DOI: 10.31038/CST.2017222
Abstract
Background: Breast cancer (BC) and its treatments lead to numerous side effects that affect a person’s life for years after treatment has ended. Research shows that regular exercise limits many of these side effects.However, less than 30% of BC survivors regularly exercise due to many barriers at both the patient and health care professional level. The purpose of this pilot trial is to assess the feasibility and effectiveness of conducting a novel KT intervention using exercise and self-management versus usual care among BC survivors.
Methods: Study Design: Pilot randomized controlled trial. Eligibility: Women older than 18 years who are currently undergoing chemotherapy treatment for BC. Intervention: The intervention group includes an 8-session multi-component intervention with a structured aerobic exercise program plus SM supervised by a physiotherapist. Randomization: Participants will be randomly allocated using a 1: 1 allocation ratio to receive the intervention of structured exercise plus SM program or usual care. Outcomes: The primary feasibility outcomes include recruitment rate, retention rate, and adherence rate. The secondary outcomes include exercise knowledge and behavior, HRQoL and resource utilization. Analysis: A blinded assessor will assess outcomes at baseline, post intervention, at 2- and 4-month follow up. Intervention feasibility and effectiveness will be assessed using descriptive statistics and analysis of covariance for continuous outcomes.
Discussion: This study aims to assess the feasibility of a novel KT intervention to close the current KT gap and increase exercise awareness for women with BC. This project will assess process and resource variables before implementation of a larger scale intervention. The overall project goal is to promote sustainable exercise behaviour to help manage the burden of BC.
Trial Registration: This trial was registered on ClinicalTrials.gov on March 21, 2017 (Identifier: NCT03087461).
Keywords:
Breast neoplasms, exercise, translational medical research, pilot study, rehabilitation
Introduction
Breast cancer (BC) and its treatments lead to numerous side effects that affect a person’s quality of life (QOL) for years after treatment has ended [1-5]. Research has shown that regular exercise limits many of these side effects and can prevent disease recurrence [5-10]. However, less than 30% of survivors participate in regular exercise [11-13]. Previous research conducted by our team has shown that over 80% of BC survivors in southwestern Ontario are unaware of the benefits of exercise and are not educated on the need to stay physically active [11], health professionals face many institutional, personal, and patient-related barriers to promoting exercise [14], and there is a need for novel knowledge translation (KT) strategies within cancer institutions that focus on easy-to-access exercise interventions and education by physiotherapists (PTs) [15].
Moreover, the societal burden of this disease is projected to increase substantially over the next two decades. The Canadian Cancer Society’s 2015 Statistics report [16] suggests that the number of new cancer cases in women will increase by 74% by the year 2032 due to the aging Canadian population. The number of new cases of BC is projected to increase by more than 10,000 in this same time period [16]. Fortunately, due to improved screening and treatment techniques, survival rates for BC are increasing [3]. However, physical and functional sequelae prevent survivors from returning to their activities associated with work, leisure, and domestic roles [3]. Exercise is an effective, safe, and cost-efficient way to manage this burden and return women to their pre-cancer activity levels. Therefore KT research is needed to determine how to best translate and integrate this research knowledge into clinical practice in order to elicit sustainable behaviour change for this population. Pilot work completed for this project has shown that in order to change clinical practice, implementation strategies using accessible exercise options and education are needed within the institution to maximize women’s engagement with exercise information provided and to partake in this behaviour [15]. Along with this, self-management (SM) programs have been shown to improve QOL and physical side effects in BC survivors, however, the implementation of these programs in clinical practice is scarce [17]. The current knowledge to practice gap in the field of BC rehabilitation shows that novel KT strategies are needed.
