taste. The study concluded that SBE-β-CD was able to mask bitter taste of quinine because of the ionic interaction between its –SO 3 2- group and the deformed –NH 3 + group from the quinine [56] (Figure 2).
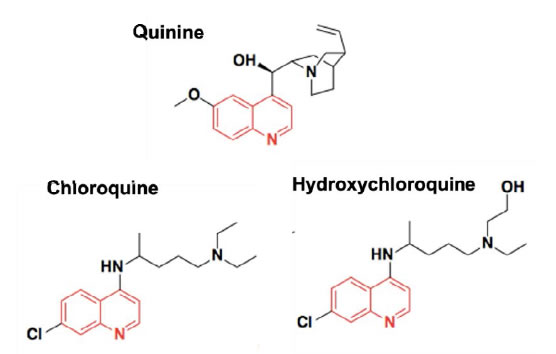
Figure 2. Chemical structures of Quinine, Chloroquine and Hydroxychloroquine.
Since the chemical structure of quinine is similar to CQ and HCQ (Figure 2), the potential of SBE-β-CD for taste masking offer a masking option. However, according to Ghateet al [57] the native SBE-β-CD is salty, which may impede it use for taste masking. Captisol® is an FDA approved SBE-β-CD. This enabling technology available to research and development through flexible licensing arrangements is claimed to be tastelessness in oral formulas. It is and has been used in drug products on the market [58, 59]. Remarkably, Joneset al [60] recently developed a novel CD, modified with mercaptoundecane sulfonic acids. This highly sulfonated CD could mimic heparan sulphates (HS) and kill most HS-dependent viruses, such as herpes simplex virus, respiratory syncytial virus, dengue virus, and Zika virus. The study indicated that the newly modified CD had a potential to act as a broad-spectrum antiviral agent. Although corona viruses were not tested, their discovery reveals a different choice, where the modified CD could mask bitter taste, and at the same time, kill viruses. It is to be noted that this is probably the furthest away from translation as this would require extensive studies to support the claim. Apart from CDs, the cucurbituril (CB) family is another emerging complexing strategy and believed to be promising and attractive for pharmaceutical development. CBs ares upramolecular host molecules or macrocycles consisting of 5 or more glycoluril units joint together by methylene linkages [61]. CBs can also accommodate lipophilic drug moieties inside their hydrophobic cavities [62]. Currently, there is no literature about the HCQ-CB complexation or the CQ-CB complexation. However, it was confirmed by Boraste, Chakraborty et al [63] that CB7, which connects 7glycoluril units, was able to form a stable and a complete inclusion complex with quinine at a ratio of 2:1 (CB7 to quinine). Compared with CDs, CB7 was demonstrated to not only encapsulate the small aliphatic ring of quinine, but also fit the large quinoline ring into its cavity. This process was accomplished by binding the aliphatic ring of quinine to one CB7, while the quinoline moiety of quinine entered into a second CB7 cavity, at a lower pH. Accordingly, it is hypothesized that it could be possible for CQ and HCQ to form complete inclusion complexes with CBs. In this way, interactions between bitter drugs and taste receptors could be inhibited and taste masking achieved. However, before they are ready to be used in practice or tested in humans, there are number of issues that needs to be addressed including safety (Figure 3).
