Abstract
The ammonia gas-tolerant bacterium Paenibacillus lentus NH33 (hereafter referred to as NH33) was isolated using minimal medium containing glucose in the presence of 1300 ppm of ammonia gas. NH33 showed no growth in the absence of gaseous ammonia (undissociated ammonia), indicating that it is an obligate requiring gaseous ammonia for growth. We investigated how NH33 uses gaseous ammonia. It also grew on minimal agar medium with or without glucose in the presence of 1350 ppm of ammonia gas, indicating that it was also capable of both mixotrophic and chemolithoautotrophic growth. Under the mixotrophic condition, NH33 used gaseous ammonia as a nitrogen source, but did not use dissociated ammonia, nitrite, or nitrate; thus, it appears to use gaseous ammonia as its sole nitrogen source. Under the autotrophic condition, NH33 was capable of using gaseous ammonia as its sole energy source, but not dissociated ammonia. The ammonia gas concentration that was optimal for the growth of NH33 was determined to be 1350 and 170 ppm for the mixotrophic and autotrophic growth conditions, respectively. These growth characteristics indicated that NH33 is a novel nitrifying bacterium that uses gaseous ammonia as its sole energy and nitrogen source.
Keywords
Gaseous ammonia, Ammonia-oxidizing bacteria, Chemolithoautotrophic growth, Mixotrophic growth, Paenibacillus lentus, Nitrite
Introduction
Odors are generally attributed to the production of various volatile compounds arising from the anaerobic decomposition of fecal matter. The number of complaints regarding odors from outdoor toilets, compost facilities, and livestock farms is increasing year-by-year [1]. Deodorization technologies can be classified into three categories, i.e., technologies employing chemical [2], physical, or biological treatment methods [3]. An important advantage of biological treatment methods over physical and chemical treatment methods is that biological processes can be conducted at moderate temperatures and atmospheric pressure. We have been developing biological deodorization techniques by applying the functions of microbes, but this approach is challenging as it requires the isolation of microorganisms capable of growing in the presence of gaseous ammonia. We have isolated promising microorganisms that decrease the odor of ammonia using a newly developed culture method called the closed gaseous ammonia-exposing culture method. The isolated microorganisms were all bacterial species that could be classified into two groups: 1) hypergaseous ammonia-tolerant bacteria and 2) gaseous ammonia-requiring bacteria, which are obligates requiring gaseous ammonia as the sole nitrogen source. These newly discovered bacteria had the ability to eliminate toxic ammonia, or conversely, to assimilate toxic ammonia, which has not been seen before in bacteria. An ammonia gas-tolerant bacterium, Paenibacillus lentus NH33 (hereafter referred to as NH33), was capable of growing in minimal agar medium containing glucose in the presence of gaseous ammonia (1350 ppm), but not in the absence of gaseous ammonia. This indicated that NH33 is a special organism that requires only gaseous ammonia as the nitrogen source. NH33 had a high gaseous ammonia-absorbing rate of 2.20 mmol/1010 cells. The ammonia-eliminating property of NH33 may be useful for biological deodorization technology [4]. The forms of ammonia include undissociated ammonia (NH3), which is the gaseous form, and dissociated ammonia (NH4+), which is the ionic form [5]. Ammonia is the preferred nitrogen source for most bacteria and fungi, and plants also require ammonia from the soil as a nitrogen source. Microorganisms also use nitrite and nitrate as nitrogen sources, and these nutrients are reduced to ammonia by reductase [6]. However, ammonia is a paradoxical nutrient: although ammonia is required for growth, undissociated ammonia has a strong cytotoxic effect. Undissociated ammonia dissolves easily through the lipid barrier and across the cytoplasmic membrane, and has a detrimental effect on the growth and metabolism of organisms [7]. Well-known organisms that use ammonia are chemolithoautotrophic ammonia-oxidizing bacteria, which are among a select group of microbes that have the ability to use ammonia as the sole source of energy and reductant for growth [8]. Ammonia-oxidizing bacteria extract energy from a single inorganic source (NH3), assimilate inorganic substrates (e.g., CO2 and NH3), and use them to synthesize all of the biochemicals needed for growth. The product of ammonia oxidation is nitrite. On the other hand, anaerobic ammonium oxidation (anammox) bacteria were discovered in wastewater sludge in the early 1990s; they have the unique metabolic ability to combine ammonium and nitrite or nitrate to form nitrogen gas [9]. Nitrification is catalyzed in two steps by ammonia-oxidizing bacteria and nitrite-oxidizing bacteria that are phylogenetically not closely related, and it was thought that no organism could oxidize both substrates. However, recently, complete ammonia oxidizers (comammox) that are capable of converting ammonia to nitrate within a single organism were unexpectedly discovered within the genus Nitrospira [10].