A pilot study is an, “investigation designed to test the feasibility of methods and procedures for later use on a large scale or to search for possible effects and associations that may be worth following up in a subsequent larger study” [18]. For this project, a pilot study is needed as the first step in order to assess process and resource variables before implementation of a large scale intervention [19]. Process variables include measuring recruitment rate, retention rate, and adherence rates to the intervention provided [19]. Resource variables include determining the centers willingness and capacity to house a specific intervention, equipment availability, intervention location, and budget concerns [19]. There is currently a lack of pilot trials for a novel KT intervention of this sort and therefore this pilot study will aid in shaping and guiding a larger, phase III trial.
Methods & materials
Study Purpose:
The purpose of this study is to determine whether KT strategies, focusing on accessible exercise locations and SM education by PTs using technology, are feasible and impact exercise knowledge and behaviour, QOL, and need for additional health care services among women with BC. Specifically, the objectives of this project are to: (1) Determine the feasibility (through recruitment, retention and adherence rates) of providing a complex KT intervention designed specifically for women with BC using technology, and (2) Determine preliminary estimates of effects of the KT intervention on levels of exercise knowledge and behaviour, health related quality of life and, resource utilization, among BC survivors over a four month period.
Study Design & Participants:
This study is a pilot randomized controlled trial. Eligible participants will include community-dwelling, English-speaking women, over 18 years, who are currently undergoing chemotherapy for Stage 1-3 BC and have been cleared by their oncologist to participate in moderate intensity aerobic exercise. Participants will be excluded from the study if they have another chronic disease, cognitive impairment or injury that prevents them from participating independently in moderate intensity exercise.
Recruitment:
Medical oncologists and Primary Care Nurses at the Juravinski Cancer Centre (JCC) will recognize possible participants for this study within their patient caseload. For those they think are eligible, the health care professional will briefly discuss the study with their patient and get consent for the patient to be contacted by a member of the research team. Possible participants will be contacted by phone to discuss eligibility and potential study enrollment. The Hamilton Integrated Research Board approved this study (reference #: 3124) in April 2017. All participants will provide written informed consent on the approved consent forms prior to enrollment in this study.
Intervention:
This pilot project will implement a multi-dimensional KT intervention including an exercise and SM program. Refer to Figure 1 for study flow chart.
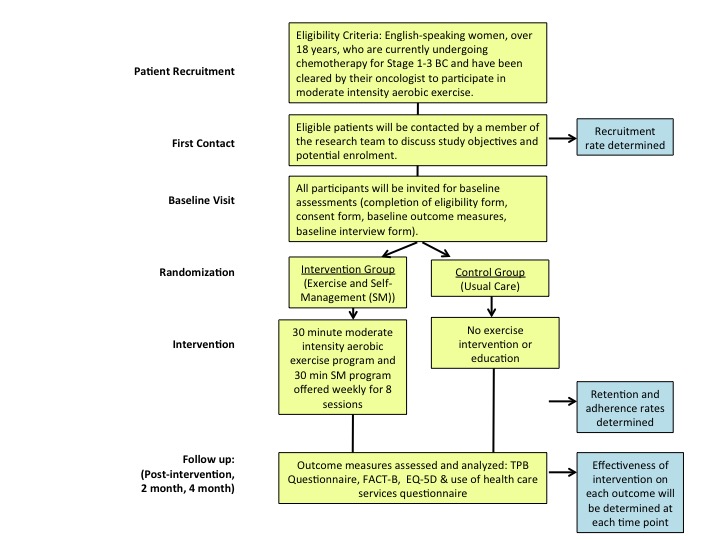
Figure 1. Study Flow Chart
The exercise intervention will involve an evidence-based moderate intensity aerobic exercise program, using recumbent bikes, delivered within the cancer institution. Results from participants in our focus group run during the pilot work for this project suggest that exercise programs should be delivered in the institution where women are waiting for their chemotherapy. Delivering the program in this environment will increase the accessibility of the services and we anticipate this approach will increase exercise awareness. Participants will take part in the 30-minute, moderate intensity (50-70%HRmax or 4-6/10 on Rate of Perceived Exertion scale) aerobic exercise program for 8 sessions. The intervention will be supervised by a PT educated in cancer rehabilitation and who has been trained in the specific protocol used and in working with women with BC.
