Abstract
The objective of the present opinion paper was aimed to present an at glance review of B cell immunology in COVID-19. B cell system is important both in human health and disease. B cells in health interplayed a role in organization of lymphoid tissue and regulation of lympho-angiogenesis. The antigen presenting, antibody producing and cytokine producing subsets of B cells are crucial for the activities of human immune system both in health and disease. The memory B cell functions and counts are acting as a valid probe for vaccine efficacy, vaccine breakthrough infections, severe infection form and hypoxic severe infection form of COVID-19. B cell depletion serves as indicator for septic COVID-19 disease conditions.
Keywords
B cell, Breakthrough, COVID-19, Deletion, Hypoxia, Vaccine
Introduction
Bone marrow plays a niche for the leukocyte primordium and a site where they differentiated. This primordium is the pluripotent stem cell that serves as the mono-chotomus origin of the immune cells. Stem cells are differentiated through the action cytokine into the tri-chotomus progenitor cell lines. The myeloid progenitor cell which forms the origin of granulocytes, the lympho-myeloid progenitor cell, the origin of mononuclear cell system and the lymphoid cell progenitor which constitute the origin of lymphocytes. Macrophages, T cells and B cells are forming the functional back bone of the immune system. B cell system displayed an array of immune functions in human health and disease. The present opinion paper concerned with the role played by B cell system in COVID-19 immunity [1].
B Cell Immunology
B cell can have two main subsets B1 and B2 [2]. Though, functionally they were subset as systemic and mucosal B cells. B cell may display; antibody production, cytokine production [3] and antigen presentation [4,5]. This story is ensemble under the umbrella of B cell system. B cell descends from the lymphoid cell progenitor of the pluri-potent haemo-poietic stem cell. In general lymphocytes were considered from the morphological point of view as more heterogeneous than mono and poly-nuclear leukocytes. The ratio of nucleus to cytoplasm is large. They are devoid from Golgi apparatus and from endoplasmic reticulum. Their mobility is rather slower than other leukocytes and has amoebic movement fashion. B cells bear surface Ig that acts as a recognition molecules and IgFc fragment receptor. B cells are devoid from thymus antigens. They have transmembrane cluster of differentiation; CD19, CD20, CD22, CDR1, CDR2, CD5, CD35, BCR and B7 (Table 1) [6].
Table 1: Developmental B cell phases and their receptors
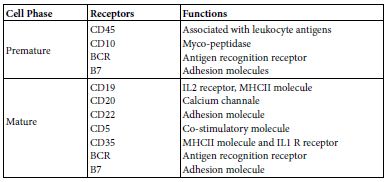
Maturation of B cell from the B lymphocyte progenitor may establish in bone marrow or it may migrate as an immature B lymphocyte to spleen or to other peripheral lymph nodes and maturate there. The onto-genic maturation steps starts as; progenitor the primordium, early Pro B, Bro B, pre B to immature B cell then to mature B cells. Mature B lymphocytes when stimulated by an antigen it well grow, differentiate and expand the transform to an effector plasma cells producing either antibodies and/or cytokines IL10 and IL12. As well memory B cells, Memory B cells are elected from those cells that undergoes heavy chain translocation and V fragment amplifying mutation which lead to the production of high affinity cells producing Ig G, Ig A, IgE, their BCR have high affinity for antigen binding [7-9].
B Cell Molecular Immunology
The molecular genetic system of B cells contains an array of gene sets that encodes for the various immune functions like those encoding genes for mitotic cell cycle, Ig gene superfamily, cytokine production genes, antigen presenting genes and the apoptosis encoding genes. The ontogeny of the different cell differentiation phases based on molecular genetic mechanisms like; gene re- arrangement, gene exclusion, alternative splicing and apoptotic gene expression. B cell on antigenic epitope stimulation, the epitope triggers naïve B cells to be activated for growth, proliferation, expansion and change to effector plasma cell and memory B cells.