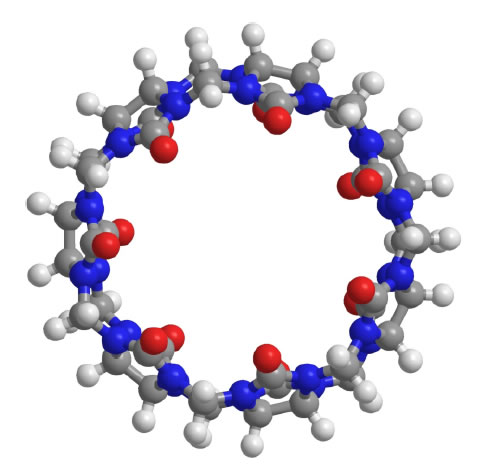
Figure 3. Molecular structure of CB7
Crystal engineering strategies
Pharmaceutical cocrystals are molecular crystals that are multi-component crystalline substances, where one of the components is an API and the other component/components are crystalline substances that have been approved by the regulatory bodies within the jurisdiction of commercial use; these species are known as the cocrystal former or the conformer [64]. Co-crystallization is one of the emerging crystal engineering techniques for modulating pharmaceutical performance through controlling solid-state properties of APIs and expanding the access to new solid forms differing in structures. The approach relies on the self-assembly of a bitter-tasting drug and a taste-masking agent, whereby the molecules are held together by non-covalent interactions including hydrogen and halogen bonds. Co-crystallization can modify different physicochemical properties of the APIs, without any change in their activity, such as improving the solubility of poorly soluble drugs, masking the bitter taste, increasing chemical stability and decreasing hygroscopicity, enhancing manufacturability as well dissolution rate and bioavailability [65]. Investigations into the solid-state landscape of CQ and HCQ have demonstrated the advantages of complexation within the crystal lattice. They have distinct acid base properties and hydrogen bond abilities that makes them suitable to form salts and co-crystals. The only crystal form of HCQ currently on the market is in the form of the sulphate salt [66]. Whereas CQ is on the market as the stand-alone API or as the phosphate, sulphate or hydrochloride salt [67, 68]. One study demonstrated how improvements can be made to these solids using crystal engineering to enhance the physicochemical properties and pharmacological activity of these co-crystallised compounds over parent compound. CQ and HCQ are both part of the quinolone family and are closely related to quinolone. Baruah et al [69] showed that salts and cocrystal of quinoline with hydroxyaromatic carboxylic acids enhance antimalarial activities over parent compounds. However, there is no literature to support the application of crystal-engineering approach to taste masking neither of CQ or HCQ. Research into the preparation of multicomponent crystals involving other APIs containing quinoline moieties suggest that this group of compounds could either partake in salt formation or cocrystal formation to improve API taste depending on the type of cocrystal or salt former used [70-72].
Alternative routes of administration to address formulation challenges
Rectal drug delivery
To subsequently tackle at the same time the bitter taste issue and high dose administration whilst still being applicable to all patient groups with possible comorbidities/polypharmacy, the rectal route may be the most appropriate alternative administration route. Symptomatic relief would also be achieved and allow critically ill/unconscious patients in ICU to be treated equally. Suppositories are often used as drug delivery system in case of nausea or vomiting, in case of oral administration rejection due to the bad taste or in case a medication is readily decomposed in gastric fluid. Published studies showed that CQ given in suppositories with the same dose as oral formulations reached lower blood concentrations and it was slower to produce the same antimalarial effects than when administered orally [73-76]. Tjoenget al [77] performed comparative bioavailability studies of rectal and oral formulations of CQ in healthy volunteers. The study demonstrated that the relative bioavailability of CQ 500 mg suppositories varied between 10-53% compared to a tablet formulation in adults. Onyeji at al [75] demonstrated that the bioavailability of chloroquine 100 mg suppositories was 63.4 +/- 8.8% (mean +/- SEM) relative to the tablet formulation. Bruce-Chwatt et al [73] observed that only when the same dose of CQ 300mg tablets was administered rectally over a 5 days period, it was able to reach the same parasite clearance of the oral dose but more slowly. The study concluded that CQ given by mouth was better and faster absorbed compared to the rectal route. However, in all these studies, no consideration was drawn on formulation improvements that could enhance the rectal release and adsorption of CQ from the suppository. Suppository are made of relatively low-cost excipients but their manufacture can be more challenging than other common dosage forms (tablets, liquids): they may need temperature-controlled storage depending on melting point and humidity control may be required during manufacture [78]. The nature of the base and the surfactant content in the suppository composition need to be carefully chosen to obtain the optimal mechanical and drug release properties of CQ [79]. Redgon et al [80] assessed different lipophilic and hydrophilic bases for CQ phosphate. They found that the hard-fat base Witepsol H15 was the best in terms of disintegration times, a with good storage conditions, was also suitable for countries with a continental climate. Onyeji et al [81] on the other hand, studied the effect of absorption-enhancing agents, non-ionic surfactants and sodium salicylate, on the in vitro release characteristics of CQ from polyethylene glycol (1000:4000, 75:25%, w/w) suppositories. The