Abstract
There have been great advances in the bone-grafting field. Grafts to enhance porosity, mechanical strength, and compatibility are currently being created and studied. As has been stated in previous literature, porosity of a bone-grafting scaffold is essential for the infiltration of cells and nutrients; which will enhance the compatibility. Currently, most researchers are looking at the introduction of various calcium phosphates to scaffolds and the use of bioactive glass to enhance the mechanical integrity of the graft. Reviews on such bone grafts are described in this article (Figure 1).
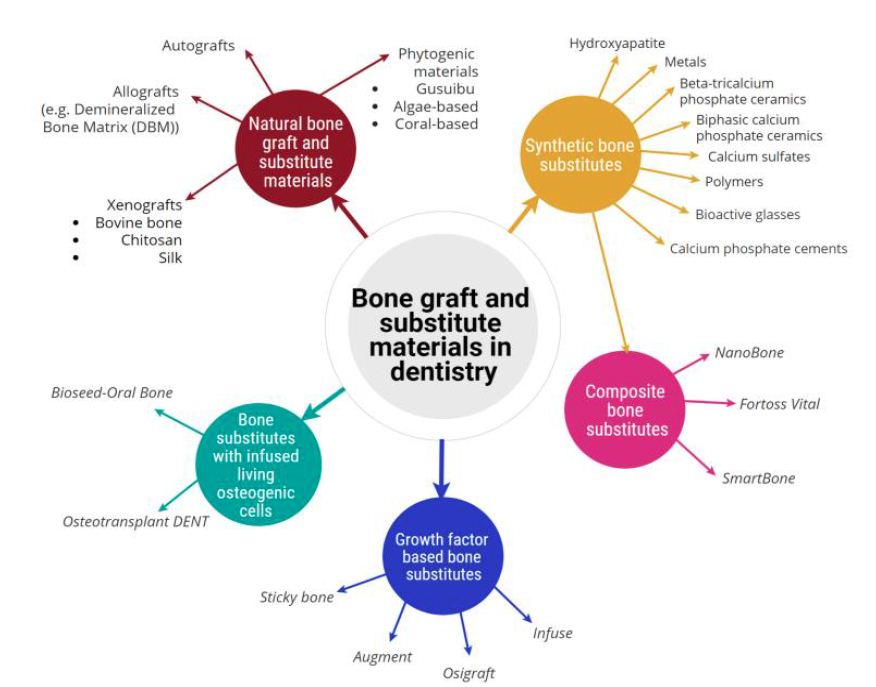
Figure 1: Classification of bone graft and substitute materials used in dentistry, broadly classified into five categories and showing their associated sub-categories [30].
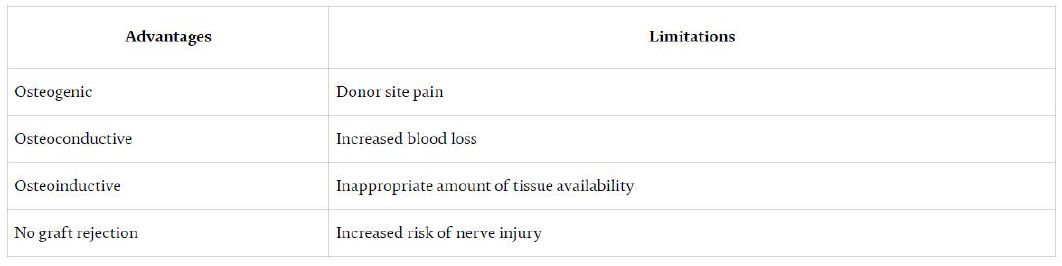
Bone grafting is a surgical procedure that rebuilds bone by transplanting bone tissue. A dental bone graft is often required, if a patient has lost one or more adult teeth or has developed gum diseases as both problems can cause bone loss. After tooth loss, bone resorption is irreversible, leaving the area without adequate bone volume for successful dental procedures. Bone grafting is the only solution to reverse dental bone loss and is a well-accepted procedure. Bone grafts are used as pillar and scaffold over which regeneration and healing takes place. A dental graft adds volume and density to the jaw in areas where bone loss has occurred. Bone Grafting techniques have been used by specialists for more than 100 years. Many factors are involved in the successful incorporation of a graft material, including graft type, preparation site, vascularity, mechanical strength and pore size of the materials. These parameters make the use of bone substitutes challenging in terms of reliability and Predictability.1 Bone grafts are generally evaluated based on their osteogenic, osteoinductive or osteoconductive potential. Materials to be grafted can be obtained from the same person (autograft), from a different person of the same species (allografts), or from a different species (xenografts) (Table 1).