Early in the Immune response time curve B cell produce IgM then class switched to other isotype. IL4, IL10, TGFB and IFN gamma promotes class switching [7]. When antigen primed B cell acts as an antigen presenting cell, it well take up and process antigen then present it onto their surface with MHCII molecules to be presented to T helper cell. It appear that there was a shift from antibody production to APC function. Primed B cells that are transformed to plasma cells are devoid from either IgM or IgD. Memory B cells once reactivated will perform class switching of Ig iso-types. BCR are composed of two identical Ig heavy and two identical light chains together with Ig alpha and Ig beta chains that transmit signals to the cell interior when legends are bound to BCR. Each chain has amino and carboxy termini. Domains are found on the amino termini of each chain. The epitope specificity of Ig molecule of BCR is determined before they are facing the antigen. The number of the possible BCR-epitope binding specificities high exceeds that number of genes forming human genome. This present a paradox. Such paradox can be solved through molecular re-arrangement, splicing and /or gene exclusion. The Kappa light chain encoding genes are mapped onto chromosome 2. While, those encoding lambda light chains are mapped onto chromosome 22. The gene cluster encoding is located onto chromosome 14. The potential antigen binding segment combinations are greater than 26 million. The heavy and light chain gene segments for both variable and constant regions are rearranged, transcribed into RNA and translated into single heavy or light chain polypeptide. The restriction of VLCL, and VHCH expression to a single member of each of the involved chromosome pairs is termed as allele exclusion. However, the collective combination of all of B cell means that both maternal and paternal of allele genes are expressed within any particular individual. There has been a guess that each B cell has the ability to produce a range of individual antigen specific receptor capable for binding as many as ten different epitopes. Significant part of this BCR epitope diversity are attributed to chromosomal DNA rearrangement. BCR represent a case of natural construct of a set of gene clusters for both of the heavy and light chains in which each set of gene clusters construct RNA transcript that express as a single polypeptide. Then these polypeptides undergo post-translational assembly. A single B cell synthesizes Ig of one single specificity at a time. This is because of its nature of combination of VL and VH regions at the effect of allelic exclusion. Un-stimulated B cell synthesizes and display monomeric IgM and IgD on their surfaces. Upon stimulation B cell may change their iso-type but not the epitope specificity of the Ig they produce. This process is known as iso-type switching. Iso-type switching influences the ultimate nature of humoral immune responses. Memory B cell producing IgM can undergo further DNA re-arrangement to change the Ig iso-type they produce [7-11].
Antigen Presenting B Cell
B lymphocytes including B1 may act as antigen presenting cell. When they do so B cells use specialized MHCII complex antigen presentation pathway to process BCR bound and internalized protein antigens and present them to CD4 T cells. Such processes are performed through several efficient molecular mechanisms in a stepwise manner as; (i) antigen capture and uptake, (ii) intersection of the internalized antigen-BCR complexes with MHCII complexes,(iii) generation and regulation of MHCII-peptide complexes, (iv) exo- cytotic transport for presentation of MHCII-peptide complexes at the surface of B cells and (v) activation of CD4 T cells. Collectively, these molecular mechanisms affect both of the fate of lymphocyte and shape the immune response [4,5,12].
Antibody Producing B Cells
A naïve B cell subset, on priming with SARS-COV-2 antigenic epitope(s) in continuum with in-vivo, ex-vivo and in-vitro settings will be activated through; cell growth, proliferation, expansion and transition into plasma cell producing antibodies specific for spike, receptor binding domain and other epitopes. In a study performed in 2020 workers isolate 61 SARS-COV-2 neutralizing monoclonal antibodies from five hospitalized severe COVID-19 infection. Of which 19 were potent neutralizing to authentic SARS-COV-2 in-vitro [13,14].
Cytokine Producing B Cells
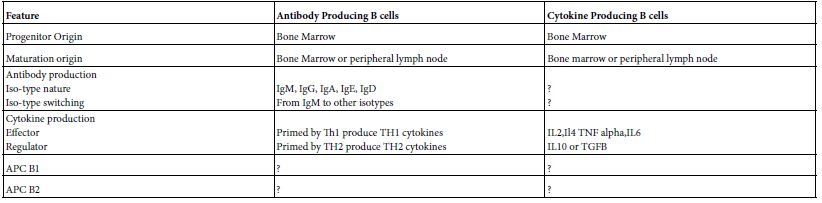
B cell performed an array of immune functions both in human and mammals independent on that of antibody formation. Like; tolerance, tumor rejection, immune response suppression autoimmunity, organization of lymphoid tissue and regulate lymphoangiogenesis. Broadly speaking it can be bio-typed into antibody producing and cytokine producing. Cytokine producing B cells are ramified into an effector and regulator B cells. The cytokine profiles of the effector are somewhat different from regulator B cells. The ontogenic origin of antibody producing B cells is different from that of cytokine producing B cells (Table 2) [3].