The SM component will include educational modules created by a PT. Participants will view these 30 minute modules prior to each exercise intervention, over the same 8 sessions. Content provided within the program will include information on the benefits of exercise during and after BC treatment, safe exercise prescription, how to self-monitor exercise levels, action planning for specific exercise strategies, and precautions related to exercise and BC. Refer to Table 1 for details of SM content for each week of the program. A variety of tools will be used in the SM program, including a mobile app and e-health resources for BC. Having numerous sessions will allow the PT to provide consultation in respect to exercise adaptation, parameters, and programming in order to facilitate long-term exercise engagement and participation. Adherence and fidelity to the specified exercise and self-management protocol will be monitored through random observation by study investigators. (Figure 1, Table 1).
Table 1. Self-Management Content
| Session | Content |
| 1 | Introductions.
What are the side effects of treatment? Why do they happen? Benefits of exercise for women with breast cancer. Types of exercise and safety precautions during exercise (how to monitor BP, HR, RPE) What is self-management? How to participate in effective self-management. Self-management and breast cancer. Introduction to goal setting/action planning. |
| 2 | Review of previous week goal/action plan.
The importance of posture for women with breast cancer (common postural issues, how to assess posture, how to ensure optimal posture). Relaxation and breathing techniques to manage anxiety and stress. Set goal/action plan for week. |
| 3 | Review of previous week goal/action plan.
Appropriate exercise techniques to maintain/increase endurance: – Description of aerobic exercise – Types of aerobic exercise – Parameters for aerobic exercise Set goal/action plan for week. |
| 4 | Review of previous week goal/action plan
Appropriate exercise techniques to maintain/increase strength – Description of strengthening exercise – Types of strengthening exercise – Parameters for strengthening exercise Set goal/action plan for week. |
| 5 | Review of previous week goal/action plan
Other forms of Exercise (flexibility, yoga, Tia Chi, etc) Appropriate exercise techniques to maintain/increase flexibility: – Description of flexibility exercises – Types of flexibility exercises – Parameters for flexibility exercise Description of other forms of exercise: – Types of other forms of exercise – Parameters of other forms exercise Set goal/action plan for week. |
| 6 | Review of previous week goal/action plan.
Self-monitoring physical activity levels: – Introduction to Breast Cancer Physio Guide (App) – Introduction to Stanford Action Planning App – Other techniques to monitor physical activity levels Set goal/action plan for week. |
| 7 | Review of goal/action plan.
Communicating with others (family, health professionals) about exercise and physical activity. Available exercise programs in the community. How to move forward: how to evaluate progress. Set goal/action plan for week. |
| 8 | Review of goal/action plan.
Summary of self-management program. How did you use self-management information? Questions/comments. |
Outcomes:
Primary Outcomes: The feasibility and effectiveness of the KT intervention will be assessed using quantitative outcomes. The primary outcomes of feasibility variables will be assessed at baseline and post intervention, where applicable. Feasibility will be assessed by measuring recruitment (percentage (%) of eligible patients recruited), retention (% of consented patients who complete the intervention), and adherence rates (% of sessions attended) to the intervention).
Secondary Outcomes: Secondary effectiveness outcomes will be assessed at four time points: baseline, post intervention, and at 2 and 4 month follow up. At baseline, participants will be instructed on how to complete each self-report measure by an assessor blinded to participant group allocation. All post-intervention, 2 and 4 month follow up, assessments will be mailed to participants to complete and return to study investigators using pre-paid postage envelops. No identifiers will be used on these assessments and therefore assessors performing the analysis of data will be blinded to participant group allocation. Hard copies of the completed outcome measures will be stored in a locked filing cabinet only accessible by the study investigators at McMaster University and data entered into statistical analysis software will be stored on a password protected computer.
Level of exercise knowledge and behaviour will be assessed using a Theory of Planned Behaviour (TPB) [20] based questionnaire. The TPB has been used extensively to determine levels of intention and behaviour for various health behaviours, including exercise [21,22].