Table 1: Some of the common advantages and disadvantages associated with autograft [29]
History
The use of bone grafts for reconstructing intra-osseous defects produced by periodontal disease dates back to Hegedus in 1923. It was then revived in 1965 by Nabers and O’Leary.Buebe and Silvers used boiled cow bone powder to successfully repair intra-bony defects in humans. Force berg used Ox purum in 11 human intra-bony defects. Melcher and Dent used an organic bone in bovine bone in bone defects, which showed sequestration and slow resorption militated against the use of organic bone. Scopp used Boplant bovine bone and reported pocket depth reduction at 6 months. Now, with the introduction of advanced bone grafting techniques, it is possible to increase the volume, width, and height of bone in deficient areas [1-5].
The biologic mechanisms that provide a rationale for bone grafting are osteoconduction, osteoinduction and osteogenesis [6].
Osteogenesis
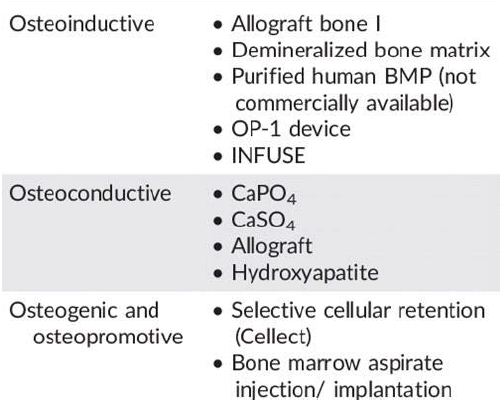
Osteogenesis is the ability of the graft to produce new bone and this process is dependent on the presence of live bone cells in the graft i.e. It occurs when vital osteoblast, originating from bone graft material, contributes to the new Growth of new bone along with bone formation. Osteogenic graft material contain viable cells with the ability to form bone (osteoprogenitor cells) or the potential to differentiate into bone forming cells including Osteogenic precursor cells. Osteogenesis is a property found only in fresh autologous bone and in bone marrow cells.
Osteoconduction
It is a physical property of a bone graft material to serve as a scaffold for viable bone healing and new bone growth, which is perpetuated by the native bone. It allows for the growth of neovasculature and infiltration of osteogenic precursor cells into the graft site.Osteoconductive properties are found in cancellous bone autograft and allograft demineralized bone matrix, hydroxyapatite, collagen and calcium phosphate. Osteoblast forms the margin of defect that is being grafted, Utilizing the bone graft material as a framework upon which to spread and generate new bone. In the very least, a bone graft material should be osteoconductive.
Osteoinduction
Osteoinduction is the ability of graft material to induce stem cells to differentiate into mature bone cells.The process is typically associated with presence of bone growth factors within the graft material or a supplement to bone graft.It Involves stimulation of osteoprogenitor cells to differentiate into osteoblast and then begin formation of new bone. The most widely studied type of osteoinductive cell mediator is BMP.4 A bone graft material that is osteoconductive and Osteoinductive will not only serve as a scaffold for currently existing osteoblasts, but will also trigger formation of new osteoblasts promoting faster integration of the graft.
Osteo Promotion
It involves Enhancement of osteoinduction without possession of osteoinductive properties.For example, enamel matrix derivative enhances the osteoinductive effect of the demineralized freeze dried bone allograft (DFDBA) but will not stimulate bone graft alone (Figure 2).
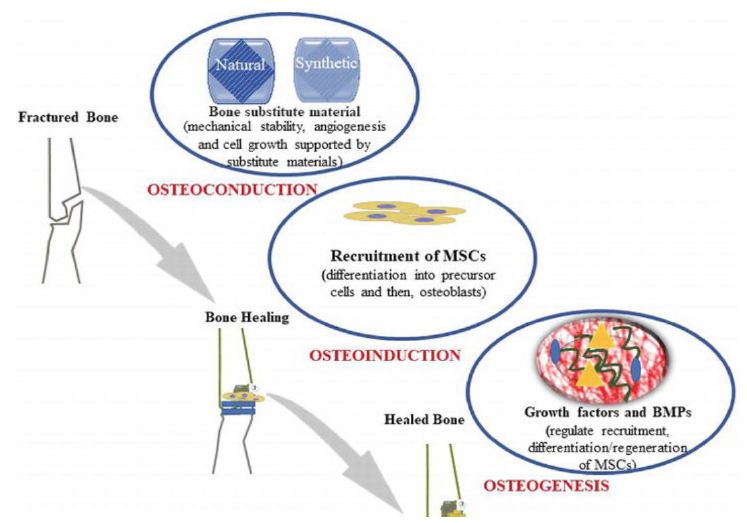
Figure 2: Schematic representation shows the process of bone graft substitutes [29]
Classification of Bone Graft [7]
Based on the type of graft used:
- Particulate
- Putty
- Block.