Table 2: Immune Functions of B cells
Proposed COVID-19 Immunity
It seems to be that the nature of human immunity; during SARS- COV-2 infections, recovery and post-infection is somewhat different from other human viral infections. Collective scientific task has been made by STAT scientists [15] proposed four scenarios for immunity to SARS-COV-2 infections. These are; sterilizing, functional, waning and lost. Other idea tempted to put-down a collective scenario that mix up more than one of the mentioned scenarios. Sterilizing; The immune system of the host is armed to a foe and able to fend it off before infection take a hold. Functional; The immune system produce specific antibody responses but the antibodies wan on time post-infection but many cellular responses are not waning. Under this scenario, people whose immune system have been primed to recognize and fit the virus in continuum with either infection or vaccination could contract it again in the future but with either mild or symptomless. Waning; In this scenario people who have been infected or vaccinated would lose their protection overtime. But even if immunity wans, reinfection would be less severe and be a variation to the functional immunity. Lost; In this scenario people who have been infected would lose all of their immunity against the virus within some time frame. A re- infection after that point would be like that of first infection. Mixed Scenario; The overall picture of human immunity to SARS-COV-2 infection will be mixed. Some people will have sterilizing but most will fit about functional or waning immunity. Neither sterilizing nor lost immunity gains support by the scientific immunity [15].
Evidence Based COVID-19 Immunity
Collective local experience from the in-practice COVID-19 patient some of them recover with use of convalescent sera others not. Most patient produce specific antibodies but few fail to raise antibodies though they clinically infected [unpublished data, early in the waves of the pandemic]. The to date information in this context indicated that both humoral and cellular immunity can be lasting up to eight months post infection [14,16-19]. Effective effector B, memory B, effector T and memory T cells are involved in the protection against COVID illness. Breakthrough infection during post-vaccination periods could function exactly the same as a booster immunization. Though this may be a theoretical concept rather than practical one [20].
Memory B Cell Probe COVID-19 Vaccine Efficacy
In an experiment two groups of human subjects were assigned as; SARS-COV-2 naïve 33, and SARS-COV-2 recovered 11. Antibody and antigen specific B cells were mapped overtime. SARS-COV-2 naïve group were found to be requiring both vaccine doses for antibody response. Likewise memory B cells tracing have shown full length spike protein and spike receptor binding domain were detectable at second mRNA vaccine shot. While in SARS-COV-2 recovered group antibody and B cell responses were significantly boosted after first vaccine dose. Second dose neither increase antibody nor B cells. First vaccine dose strongly correlated with the pre-existing memory B cells in recovered group. COVID mRNA vaccine priming to individuals have shown distinct response based on prior SARS-COV-2 exposure. Memory B cells could be used as a probe for vaccine efficacy both in naïve and recovered COVID-19 individuals [21].
Memory B Cells Breakthrough Infection
In an experimental setting in which two group of patients. One vaccine breakthrough infection 55 patients and the second vaccinated close contacts who did not contract infection 88 subjects. Vaccine breakthrough infection was sharing lower memory B cell frequency but high antibody in plasma cell and those produced by memory B cells. Inflammatory cytokines, the IL1B and TNF were lowered in vaccine breakthrough infection than that infection of similar settings. Hence, lower memory B cells are correlated with vaccine breakthrough infections of COVID-19 [22].
Memory B Cells and Severity
In a retrospective study that was planned as 208 laboratory confirmed COVID-19 patients in which severity was checked on a scale graded from 0-10 severity score. Two parameters were adopted for evaluation of severity; the memory B cell count and the serum immunoglobulin levels. The age range for those patients 35-63 years with median of 50 years of which 88 were females (42%). The survivors were 191:208(91.82%) and diseased were 17:208(8.18%). The severity was ranged from6-8 with a median of 8 in deceased and 0-2 with median of 1 in survivors. Significant low levels of total B, naïve B, switched memory B, and serum immunoglobulin levels; IgA, IgG1, IgG2 in deceased than in survivors. Negative significant correlation between serum Igs and memory B cells was evident. The prognosis of COVID-19 disease is associated with B cell subsets and serum immunoglobulin levels [23].
B Cell and Hypoxia
In an experimental setting including COVID-19 patients with variable severity and hypoxia and genetically modified VHL deficient mice kept in hypoxia exposed mice. Blood was collected from those patients for detailed B cell phenotypes in peripheral blood lymphocytes using flow cytometery. Single cell transcription and whole blood sequence analysis were done to evaluate the impact of hypoxia on patients B cells and same was done onto the genetically modified hypoxia kept mice. There was an early and perminant defects in B cell subsets in moderate to severe COVID-19 patients including marginal zone-like, memory and transitional B cells. Similar findings were noted on genetically modified hypoxia kept mice. To this end hypoxia may contribute to pronounce and persistent defects of B cell pathology observed both in COVID hypoxic patients and hypoxia kept mice [24].