Quality of life will be measured using the FACT-B [23], a selfreport measure designed to assess multi-dimensional QOL specifically for women with BC.
Need for additional health care services will be measured using the EQ-5D [24] and a piloted self-report questionnaire assessing health care facility visits, doctor visits, procedures received, support services used, loss of work, and prescription medications used.
Sample Size:
Debate exists as to whether sample size calculations for pilot studies are necessary. Some authors suggest that no calculation is needed as long as the pilot study is large enough to provide useful information about the aspects that are being examined for feasibility [19]. However other authors suggest using a percentage of the sample required for a full study [25,26], or to use a confidence interval (CI) to establish feasibility [19,26]. For this project we have decided to calculate sample size based on the proportion of success of the primary outcome of feasibility (using estimates for adherence rates) [19,25,26].
Therefore, using a Z value from the standard normal distribution to reflect a 95% confidence interval (1.96), E as the desired margin of error (0.2), and p as the proportion of successes in the population (0.75-estimated adherence rates), the sample size for this pilot study will be at least 18 participants (9/group). With an expected drop out rate of 25%, based on previous exercise based literature with this population, the final sample size for this project should be 23 participants. In order to ensure an even number of individuals can be randomized to each group, this will be rounded up to a total of 24 participants (12/group).
Randomization-Sequence Generation:
Prior to participant randomizations, all eligible participants will complete the following forms: (a) patient information form, (b) Godin leisure time exercise questionnaire, (c) consent form, and (d) baseline measures. All participants will be informed verbally in person and in writing that they have equal chance of being randomized into the intervention or control group. They will not be made aware of the study hypotheses. Randomization to intervention or control group will be completed by a member of the research team who is independent of the intervention on a record by record basis using a computer software program (STATA/MP v14). This researcher will remain blind to the identity of each treatment group (by randomizing only to group A or B) during the randomization process. Participants may withdraw from the study at any time. Investigators may withdraw a participant from the research study if circumstances arise which warrant doing so (for example, safety).
Allocation Concealment & Implementation:
Allocation of participant randomization will be concealed in sequentially numbered, opaque, sealed envelops. The envelops will be opened sequentially by the researchers only after participant details have been written on the envelop by the researcher who completed randomization. If the participant is allocated to the intervention group, they will receive a phone call to organize details of the first intervention session (such as time and location).
Blinding:
Due to the nature of this knowledge translation study, participants and persons administering the intervention will not be blinded to group assignment. However, the assessor receiving the self-reported outcome measures will be blind to group allocation and will not be involved in running of the intervention. A researcher blinded to the group allocation of the participants will conduct all statistical analysis.
Statistical Methods:
Participant characteristics will be analyzed at baseline to ensure no significant differences exist between groups. Means and standard deviations (SD) will be used to report continuous variables and t-tests will be used to assess differences between the two groups for these variables. Frequencies will be used to report categorical variables and Pearsons X2 test will be used to assess differences between groups for these variables. All statistical analysis will be completed using STATA/ MP 14. Refer to Table 2 for a summary table of study objective and methodology.
Research Questions 1: Descriptive statistics will be used to measure feasibility (recruitment rate, retention rate, and adherence rates). Recruitment rates will be calculated by determining the percentage of eligible patients that were actually enrolled in the study. A recruitment log will be kept, detailing reasons for non-participation of eligible patients. Retention rate will be defined by calculating the percentage of enrolled patients who complete the intervention. Adherence rates will be calculated as a percentage of total sessions attended. Attendance will be tracked on the feasibility data collection sheet and reasons for non-participation on scheduled intervention days will be documented using an adherence log.