study concluded that the incorporation of 4% Tween 20 or 25% sodium salicylate improved the invitro release of CQ from the suppository. Considering these adjuvants also have absorption-promoting properties, association of the improved in-vitro release with enhanced in vivo availability is envisaged but would require a PK study for confirmation. Thus, results of this study serve as a guide in the selection of an optimal formula regarding the type and concentrations of absorption enhancers required for optimization of CQ release and a possible enhancement of rectal absorption of the drug. Finally, Okubanjoet al [79] studied the effects of interacting variables on the mechanical and release properties of CQ phosphate suppositories. A23 factorial experiment was designed to study the effects of the type and nature of the base, the concentration of surfactant and storage conditions. The study demonstrated that the presence and concentration of surfactant was the main individual variable affecting the release properties of suppository formulations. The addition of surfactants increased the crushing strength and decreased the dissolution times of CQ suppositories. Also, the type of suppository base played a role in the modification of the mechanical and release properties. Witepsol H15, as previously highlighted by Redgon et al [80] was better than Suppocire AS2 in increasing the crushing strength and dissolution rates and decreasing the dissolution times while storage conditions had the lowest effect. In the past, the rectal administration of CQ, but not HCQ, was explored for the treatment of malaria. Evidence tends to suggest that the use of rectal formulations for the administration of CQ could be re-considered as an alternative route to the oral administration to overcome the problems associated with this route. Due to the similarity of the 2 compounds, it is speculated that HCQ could also be a good candidate to be reformulated for rectal administration. It would be interesting to explore more recent advances introduced for the release and adsorption of drugs form rectal suppositories, such as the use recto dispersible dosage forms with non-melting excipients [82] or the use of hollow-type suppositories which have been developed to enhance the adsorption of various drugs [47]. The use of rectal formulations could be particularly useful for the treatment of certain patients’ groups affected by COVID-19, such as those with swallowability difficulties, critically ill patients, unconscious or vomiting patients or pediatric patients from birth. Although speculatively, by this route, there may be the added advantages of attaining the necessary higher plasma concentrations with a lower drug dose (yet high doses of API can be delivered rectally) as it generally avoids first pass metabolism. Finally, it is suitable for APIs that are gastro-irritant and could speculatively again help with some GI side effects. Socio-cultural norms drive recommendations regarding the knowledge, attitude, preference, and behaviour of people. To implement rectal delivery of CQ and HCQ, beside positive pharmacological outcomes, it would be important to consider patients’ real barriers versus cultural, perceived barriers or lack of understanding of the potential of this mode of administration [83]. Two further routes of administration for CQ and HCQ have been recently re-proposed as alternatives to the oral drug delivery.
Pulmonary drug delivery
An aerosolized formulation of HCQ was developed and tested in early phase clinical trials by the American company APT Pharmaceuticals. For the anti-inflammatory effect of HCQ, this formulation was developed for the potential treatment of respiratory diseases such as asthma, chronic obstructive pulmonary disease (COPD), rhinitis and severe acute respiratory syndrome (SARS). The drug was delivered by using an aerosol generating system (AERx®) for pulmonary delivery developed by Aradigm Corporation of Hayward, to maximize drug delivery in a patient-friendly format. By using this targeted delivery system, the company believed that the aerosolized dosage form and the use of the pulmonary route could achieve a faster onset of action, within hours, and greater therapeutic effects than conventional oral therapy at substantially lower systemic doses [84]. However, despite the favourable results showed in a phase 1 clinical study, the subsequent phase 2a data failed on efficacy endpoints and the study was stopped. In light of the potential role that CQ and HCQ might have as potential candidate therapies for COVID-19, Klimke et Al [85] re-proposed the use of them as pulmonary aerosol in a dosage of 2-4 mg of HCQ per inhalation for reduction and prevention of severe symptoms after SARS-CoV-2 infection. They speculated that by administering the drug targeting directly the lung, a lower drug dosage would be required to reach the optimal concentrations, compared to the oral route and avoid the oral side effects. Based on their speculations, the 2 authors decided to inhaled themselves tolerability and safety of 1 mg of HCQ in 2ml sodium chloride 0.9%, b.i.d. increased to 4 mg daily over one week. The dose was deemed well tolerated, with after 4 days still the feeling of a transient bitter taste in the mouth, which lasted 2-3 hours after each dosing. However, no efficacy data are available to validate their hypothesis.