B Cell and Sepsis
In a clinical settings of an abscess forming super-infection viral COVID-19 pneumonia. Bone marrow and splenic B cell count have shown severe B cell loss in bone marrow or spleen in 64% of the of the patients. This was reflected by peripheral blood lymphopenia. B cell loss was associated with higher pulmonary SARS-COV-2 burden and only with marginal decrease in T cell counts. Study suggests the presence of sepsis related immunodeficiency in sever COVID-19 pneumonia with super-infection [25].
B Cell in COVID-19 with Seasonal Coronavirus
In this clinical setting tempts were made to evaluate the role of the pre-existing seasonal endemic coronavirus B cell on the development of sars cov-2 specific IgG responses. The tried parameters were; kinetics, breadth, magnitude and levels of cross-reactivity of IgG antibodies against SARS-COV-2 and heterologous corona virus at the clonal levels in patients with mild or severe COVID-19 and controls. Assessment were made onto antibody reactivity to nucleo- capsed and spike antigens and correlate this with IgG responses to SARS-COV-2 neutralization. Patients with COVID mounted a mostly type specific SARS-COV-2 responses. IgG clones directed against seasonal coronavirus were boosted in patients with severe covd-19. these boosted clones did not neutralize SARS-COV-2. Such findings indicate a boost of poorly protective COVID-19 specific antibodies in patients with COVID-19 that correlate with disease severity, reveals “Original antigenic sin” [26].
Relaxing B Cell Tolerance
Five groups of individuals were the test and controls. First COVID-19 convaluscent, second mild COVID-19, third severe COVID-19 fourth COVID-19 vaccinated and fifth control. Blood were collected from these groups. Mononuclear cells were separated from the test and control blood samples. Flow cytometery was performed on these preparations. Anergic B cells were found in high frequency in severe COVID-19 patients as compared to mild cases. They were in activated state displaying reduced inhibitory receptor expression and restored BCR signaling indicative of breach of anergy during viral infection, supported by increment shift in autoantibody levels. This together with phenotypic and functional alterations significantly correlated with hyper-inflammation in severe SARS-COV-2 infection. Hence, B –ND undergoes relaxation in their peripheral tolerance in severe COVID-19 [27].
Memory B Cell Pulmonary Subsets
Memory B cells are immune cells produced primarily in the lymph node and spleen. They persist for long time there in these regions and retain the memory of the infectious agents. If the subject body facing with same agent in future. These cells immediately mobilized and rapidly reactive immune system for effective protection. Recently workers have been documented the presence of memory B cells in lungs of laboratory animal models. Whereby, the laboratory animal model infected with the virus as influenza or COVID-19. Ten weeks later the virus eliminated from the body of infected animals. Memory B cells were tracked in lungs of the infected animals using flurescent markers followed by single cell transcriptomic analysis. Such techniques enable localizing memory B cells in lung and their gene expression profile cell by cell. Results have shown groups of memory B cells in the bronchial respiratory mucosa in two subpopulations of different gene expression profile and functions. The Bona fide and Bystandard subsets. The bona fide subset showed high affinity to virus and trigger their appearance immediately on entry and infection. While the bystandard is not directly recognize the virus but bind to the receptor of the immune complexes formed by the antibodies produced by bona fide cells. Both of the subsets exhibit synergistic function as a two tiers system [28].
Conclusions
B cell immunology of COVID-19 is in the forefront and current scientific mode. Memory B cell was found pathognomic with an array of human COVID-19 pathological changes. The changes cover both subnormal count of the B cell subsets and cell defects in different COVID-19 disease forms.
References
- Abbas AK, Lichtman AH, Pillai S (2015) Cellular and Molecular Immunology, 8th Elsevier Saunders, Phildelphia.
- Allman D, Pillai S (2008) Peripheral B cell Curr opin Immunol 20: 149-157. [crossref]
- Lund FE (2008) Cytokine producing B lymphocytes-key regulator of Curr Opin Immunol 20: 332-338. [crossref]
- Popi AF, longo-Maugeri IM, Marino M (2016) An overview of B1 cells as an antigen presenting cells. Front Immunol 7: 138. [crossref]
- Adler LN, Jiang W, Bhamidipati K, Millican M, Macaubas C, et al. (2017) Other functions: class II restricted antigen presentation by B cells. Immunol 8: 319. [crossref]
- Shnawa IMS (2014) Dijla-ALWadah Publishing House. Iraq-Jordan.