Table 2. Summary Table
| Objective | Hypothesis | Outcome | Analysis Method |
| Determine the feasibility (through recruitment, retention and adherence rates) of providing a complex KT intervention including accessible exercise programs delivered within the cancer institution and a SM program designed specifically for women with BC using technology | Implementing and providing a complex KT intervention for women with BC is feasible (recruitment, retention, and adherence rates are 50%, 75%, and 75% respectively). | Recruitment Rate | Descriptive statistics (percentage (%) of eligible patients recruited). Recruitment logs will be kept, detailing reasons for non-participation of eligible patients. |
| Retention Rate | Descriptive statistics (% of consented patients who complete the intervention). | ||
| Adherence Rate | Descriptive statistics (% of sessions attended). Adherence rates will be tracked using the data collection sheet. Reasons for non-participation on scheduled intervention days will be documented using adherence logs. | ||
| Determine preliminary estimates of effects of the KT intervention of exercise plus SM versus usual care among BC survivors over a four-month period. | Compared to the control group, BC survivors participating in the KT intervention will have higher levels of exercise knowledge and behaviours and QOL, and less need for additional health care services.
|
Levels of exercise knowledge and behaviour (using a Theory of Planned Behaviour based questionnaire) | An analysis of covariance will be used to determine within and between group differences. An intention to treat analysis will be used for these analyses. |
| Health related quality of life (using the FACT-B) | |||
| Resource utilization (using the EQ-5D and a piloted self-report questionnaire assessing health care facility visits, doctor visits, procedures received, support services used, loss of work, and prescription medications used) |
Research Question 2: Effectiveness outcomes will be assessed at four time points: baseline, 12 weeks (post intervention), and at 2 and 4 month follow. An analysis of covariance will be used to determine within and between group differences. An intention to treat analysis will be used for these analyses. (Table 2)
Discussion
There is a small risk of participant injury during the exercise intervention. While it has been well documented that exercise is safe for this population if proper screening and precautions are followed, minor injuries have the potential to occur in any active intervention. In order to ensure safety, all participants will need medical clearance to participate in moderate intensity exercise from their medical oncologists and will be supervised by a physiotherapist during each session. Heart rate, blood pressure, rate of perceived exertion and oxygen saturation measurement tools will be present and used during each exercise session. While uncommon, all side effects secondary to exercise will be tracked using Exercise logs. Type, intensity, duration, and management of any side effect will be documented using these logs.
The findings of this KT pilot study will help to determine the feasibility and preliminary effectiveness of a novel implementation strategy. This project will inform a larger intervention trial which has the potential to change the way rehabilitation services are provided in clinical practice and impact all levels of BC prevention; secondary and tertiary prevention of treatment-related side effects, and primary prevention of disease recurrence through sustained behaviour change. The results from this pilot project should be interpreted with an understanding of the potential threats to the generalizability of the results. Specifically, the results of this pilot study will only be relevant to the implementation of a larger intervention at sites comparable to the JCC and will be specific to the unique characteristics of women with BC. As the intervention process and management is more extensive with larger numbers of participants, the researcher team will have to take into consideration additional time and resource needs when implement the larger scale project.
Trial status
Protocol Version Number: 3. Date: April 11, 2017. Approximate recruitment start date: June, 2017. Approximate recruitment end date: August, 2017. Any trial modifications will be updated in a timely manner on ClinicalTrials.gov and sent via email to appropriate parties.
Funding
The Hamilton Division of the Ontario Physiotherapy Association funds this project. This funding body has no role in the design of the study, collection, analysis or interpretation of data, or in writing of this or future manuscripts.
References
- Shapiro SL, Lopez AM, Schwartz GE, Bootzin R, Figueredo AJ, et al. (2001) Quality of life and breast cancer: relationship to psychosocial variables. J Clin Psychol 57: 501-519. [crossref]
- Wengström Y, Häggmark C, Strander H, Forsberg C (2000) Perceived symptoms and quality of life in women with breast cancer receiving radiation therapy. Eur J Oncol Nurs 4: 78-88. [crossref]
- Ewertz M, Jensen AB (2011) Late effects of breast cancer treatment and potentials for rehabilitation. Acta Oncol 50: 187-193. [crossref]
- Knobf MT, Sun Y (2005) A longitudinal study of symptoms and self-care activities in women treated with primary radiotherapy for breast cancer. Cancer Nurs 28: 210-218. [crossref]
- Cella D, Fallowfield LJ (2008) Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat 107:167–180.