These are available as large or small particles, a combination of porosities, and from specific locations of origin (e.g. cortical, cancellous).
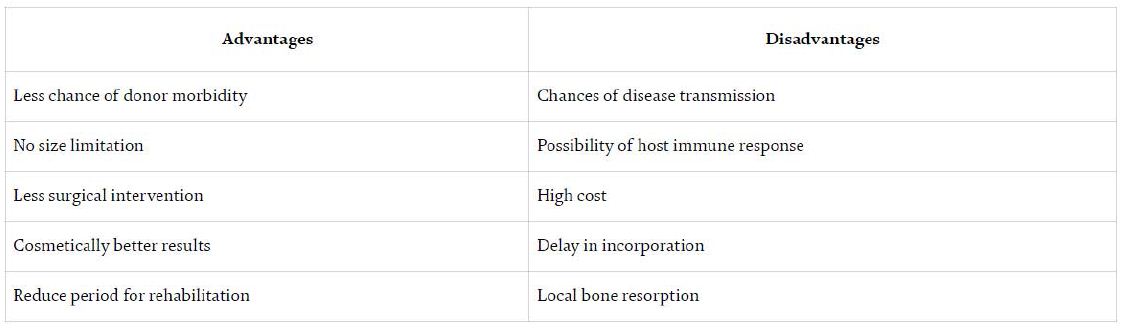
Based on the source (Table 2):
- Autograft
- Allograft
- Xenograft
- Alloplast
Table 2: Common advantages and disadvantages associated with an allograft [29]
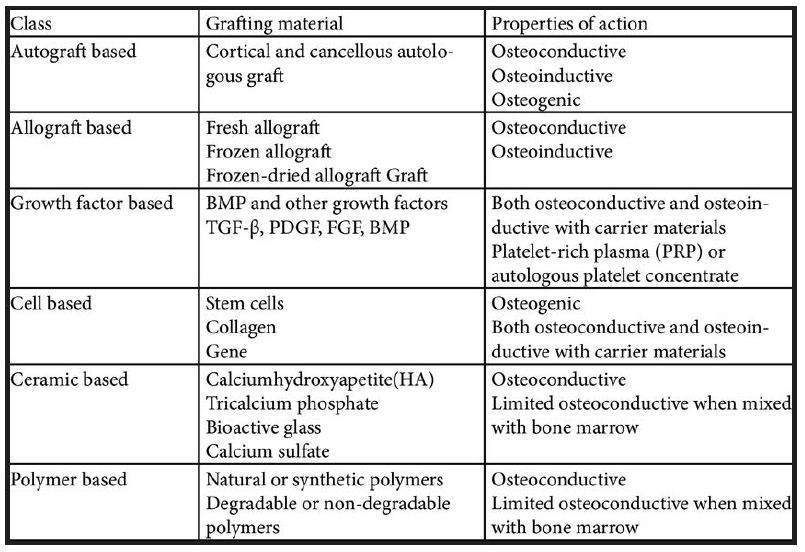
Based on Bone Graft Substitutes (Laurencin):
- Allograft based
- Factor based
- Cell based
- Ceramic based
- Polymer based.
Allograft based:
- Allograft bone used alone or in combination
- For example: allegro, orthoblast, graft-on
- Action: osteoconductive, osteoinductive
Factor based:
- Natural and recombinant growth factor used alone or in combination
- For example: Transforming growth factor-beta, platelet-derived growth factor, fibroblast growth factor, BMP
- Action: Osteoinductive, osteoinductive, and osteoconductive with carrier materials.
Cell based:
- Cells used to generate new tissue alone or seeded onto a support matrix • For example: Mesenchymal stem cells
- Action: osteogenic, both osteogenic and osteoconductive with carrier materials.