- Shnawa IMS (2013) Molecular Immunology. Lap-Lambert Academic Publication-
- Lu L (2013) Frontier in B cell Cellular and molecular Immunology 10: 95-96. [crossref]
- Alt FW, Horj T, Reth M (2015) Molecular Biology of B ElSevers Ltd.
- Scheid JF, Barnes CO, Eraslan B, Hudak A, Keeffe JR, et (2021) B cell genomics behind cross-neutralization of sars-cov-2 variant and sars-cov. Cell 164: 3205-3221. [crossref]
- Cai H, Hu J, Huang L, Gao C, Xu M, et (2022) The relationship between convergent IgA signatures and severity of covid-10 patients by next generation sequences of BCR repertior. Front Microbiol 12: 833054. [crossref]
- Frazi R, Aghbash PS, Eslami N, Azadi A, Shamek A, et (2022) The role of antigen presenting cells in pathogenesis of covid-19. Pathol Res Pract 233: 153848. [crossref]
- Ng K, Faulkner N, Cornish GH, Rosa A, Harvey R, et (2020) Pre-existing and de- novo humoral immunity is sars-cov-2 in humans. Science 370: 1339-1343. [crossref]
- Liu L, Wang P, Nair MS, Yu J, Rapp M, et al. (2020) Potent neutralizing antibodies directed to multiple epitopes on sarscov-2 Nature 584: 450-456. [crossref]
- Branswell H (2020) Four scenarios on how we might develop immunity to covid- 19-STAT.
- LeBert N, Tan AT, Kunasegaran I, Tham CYL, Hafezi M, et (2020) T cell immunity in cases of covid-19 and sars and uninfected controls. Nature 584: 457-462. [crossref]
- 17-Gerfoni A, Weiskopf D, Sydney I, Mateus J, Dan JM, et al. (2020) Target T cells responses to sars-cov-2 corona virus I humans with covid-19 disease and unexposed individuals. Cell 181: 1489-1501. [crossref]
- Gallais F, Velay A, Wending MJ, Partisani M, Sibilia J, et al. (2020) Intrafamilial exposure to sars-cov-2 induce cellular immune responses without seroconverstion, France. Emerg Infect Dis 27: 113-121. [crossref]
- Wise J (2020) Covid-19: T cells responses for at least six months after infection study shows. BMJ 371:4257.
- Smith J (2021) Is covid-19 exposure post-vaccination, a booster or a risk. Oper in Coronavirus.medium.com
- Goel RR, Apostolidis SA, Painter MM, Mathew D, Pattekar A, et (2021) Distinct antibody and memory B cell responses in sars-cov-2 naïve and recovered individuals after mRNA vaccination. Sci.Immunol 6: 6950. [crossref]
- Tay MZ, Rouers A, Fong SW, Goh YS, Chan YH, et (2022) Decreased memory B cell frequencies in covid-19 delta variant vaccine breakthrough infection. EMBO Mol Med 14: 152272. [crossref]
- Colkesen F, Kurt EK, Vatansev H, Korkmaz C, Colkesen F, et al. (2021) Memory B cell and serum immunoglobulins are associated with disease severity and mortality in patients with covid-19. Postgrad Med J 98: 765-771. [crossref]
- 24-Kotagiri P, Mescia F, Hanso AL, Turner L, Bergamaschi L, et 2022.The impact of hypoxia in covid-19.EbioMedicine 77: 103878. [crossref]
- Ihlow J, Michaelis E, Greuel S, Heynol V, Lehmann A, et al. (2021) B cell depletion and signs of sepsis-aquired immunodeficiency in bone marrow and spleen of covid-19 Int Infect Dis 103: 628-635. [crossref]
- Aguilar-bretones M, Westerhuis BM, Raadsen MP, Bruin ED, Cahndler FD, et al. (2021) Seasonal coronavirus specific B cells with limited Sars-cov-2 cross-reactivity dominate the IgG responses in severe covid-19. J Clin Invest 131: e150612. [crossref]
- Castleman MJ, Stumpf MM, Therrien NR, Smith MJ, Lesteberg KE, et (2022) Sars- cov-2 infection relaxes peripheral B cell tolerance. J Exp Med 219: e20212553. [crossref]
- Greggoire C, Spinelli L, Villazala-Merino S, Gill L, Holgado MP, et (2022) Viral infection engenders bona fide and bystander subsets of lung-resident memory B cells through a permissive mechanism. Immunity 55: 1216-1233. [crossref]