Transdermal Drug Delivery
A study by Musabayane et al [86] investigated the potential application of pectin hydrogel patch as a matrix polymer for transdermal administration of CQ with Dimethyl sulphoxide as a penetration enhancer. They tested on rats the effects of CQ via intravenous infusion and the patch applied on shaved area during the 1 h 20 min. The results (plasma profile) showed good potential for transdermal delivery of CQ. However, the loading efficiency was only 46% of the theoretical 10 g. Moreover, the dose administered (16 µg/kg) was much lower than those previously used in rats (20–25 mg/kg), and in man (300 mg/kg). Glan is Pharmaceuticals Inc. recently obtained the rights for a US provisional patent for a transdermal drug delivery system of HCQ as a potential treatment for COVID-19. They suggest that controlled transdermal delivery could provide constant drug plasma concentrations for pre-determined periods of time, potentially reducing side effects associated to the oral delivery. However, so far, it seems that only literature search and pre-formulation studies have been done in collaboration with Reformulation Research Laboratories Inc. but no related clinical information are available [87]. The development of transdermal formulations [88] of CQ and HCQ with novel strategies would need to be carefully studied in order to address some challenges such as the hydrophilic nature, the need to administer larger doses of drug, and potential skin irritation due to enhancers or other additives added to the transdermal formulation.
Conclusion
COVID-19, officially designated as severe acute respiratory syndrome-related corona virus SARS-CoV-2 currently represents a pandemic threat to global public health. Researchers are leaving no stone unturned in an effort to understand this new emergent disease and uncover existing drugs with therapeutic potential for COVID-19. CQ and HCQ, antimalarial drugs are among the existing drugs being investigated in clinical trials as a possible treatment protocol for COVID-19.The clinical evidence base is currently limited and there is hope that the ongoing clinical trials may unfold the missing evidence if these antimalarial drugs could be effective in changing the disease course in patients with COVID-19. Although it is the elderly and those with underlying health conditions find themselves most gravely affected by COVID-19, the virus does not discriminate by age. All age groups are at risk and more likely to require drugs such as CQ or HCQ in an hospital setting. A formulation which does not rely on swallowing a bitter tablet or suspension and that would suit both ambulatory and critical care settings, would be welcomed. CQ and HCQ, in currently available forms (tablet and syrup) are inundated with challenges in terms bitter taste, high dose etc. This review has outlined different reformulation approaches that could be utilised for taste masking the CQ and HCQ and alternative routes of administration to surpass the problems associated with the oral administration. We hope that teaching these old drugs new tricks may represent an opportunity to address the pharmaceutical challenges and deliver better health to patients with COVID-19 and/or even for antimalarial treatment.
References
- WHO. Landscape analysis of therapeutics. 2020.
- Gao J, Tian Z, Yang X (2020) Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Bioscience trends 14: 73-74. [crossref]
- Gautret P, Lagier J-C, Parola P, Meddeb L, et al. (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. International journal of antimicrobial agents. [crossref]
- Schlagenhauf P, Grobusch MP, Maier JD, Gautret P (2020) Repurposing antimalarials and other drugs for COVID-19. Travel Med Infect Dis. [crossref]
- Keyaerts E, Vijgen L, Maes P, Neyts J, Van Ranst M (2004) In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochemical and biophysical research communications 323: 264-268. [crossref]
- Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, et al. (2005) Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virology Journal 2. [crossref]
- Wang M, Cao R, Zhang L, Yang X, Liu J, et al. (2020) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell research. 30: 269-271. [crossref]
- WHO (2015) 19th WHO Model List of Essential Medicines.