Ceramic based:
- Includes calcium phosphates, calcium sulfate, and bioactive glass used alone or in combination
- For example: Osteograft, osteoset, Novabone • Action: Osteoconductive, limited osteoinductive when mixed bone marrow.
Polymer based:
- Includes degradable and nondegradable polymers used
- For example: Cortoss, OPLA, Immix
- Action: Osteoconductive, bioresorbable in the degradable polymer (Table 3).
Table 3: Bone graft and bone graft substitutes
Indications of Bone Grafts
- Deep intraosseous defects-two-walled and three-walled defects
- Tooth retention
- Support for critical teeth-abutment tooth
- Bone defects associated with juvenile periodontitis
- Esthetics (shallow intraosseous defects)
- Furcation defects-Grade II, III furcation
- Ridge augmentation
- Sinus lifting procedure
- Regeneration around implants
- Filling donor site bone defects (Figure 3) [8].
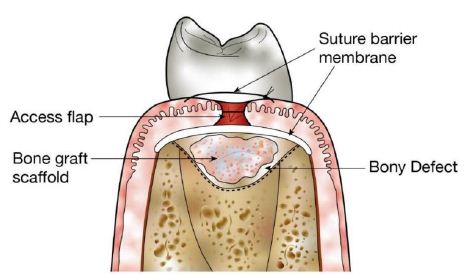
Figure 3: Use of structural scaffolds to restore bony defects. Diagram shows placement of a bone graft scaffold within a bony defect in alveolar bone following surgical generation of an access flap.
Ideal Requisites of Bone Grafts
- Osteoinductive property
- Non-toxic
- Resistant to infection
- No root resorption or ankylosis
- Non-antigenic and biologic compatibility
- Easily adaptable and available
- Predictability
- Strong and resilient
- Require minimal surgical intervention
- Rapid vascularization
- Should stimulate new attachment and be able to trigger osteogenesis [9].
Bone Morphogenic Protein (BMP)
BMP’s are members of the family of transforming growth factors. 15 different bmp’s have been identified all having different degrees of cellular activity, including cartilage or bone inducing properties. Two recombinant proteins are available at present- recombinant human bone morphogenic protein (rhBMP-2) and (rhBMP-7). Two rhBMP associated carrier systems have received approval from the US Food and Drug Administration. 1) Osteogenic protein-1 (OP-1) consists of rhBMP-7 and bovine collagen (Stryker Biotech Hopkinton, Massachussetts) 2) InFuse System (Medtronic Sofamor Danek Warsaw, Indiana) consists of rhBMP-2 on an absorbable bovine type I collagen sponge carrier. BMP product is packaged as a lyophilized powder in a sterile vial which can be reconstituted with sterile water and applied to the carrier (Table 4) [10].
Table 4: Bone graft Substitutes [31]
Platelet Rich Plasma (PRP)
PRP is a source of platelet derived growth factor (PGDF) and transforming growth factor beta (TGF-b) that is obtained by sequestering and concentrating platelets by a process of gradient density centrifugation [11].
Platelet Derived Growth Factor (PDGF)
PDGF, a glycoprotein has a molecular weight of approximately 30kd. It was first described in the alpha granules of platelets, but can also be synthesized and secreted by cells like macrophages and endothelium. There are approximately 0.06ng of PDGF per one million platelets, a fact that emphasizes this molecule’s great potency. Its mechanism is to activate cell membrane receptors on target cells, which results in the development of high-energy phosphate bonds on internal cytoplasmic signal proteins which then activate the signal proteins which initiate a specific activity within the target cell. The most specific activities of PDGF are mitogenesis, angiogenesis and macrophage activation [12,13].
TGF-b
The term transforming growth factor beta is applicable to the superfamily of growth and differentiating factors. Bone morphogenic protein (BMP) is a member of this family and contains at least 13 BMPs. TGF-b1 and TGF-b2 are proteins that have molecular weight of approximately 25kd [14]. Like PDGF, they are synthesized and found in macrophages as well as in other cell types. When released by platelet degranulation or actively secreted by macrophages, they act as paracrine growth factors and affect cells such as fibroblasts, marrow stem cells and preosteoblasts. Each of these target cells has the ability to synthesize and secrete its own TGF-b proteins. TGF-b therefore represents a mechanism for sustaining a long term healing process and even develops into a bone remodeling factor. The most important functions are chemotaxis and mitogenesis of osteoblast precursors. They also have the ability to stimulate osteoblast deposition of the collagen matrix of wound healing and bone. In addition TGF-b inhibits osteoclast formation thus favoring bone formation over resorption [15].