- Cormie P, Pumpa K, Galvao D, et al. (2013) Is is safe and efficacious for women with lymphedema secondary to breast cancer to lift heavy weights during exercise: a randomized controlled trial. J Cancer Surviv 7:413-424.
- Pastakia K, Kumar S (2011) Exercise parameters in the management of breast cancer: a systematic review of randomized controlled trials. Physiother Res Int 16: 237-244. [crossref]
- Chan D, Lui L, So W (2010) Effectiveness of exercise programmes on shoulder mobility and lymphedema after axillary lymph node dissection for breast cancer: systematic review. J Adv Nurs 66:1902-1914.
- Bicego D, Brown K, Ruddick M, Storey D, Wong C, et al. (2009) Effects of exercise on quality of life in women living with breast cancer: a systematic review. Breast J 15: 45-51. [crossref]
- McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, et al. (2006) Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ 175: 34-41. [crossref]
- Fernandez S, Franklin J, Amlani N, et al. (2015) Physical activity and cancer: A cross-sectional study on the barriers and facilitators to exercise during cancer treatment. Can Oncol Nurs J 42:37-72.
- Courneya KS, Katzmarzyk PT, Bacon E (2008) Physical activity and obesity in Canadian cancer survivors: population-based estimates from the 2005 Canadian Community Health Survey. Cancer 112: 2475-2482. [crossref]
- Cheifetz O (2013) Clinician’s Commentary on Singh et al.(1.). Physiother Can 65: 192-193. [crossref]
- Smith-Turchyn J, Richardson J, McNeely M, et al. (2017) Physical activity and breast cancer: A study on the barriers and facilitators to exercise promotion from the perspective of health care professionals. Physiother Can
- Smith-Turchyn J, Richardson J, Tozer R, et al. (2016) Physical activity and breast cancer: Results of a focus group to devise novel exercise interventions for women with breast cancer. (currently under review in the journal “Rehabiliation Oncology”)
- Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian Cancer Statistics 2015. Toronto, ON, Canadian Cancer Society, 2015.
- Boogaard L, Gater L, Mori M, et al. (2016) Efficacy of self-management programs in managing side effects of breast cancer: A systematic review and meta-analysis of randomized control trials. Rehabil Oncol 34:14-26.
- Everitt B (2006) Medical Statistics from A to Z: A Guide for Clinicians and Medical Students. Cambridge University Press: Cambridge.
- Thabane L, Ma J, Chu R, Cheng J, Ismaila A, et al. (2010) A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol 10: 1. [crossref]
- Ajzen I (1991) The theory of planned behavior. Organ Behav Hum Dec 50:179-211.
- McEachan RR, Conner M, Taylor NT, Lawton RJ (2011) Prospective prediction of health- related behaviours with the Theory of Planned Behaviour: a meta-analysis. Health Psych Rev 5:97-144.
- Smith-Turchyn J, Richardson J (2015) Critical Review of the Theory of Planned Behavior to Predict and Explain Exercise Behavior in Women with Breast Cancer. Crit Rev Phys Rehabil Med 27
- Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, et al. (1997) Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol 15: 974-986. [crossref]
- Pickard AS, Wilke CT, Lin HW, Lloyd A (2007) Health utilities using the EQ-5D in studies of cancer. Pharmacoeconomics 25: 365-384. [crossref]
- Hertzog MA (2008) Considerations in determining sample size for pilot studies. Res Nurs Health 31: 180-191. [crossref]
- Cocks K, Torgerson DJ (2013) Sample size calculations for pilot randomized trials: a confidence interval approach. J Clin Epidemiol 66: 197-201. [crossref]