- WHO (2020) Medical Product Alert N°4/2020.
- WHO (2020) “Solidarity” clinical trial for COVID-19 treatments.
- Overview of planned or ongoing studies of drugs for the treatment of COVID-19. 2020.
- ClinicalTrials.gov. (2020) OVID-19 Studies from the World Health Organization Database.
- Recovery Trial (2020) Randomised Evaluation of Covid-19 Therapy.
- Institut National de la Santé Et de la Recherche Médicale (2020) Trial of Treatments for COVID-19 in Hospitalized Adults (DisCoVeRy).
- ClinicalTrials.gov. (2020) Federally-funded clinical studies related to COVID-19.
- Agency EM (2016) List of nationally authorised medicinal products. London.
- BNF (2020) Chloroquine | Drug | BNF content published by NICE: NICE.
- BNF (2020) Hydroxychloroquine Sulfate | Drug | BNF content published by NICE: NICE.
- Devaux CA, Rolain J-M, Colson P, Raoult D (2020) New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19?. International journal of antimicrobial agents. [crossref]
- Wallace DJ, Tse K, Hanrahan L, Davies R, Petri MA (2019) Hydroxychloroquine usage in US patients, their experiences of tolerability and adherence, and implications for treatment: survey results from 3127 patients with SLE conducted by the Lupus Foundation of America. Lupus Sci Med. [crossref]
- Weber JC, Alt M, Blaison G, Welsch M, Martin T, et al. (1996) [Changes in taste and smell caused by hydroxychloroquine]. Presse Med 25. [crossref]
- Braga CB, Martins AC, Cayotopa AD, Klein WW, Schlosser AR, et al. (2015) Side Effects of Chloroquine and Primaquine and Symptom Reduction in Malaria Endemic Area (Mancio Lima, Acre, Brazil). Interdiscip Perspect Infect Dis. [crossref]
- Deen JL, von Seidlein L, Dondorp A (2008) Therapy of uncomplicated malaria in children: a review of treatment principles, essential drugs and current recommendations. Trop Med Int Health. 13: 1111-1130. [crossref]
- Srinivasa A, Tosounidou S, Gordon C (2017) Increased incidence of gastrointestinal side effects in patients taking hydroxychloroquine: a brand-related issue?. The Journal of rheumatology 44. [crossref]
- Mc Chesney EW (1983) Animal toxicity and pharmacokinetics of hydroxychloroquine sulfate. Am J Med 75: 11-18. [crossref]
- Ploger GF, Hofsass MA, Dressman JB (2018) Solubility Determination of Active Pharmaceutical Ingredients Which Have Been Recently Added to the List of Essential Medicines in the Context of the Biopharmaceutics Classification System-Biowaiver. J Pharm Sci 107: 1478-1488. [crossref]
- Sharma T, MoonRajkumar S (2012) Formulation and Evaluation of Taste Masked Dispersible Tablets of Chloroquine Phosphate. International Research Journal of Pharmacy 3.
- European Medicines Agency (EMA) (2020) COVID-19: chloroquine and hydroxychloroquine only to be used in clinical trials or emergency use programmes.
- Wong YK, Yang J, He Y (2020) Caution and clarity required in the use of chloroquine for COVID-19. The Lancet Rheumatology 2.
- National Health Commission of the People’s Republic of China Interpretation of COVID-19 treatment guidelines (6th version). 2020.
- Cui C, Zhang M, Yao X, Tu S, Hou Z, et al. (2020) Dose selection of chloroquine phosphate for treatment of COVID-19 based on a physiologically based pharmacokinetic model. Acta Pharmaceutica Sinica B.
- Kapoor KM, Kapoor A (2020) Role of Chloroquine and Hydroxychloroquine in the Treatment of COVID-19 Infection- A Systematic Literature Review. medRxiv.