Biocompatible Bone Graft Material
Erbe developed a biocompatible bone graft material with a biocompatible, resorbable polymer and a biocompatible, resorbable inorganic material exhibiting macro, meso, and microporosites. This invention incorporates the benefits of inorganic shaped bodies having a macro, meso, and microporosity and polymers such as collagen. Different stoichiometric compositions of calcium phosphate such as hydroxyapatite (HaAP), tricalcium phosphate (TCP), tretacalcium phosphate (TTCP), and other calcium phosphate salts and minerals, have all been employed to match the biocompatibility, structure, and strength of natural bone. The role of pore size and porosity in promoting revascularization, healing, and remodeling of bone has been recognized as a critical property for bone grafting materials. To enhance porosity, this invention includes an oxidation- reduction product of at least one metal cation, at least one oxidizing agent, and at least one oxidization precursor anion. The reaction-product may be inorganic compositions comprising calcium phosphate, biphasic calcium phosphate, or beta tri-calcium phosphate (-TCP). The oxidationreduction product gives the present invention graft material macro, meso, and microporosity, which allow the graft material to have extraordinary absorption properties. The inclusion of a polymer, such as the structural protein collagen, lends to improved handling and flexibility. The porosity and large pore distribution (1 μm-1000μm) of these bone grafts increases their ability to imbibe fluids such as bone marrow aspirate, blood, or saline and cell loaded solutions (e.g. fibroblasts, mesenchymal, stromal, marrow and stem cells) for use in vivo. Applications of this property include the ability to incorporate growth factors such as BMP into the graft to enhance healing. The flexibility of the bone graft allows the graft to be shaped into any basic shape, including cylinder, blocks, strips, sheets, and wedges. This graft may also serve as a coating on any orthopaedic appliance. Further, unlike traditional bone graft substitutes, this invention is highly compressible and therefore can be packed to insure maximum contact with adjacent bone for beneficial healing of a bony defect [16-18].
Porous Ceramic Composite Bone Grafts
This porous ceramic composite developed by Smith incorporates biodegradable polymers (polycaprolactone) for use as a bone substitute in the field of orthopedics and dentistry or as a scaffold for tissue engineering applications. The biodegradable polymer allows for the passage and/or delivery of a variety of agents throughout the porous ceramic matrix and improves mechanical properties of the implant in vivo. A disadvantage of current commercially available bone grafts is their poor mechanical properties, which limits the use of these implants to non-load bearing applications. Therefore, the main focus of this particular bone graft is to enhance the mechanical properties through the use of a porous ceramic composite without the risk of articulating debris. The bone graft is a porous bone substitute that can limit fragmentation, and migration of debris during standard orthopedic fixation practice [19,20].
The graft, composed of a porous osteoinductive ceramic matrix and a biodegradable polymer, possesses optimum pore size, pore size distribution, porosity, and pore connectivity to promote the rapid in-growth of bone tissue upon implantation. In comparison to prior ceramic bone grafts, this graft has advantageous mechanical properties as a result of repeatedly coating the organic substrate with a mixture of thickening agents (slurries) varying in solid loading. The coated structure is heated to burn away the flexible organic foam and then sintered, thereby providing a fused, ceramic foam having many interconnected voids. When used as a biodegradable polymer coating, it helps to improve functional (mechanical) properties of the implant in vivo. In summary, the porous ceramic graft presented by Smith has numerous advantages and uses in the field of orthopedics and dentistry both in vitro and in vivo. As an implant, the graft can be used in both non-load bearing and load bearing applications [21,22].
Bioactive Bone Graft Substitute – Collagen Enhancement
Clineff proposed a biocompatible bone graft composed of resorbable calcium phosphate, resorbable collagen, and bioactive glass. The invention is a composite of biocompatible, resorbable, substantially homogeneous blend of calcium phosphate having maco-, meso-, and microporosity. The graft replicates the natural osteoactivity of native bone by the addition of a bioactive glass. Bioactive glasses explored in the invention include glass-ceramics, crystalline phase materials, and a combination of acrylic polymerizable species. The purpose of the bioactive glass is to react as it comes in contact with physiologic fluids including, but not limited to, blood and serum. The reaction of the bioactive glass and the surrounding fluid will lead to bone formation by forming an apatite layer on the surface of the graft. The bioactive glass can have a glass ceramic composition comprised of heterogeneous particles with an irregular morphology and regions of crystallinity. Similar to other biocompatible synthetics bone grafts, collagen is included to enhance the ability of the graft to be shaped or cut using various instruments such as scalpel and scissors. Some basic shapes may be a disk, semi-sphere, semi-tube, or torus. Collagen and bioactive glass is combined with calcium phosphate by blending the mixture to form a homogeneous mixture and a composite matrix of various shapes and sizes [23,24].
The proposed graft materials may act as both a barrier to prevent migration of other implants or graft materials and serve as an osteoconductive resorbable bone graft capable of promoting bone formation. The bone graft will reabsorb following delivery to the surgical site. The inclusion of a bioactive glass as an osteoinductive component is believed to be novel bone technology application [25].
Growth Factor Encapsulation System for Enhancing Bone Formation
Lu developed a bone technology, which enhances bone formation by releasing various growth factors and/or platelet-rich plasma (PRP) from a solid material. PRP is known to contain a number of autologous thombocyte growth factors that may aid in the acceleration of bone regeneration. These growth factors include platelet-derived growth factor (PDGF) and transforming growth factors (TGF-1); both are produced by platelets and released during granulation. PDGF stimulates mitogenesis of osteoblastic precursors while TGF-1 stimulates proliferation and collagen synthesis by osteoblasts and osteoblast precursors. PRP gel has most recently been used as an adhesive with cancellous bone particles in oral and maxillofacial surgery bone grafting procedures. The invention is comprised of a capsule of protein-permeable material having growth factor therein, releasable calcium alginate porous beads with encapsulated growth factors, a PRP gel, and a bone regeneration facilitating material [26].
The bone regeneration facilitating material is a solid material or scaffold, which serves as facilitator for the formation of new bone by bone-forming cells. Such materials include collagen, BioOss (calcium phosphate-based bone graft substitute), Pepgen P-15 (synthetic P-15 peptide bound to natural form of hydroxylapatite) and AlloGraft (demineralized bone matrix, allograft-based bone graft substitute). The bone graft is designed so that the contained growth factors can be released and delivered to a desired location site when implanted. The alginate porous beads having autologous PRP contained therein allows the growth factors to be released from the PRP and then released from the bead for delivery to the defect location. The controlled release of this invention is crucial to the enhancement of bone regeneration because the growth factors can be released at varying stages throughout the natural healing process. Chitosan beads are also explored and mentioned in this patent as a possible containment for growth factors/PRP. This novel hydrogel delivery system permits prolonged and modulated release of growth factors relevant to bone regeneration [27].
Polymeric Bone Defect Filler
Deslauriers propose bone defect filler for implantation in a bone defect of patients. The bone filler includes a particulate polymer distributed within a polymeric binder. The particulate polymer includes a plurality of particles, which may have the same materials as the polymeric binder. The particles within the particulate polymer may take on a variety of shapes and/or sizes to provide the bone defect filler with improved pore interconnectivity, materials expansion, and contamination characteristics. The proposed bone defect filler also maintains sufficient mechanical strength and handling characteristics for bone repair applications. The presented polymeric bone defect filler is advantageous to current synthetic nondegradable bone defect fillers that maintain their chemical and mechanical properties, such as titanium. Synthetic bone fillers may have poor tensile and shear properties. They also have poor adhesion properties and therefore can be washed out of the defect area before the in growth of new bone. Conventional bone grafting technologies such as the use of PMMA, are problematic because, as permanent bone fillers, they are not resorbable and/or cannot be molded and shaped for in situ curing. A similar bone technology to the proposed innovation is the use of particulate polymer mixed with biological fluids, but the particulate polymer and fluid mixtures tend to adhere poorly to the surround bone and also exhibit low initial structural properties, e.g. tensile and compressive, after implantation [28].