- Yao X, Ye F, Zhang M, Cui C, Huang B, et al. (2020) In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin Infect Dis. [crossref]
- Rismanbaf A, Zarei S (2020) Liver and Kidney Injuries in COVID-19 and Their Effects on Drug Therapy; a Letter to Editor. Archives of Academic Emergency Medicine 8. [crossref]
- Taccone FS, Gorham J, Vincent J-L (2020) Hydroxychloroquine in the management of critically ill patients with COVID-19: the need for an evidence base. The Lancet Respiratory Medicine. [crossref]
- Wang Y, Zhu LQ (2020) Pharmaceutical care recommendations for antiviral treatments in children with coronavirus disease. World Journal of Pediatrics 12: 1-4. [crossref]
- Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, et al. (2020) Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Medicine. 46: 854-887 [crossref]
- Sun X, Li S, Li K, Hu X (2020) Pharmaceutical care of chloroquine phosphate in elderly patients with coronavirus pneumonia (COVID-19). Aging Medicine.
- Verscheijden LFM, van der Zanden TM, van Bussel LPM, de Hoop-Sommen M, Russel FGM, et al. (2020) Chloroquine dosing recommendations for pediatric COVID-19 supported by modeling and simulation. Clinical Pharmacology & Therapeutics. [crossref]
- Electronic Medicines Compendium (EMC), Malarivon Syrup. 2018.
- EDQM (2020) European Directorate for the Quality of Medicines and Healthcare, Products and extemporaneous preparation of paediatric formulations that may be useful in the treatment of COVID-19.
- Nationwide Children’s Pharmacy Services. Compound Evaluation Form for Hydroxychloroquine. 2020.
- Mipeds Compounds. Michigan Collaborative Standardization of Compounded Oral Liquids 2013.
- Ansah EK, Gyapong JO, Agyepong IA, Evans DB (2001) Improving adherence to malaria treatment for children: the use of pre-packed chloroquine tablets vs. chloroquine syrup. Trop Med Int Health 6: 496-504. [crossref]
- Jiménez-Alonso J, Sabio J, Carrillo-Alascio P, Jiménez-Jáimez J, Ortego-Centeno N, et al. (2004) Intolerance to hydroxychloroquine marketed in Spain (Dolquine) in patients with autoimmune conditions. Revista clinica espanola 204: 588-591. [crossref]
- Pauli E, Joshi H, Vasavada A, Brackett J, Towa L (2020) Evaluation of an Immediate-Release Formulation of Hydroxychloroquine Sulfate With an Interwoven Pediatric Taste-Masking System. Journal of Pharmaceutical Sciences.109: 1493-1497. [crossref]
- Hua S (2019) Physiological and Pharmaceutical Considerations for Rectal Drug Formulations. Frontiers in pharmacology 10. [crossref]
- Walsh J, Cram A, Woertz K, Breitkreutz J, Winzenburg G, et al. (2014) Playing hide and seek with poorly tasting paediatric medicines: do not forget the excipients. Adv Drug Deliv Rev 73: 14-33. [crossref]
- Agresti C, Tu Z, Ng C, Yang Y, Liang JF (2008) Specific interactions between diphenhydramine and α-helical poly(glutamic acid) – A new ion-pairing complex for taste masking and pH-controlled diphenhydramine release. European Journal of Pharmaceutics and Biopharmaceutics. 70: 226-233.
- Agarwal R, Mittal R, Singh A (2000) Studies of Ion-Exchange Resin Complex of Chloroquine Phosphate. Drug Development and Industrial Pharmacy 26: 773-776. [crossref]
- Sivaneswari S, Karthikeyan E, Veena D, Chandana PJ, Subhashree P, et al. (2016) Physiochemical characterization of taste masking levetiracetam ion exchange resinates in the solid state and formulation of stable liquid suspension for pediatric use. Journal of Basic and Applied Sciences 5: 126-133.
- Akshay B, Akshay M, Vaishali P (2014) Formulation and Evaluation of Taste Masked Oral Suspension of Chloroquine Phosphate. Journal of Drug Delivery & Therapeutics 4: 16-24.