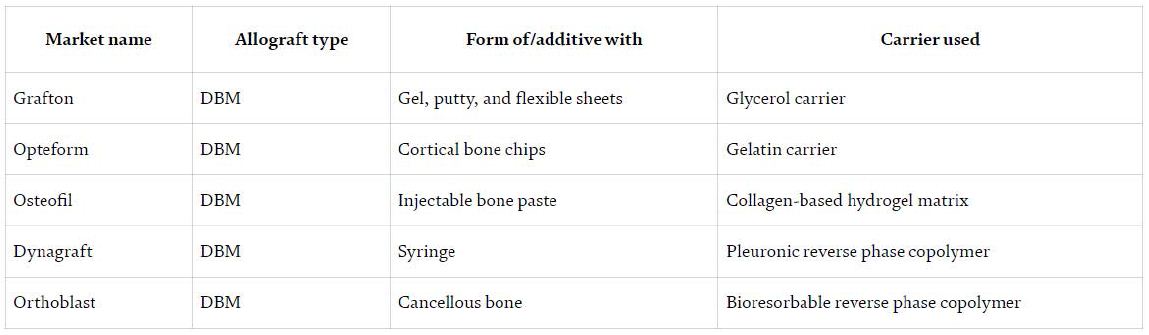
DBM possesses most of the biological properties of native bone that are important for successful bone grafting. The present bone morphogenic proteins in DBM signal stem cells to differentiate into osteoprogenitor cells to product new bone; making DBM osteoinductive. DBM is also osteoconductive in that it supports neovascularization and invasion of osteoblasts. The DBM can be made from the same species as the recipients or from a different species, with similar genetic alterations as the ATM [29]. The inventors of this bone technology are able to create ATM and DBM in multiple forms including fibers, particles, or threads. The final product or bone graft can be composed of any combinations of forms of ATM and any form of DBM (e.g. fibers of ATM and particles of DBM) and freeze dried for prolonged storage (Table 5).
This particular bone graft, held in place by sutures, can be wrapped around a bone that is damaged or that contains a defect, placed on a surface of a bone that is damaged or defected, or placed at a non-bony site to induce bone formation [30,31].
Table 5: The types of DBM bone graft substitute which is commercially available [29]
Conclusion
Bone graft and substitute materials which are either in the form of particulate or blocks are mostly used in dentistry to regenerate the missing hard tissue structures. There is a high and growing demand for new and more efficient dental grafting materials. Current bone graft and substitute materials primarily serve as a structural framework for osteo-regenerative processes that only satisfy the osteoconductivity criteria. r understanding of these materials and the growth factors at the molecular level is growing, which allows us to better control and modify their structure, understand their surface properties, and tune the interaction with other materials or physiological environment. This progress will eventually allow us to design and develop more effective dental bone substitutes. Despite the progress highlighted in this review article more work is needed to develop dental biomaterials that have a porous structure, mechanically stability, controlled degradation, and remodeling ability which is comparable with the rate of new bone formation.
References
- Fetner AE, Low SB, Wilson J, Hench LL (1987) Conducted a study to evaluate the particulate form of bioglass periodontal defects.
- Hegedus Z (1923) The rebuilding of the alveolar process by bone transplantation. Dent Cosmos 65: 736.
- Nabers CL, O’leary TJ (1965) Autogenous bone transplants in the treatment of osseous defects. J Periodontol 36: 5-14. [crossref]
- Melcher AH, Dent HD (1962) The use of heterogenous anorganic bone as an implant material in oral procedures. Oral Surg Oral Med Oral Pathol 15: 996-1000.
- Scopp IW, Morgan FH, Dooner JJ, Fredrics HJ, Heyman RA (1966) Bovine bone (boplant) implants for infrabony oral lesions. Periodontics 4: 169-176.
- Baldwin P, Li DJ, Austin DA, Mir HS, Yoon RS, et al. (2019) Autograft allograft bone graft substitutes. Clinical evidence and indication for use in the setting of orthopedic traumatic surgery. J Orthop Trauma. [crossref]
- Mellonig JT (1992) Autogenous and allogeneic bone grafts in periodontal therapy. Crit Rev Oral Biol Med 3: 333-352.
- Borghetti A, Novakovitch G, Louise F, Simeone D, Fourel J (1993) Cryopreserved cancellous bone allograft in periodontal intraosseous defects. J Periodontol 64: 128-32. [crossref]
- Jangid MR, Rakhewar PS, Nayyar AS, Cholepatil A, Chhabra P (2016) Bone Grafts and bone graft substitutes in periodontal regeneration: A review. Int J Curr Res Med Sci 2: 1-7. [crossref]
- Piattelli M, Favero GA, Scarano A, Orsini G, Piattelli A (1999) Bone reactions to anorganic bovine bone (Bio-oss) used in sinus augmentation procedures: A histologic long-term report of 20 cases in humans. Int J Oral Maxillofac Implants 14: 835-40. [crosssref]
- Mahesh J, Mahesh R, John J (2012) Predictability of bone regeneration in periodontal surgery – A review. IOSR J Dent Med Sci 2: 46-50.