- Arima H, Higashi T, Motoyama K (2012) Improvement of the bitter taste of drugs by complexation with cyclodextrins: applications, evaluations and mechanisms. Therapeutic delivery 3: 633-644. [crossref]
- Shah PP, Mashru RC (2010) Palatable reconstitutable dry suspension of artemether for flexible pediatric dosing using cyclodextrin inclusion complexation. Pharmaceutical development and technology 15: 276-285. [crossref]
- Guo Z, Wu F, Singh V, Guo T, Ren X, et al. (2017) Host-guest kinetic interactions between HP-β-cyclodextrin and drugs for prediction of bitter taste masking. Journal of pharmaceutical and biomedical analysis 140: 232-238. [crossref]
- Woertz K, Tissen C, Kleinebudde P, Breitkreutz J (2010) Rational development of taste masked oral liquids guided by an electronic tongue. International journal of pharmaceutics 400: 114-123. [crossref]
- Das O, Ghate VM, Lewis SA (2019) Utility of Sulfobutyl Ether beta-Cyclodextrin Inclusion Complexes in Drug Delivery: A Review. Indian Journal of Pharmaceutical Sciences 81: 589-600.
- DeDora DJ, Suhrland C, Goenka S, Mullick Chowdhury S, Lalwani G, et al. (2016) Sulfobutyl ether β‐cyclodextrin (Captisol®) and methyl β‐cyclodextrin enhance and stabilize fluorescence of aqueous indocyanine green. Journal of Biomedical Materials Research Part B: Applied Biomaterials 104: 1457-1464. [crossref]
- Beig A, Agbaria R, Dahan A (2015) The use of captisol (SBE7-β-CD) in oral solubility-enabling formulations: comparison to HPβCD and the solubility–permeability interplay. European Journal of Pharmaceutical Sciences 77: 73-78. [crossref]
- Jones ST, Cagno V, Janeček M, Ortiz D, Gasilova N, et al. (2020) Modified cyclodextrins as broad-spectrum antivirals. Science Advances 6. [crossref]
- Nau WM, Assaf K, Das D (2019) Applications of Cucurbiturils in Medicinal Chemistry and Chemical Biology. Frontiers in chemistry 7. [crossref]
- Wheate NJ, Limantoro C (2016) Cucurbit [n] urils as excipients in pharmaceutical dosage forms. Supramolecular Chemistry 28: 849-856.
- Boraste DR, Chakraborty G, Ray AK, Shankarling GS, Pal H (2017) pH‐Responsive Interaction of Fluorogenic Antimalarial Drug Quinine with Macrocyclic Host Cucurbit [7] uril: Modulations in Photophysical and Acid‐Base Properties. ChemistrySelect 2: 5128-5142.
- Berry DJ, Steed JW (2017) Pharmaceutical cocrystals, salts and multicomponent systems; intermolecular interactions and property based design. Advanced Drug Delivery Reviews 117: 3-24. [crossref]
- Karimi-Jafari M, Padrela L, Walker GM, Croker DM (2018) Creating Cocrystals: A Review of Pharmaceutical Cocrystal Preparation Routes and Applications. Crystal Growth & Design 18: 6370-6387.
- Semeniuk A, Kalinowska-Tluscik J, Nitek W, Oleksyn BJ (2008) Intermolecular interactions in crystalline hydroxychloroquine sulfate in comparison with those in selected antimalarial drugs. Journal of Chemical Crystallography 38: 333-338.
- Preston HS, Stewart JM (1970) The crystal structure of the antimalarial chloroquine diphosphate monohydrate. Journal of the Chemical Society D: Chemical Communications.
- Groom CR, Bruno IJ, Lightfoot MP, Ward SC (2016) The Cambridge Structural Database. Acta Crystallographica Section B 72: 171-179.
- Baruah J, Khakhlary P, Holland S, van Zyl RL (2016) Potent Anti-Malaria Salts, Co-Crystals and Derivatives of Aminoquinolines with Hydroxyaromatic Acids. Pharmaceutical Bioprocessing 4: 31-37.