- Ashman A (1992) The use of synthetic bone materials in dentistry. Compendium 13: 1020.
- Gross JS (1997) Bone grafting materials for dental applications: A practical guide. Compend Contin Educ Dent 18: 1013-8, 1020-2. [crossref]
- Yagihashi K, Miyazawa K, Togari K, Goto S (2009) Demineralized dentin matrix acts as a scaffold for repair of articular cartilage defects. Calcif Tissue Int 84: 210-20. [crossref]
- Ritchie HH, Ritchie DG, Wang LH (1998) Six decades of dentinogenesis research. Historical and prospective views on phosphophoryn and dentin sialoprotein. Eur J Oral Sci 106: 211-20.
- Oonishi H, Kushitani S, Yasukawa E (1997) Particulate bioglass compared with hydroxyapatite as a bone graft substitute. Clin Orthop Relat Res 334: 316-25. [crossref]
- Ten Huisen KS, Brown PW (1998) Formation of calcium-deficient hydroxyapatite from alpha tricalcium phosphate. Biomaterials 19: 2209-17. [crossref]
- Eppley BL, Pietrzak WS, Blanton MW (2995) Allograft and alloplastic bone substitutes: A review of science and technology for the craniomaxillofacial surgeon. J Craniofac Surg 16: 981-989. [crossref]
- Harris RJ (2004) Clinical evaluation of a composite bone graft with a calcium sulfate barrier. J Periodontol 75: 685-692. [crossref]
- Hench LL (2006) The story of bioglass. J Mater Sci Mater Med 17: 967-978. [crossref]
- Stavropoulos A, Geenen C, Nyengaard JR, Karring T, Sculean A (2007) Oily calcium hydroxide suspension (Osteoinductal) used as an adjunct to guided bone regeneration: An experimental study in rats. Clin Oral Implants Res 18: 761-767.
- Giannoudis PV, Dinopoulos H, Tsiridis E (2005) Bone substitutes: An update. Injury 36: S20-7.
- Louis PJ, Gutta R, Said-Al-Naief N, Bartolucci AA (2008) Reconstruction of the maxilla and mandible with particulate bone graft and titanium mesh for implant placement. J Oral Maxillofac Surg 66: 235-45.
- Soga lA, Tofe AJ (1999) Risk assessment of bovine spongiform encephalopathy transmission through bone graft material derived from bovine bone used for dental applications. J Periodontol 70: 1053-63. [crossref]
- Brunel G, Brocard D, Duffort JF, Jacquet E, Justumus P, et al. (2001) Bioabsorbable materials for guided bone regeneration priorto implant placement and 7-year follow-up: report of 14 cases. J Periodontal. 72: 257-64. [crossref]
- Pieri F, Corinaldesi G, Fini M, Aldini NN, Giardino R, et al. (2008) Alveolar ridge augmentation with titanium mesh and a combination of autogenous bone and anorganic bovine bone: A 2-year prospective study. J Periodontol 79: 2093-103. [crossref]
- Trombelli L, Farina R, Marzola A, Itro A, Calura G (2008) GBR and autogenous cortical bone particulate by bone scraper for alveolar ridge augmentation: A 2-case report. Int J Oral Maxillofac Implants 23: 111-6. [crossref]
- Blanco J, Alonso A, Sanz M (2005) Long-term results and survival rate of implants treated with guided bone regeneration: A 5-year case series prospective study. Clin Oral Implants Res 16: 294-301. [crossref]
- Application of Bone Substitutes and Its Future Prospective in Regenerative MedicineUjjwal Ranjan Dahiya, Sarita Mishra and Subia BanoSubmitted: August 29th, 2018 Reviewed: February 11th, 2019.
- Rusin Z, Ruijia Y, Paul R, Cooper, Zohaib K, et al. (2021) Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 26: 3007. [crossref]
- Donimukkala BR, Chandrasekharan N, Meghana G (2018) Bone Substitutes used in Implant Dentistry.