- Ramon G, Davies K, Nassimbeni LR (2014) Structures of benzoic acids with substituted pyridines and quinolines: salt versus co-crystal formation. CrystEngComm 16: 5802-5810.
- Romañuk CB, Garro Linck Y, Chattah AK, Monti GA, Cuffini SL, et al. (2010) Crystallographic, thermal and spectroscopic characterization of a ciprofloxacin saccharinate polymorph. International Journal of Pharmaceutics 391: 197-202.
- Li J, Fu X, Li J, Kong M, Yu H, Wang J, et al. (2017) Quinine acesulfamates. Crystal Growth & Design 17: 58-66.
- Bruce-Chwatt LJ, Gibson FD (1959) Chloroquine per rectum for malaria in children. British medical journal 1: 894-896. [crossref]
- Westman L, Kamanda S, Hellgren U, Ericsson Ö, Rombo L (1994) Rectal administration of chloroquine for treatment of children with malaria. Transactions of The Royal Society of Tropical Medicine and Hygiene 88. [crossref]
- Onyeji CO, Osilana AO, Ogunbona FA, Akala E (1996) Chloroquine bioavailability following rectal administration in man. European journal of pharmaceutics and biopharmaceutics 42: 204-207.
- Antia-Obong OE, Alaribe AA, Young MU, Bassey A, Etim BV (1995) Chloroquine phosphate suppositories in the treatment of childhood malaria in Calabar, Nigeria. Current Therapeutic Research 56: 928-935.
- Tjoeng MM, Hogeman PHG, Kapelle H, De Ridder MLJ, Verhaar H (1991) Comparative bioavailability of rectal and oral formulations of chloroquine. Pharmaceutisch Weekblad 13:176-178. [crossref]
- Gerrard SE WJ, Bowers N, Salunke S, Hershenson S (2019) Innovations in Pediatric Drug Formulations and Administration Technologies for Low Resource Settings. . Pharmaceutics 11. [crossref]
- Okubanjo OO, Odeku OA (2009) Effect of interacting variables on the mechanical and release properties of chloroquine phosphate suppositories. Acta Pharmaceutica Sciencia 51: 281-288.
- Regdon JRG, Schirm I, Pittmann A, Regdon G (1995) Production technology and in vitro study of rectal suppositories containing chloroquine phosphate. Acta pharmaceutica Hungarica 65: 45-50. [crossref]
- Onyeji CO, Adebayo AS, Babalola CP (1999) Effects of absorption enhancers in chloroquine suppository formulations: I: In vitro release characteristics. European journal of pharmaceutical sciences 9: 131-136. [crossref]
- Kauss T, Langlois M-H, Guyonnet-Dupérat A, Phoeung T, Xie XY, et al. (2019) Development of rectodispersible tablets and granulate capsules for the treatment of serious neonatal sepsis in developing countries. Journal of pharmaceutical sciences 108: 2805-2813.
- Jannin V, Lemagnen G, Gueroult P, Larrouture D, Tuleu C (2014) Rectal route in the 21st Century to treat children. Advanced drug delivery reviews 73: 34-49. [crossref]
- Research Corporation Technologies, APT Completes Phase I Studies with Aerosolized Hydroxychloroquine for Asthma 2004.
- Klimke A, Hefner G, Will B, Voss U (2020) Hydroxychloroquine as an Aerosol Might Markedly Reduce and Even Prevent Severe Clinical Symptoms after SARS-CoV-2 Infection. Medical Hypotheses. [crossref]
- Musabayane C, Munjeri O, Matavire T (2003) Transdermal delivery of chloroquine by amidated pectin hydrogel matrix patch in the rat. Renal failure 25: 525-534. [crossref]
- Codebase Ventures Inc., Codebase to Invest in Company Developing Hydroxychloroquine Transdermal Treatment 2020.
- Prausnitz MR, Langer R (2008) Transdermal drug delivery. Nat Biotechnol 26: 1261-1268. [crossref]