Ammonia has nutritional roles both as a nitrogen source and an energy source [11]. Ammonia-oxidizing bacteria use ammonium ions not only as an energy source, but also as a nitrogen source. NH33 is a previously unknown obligate that specifically requires gaseous ammonia for growth. In this study, to understand how NH33 utilizes gaseous ammonia, we investigated the autotrophic and mixotrophic growth properties of NH33 [12,13], including its growth in the presence of gaseous ammonia, and its energy and nitrogen sources.
Materials and Methods
Mixotrophic Culture
To prepare minimal medium containing glucose (MMG) agar plate, 15 mL of agar (Wako Pure Chemical Industries, LTD., Osaka, Japan) solution (1.5% final concentration) was sterilized by autoclaving, then (10x) minimal medium concentrate solution and (10x) glucose concentrate solution were added, and the agar was poured into Petri dish. This medium contained no nitrogen source. One liter of MMG (pH7.0) contained 2 μg of biotin, 400 μg of calcium pantothenate, 2 μg of folic acid, 2000 μg of inositol, 400 μg of niacin, 200 μg of p-aminobenzoic acid, 400 μg of pyridoxine hydrochloride, 200 μg of riboflavin, 400 μg of thiamin hydrochloride, 500 μg of boric acid, 40 μg of copper sulfate, 100 μg of potassium iodide, 200 μg of ferric chloride, 400 μg of manganese sulfate, 200 μg of sodium molybdate, 400 μg of zinc sulfate, 1.0 g of monopotassium phosphate, 0.5 g of magnesium sulfate, 0.1 g of sodium chloride, 0.1 g of calcium chloride, and 5.0 g of glucose. One mg of NH33 was added to 1 mL of sterilized water and suspended until homogenous (NH33 suspension). The NH33 suspension (50 μL/plate) was spread onto MMG agar plates without lids. The one plate was placed in an airtight plastic chamber (7.0 L) with ammonium hydrogen carbonate powder (0.178 g), and incubated at 40°C for 3 to 12 days (Figure 1). The ammonium hydrogen carbonate had completely sublimated after 2 to 3 h, and the ammonia gas concentration in the chamber reached 1350 ppm.
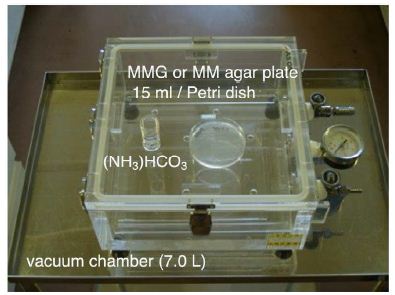
Figure 1: Photograph of the closed gaseous ammonia exposure culture system. A MM agar plate for chemolithoautotrophic growth or a MMG agar plate for mixotrophic growth was placed in the 7-L chamber with ammonium hydrogen carbonate powder ((NH3)HCO3). The chamber was tightly closed, and incubated at 40oC. The ammonium hydrogen carbonate had completely sublimated during the 2.5- to 3-h incubation period to provide a gaseous mixture including gaseous ammonia. The amount of ammonium hydrogen carbonate powder in the chamber was adjusted to change the concentration of gaseous ammonia for the experiments.
Autotrophic Culture
To prepare minimal medium (MM) agar plate, 15 mL of agar solution (1.5% final concentration) was sterilized by autoclaving, then (100x) minimal medium concentrate solution was added, and the agar was poured into Petri dish. The MM contained no nitrogen and carbon source. One liter of MMG (pH7.0) contained 2 μg of biotin, 400 μg of calcium pantothenate, 2 μg of folic acid, 2000 μg of inositol, 400 μg of niacin, 200 μg of p-aminobenzoic acid, 400 μg of pyridoxine hydrochloride, 200 μg of riboflavin, 400 μg of thiamin hydrochloride, 500 μg of boric acid, 40 μg of copper sulfate, 100 μg of potassium iodide, 200 μg of ferric chloride, 400 μg of manganese sulfate, 200 μg of sodium molybdate, 400 μg of zinc sulfate, 1.0 g of monopotassium phosphate, 0.5 g of magnesium sulfate, 0.1 g of sodium chloride, and 0.1 g of calcium chloride. The NH33 suspension (50 μL/plate) was spread onto MM agar plates without lids. The plates were placed in an airtight plastic chamber (7.0 L) with ammonium hydrogen carbonate powder (0.178 g), and incubated at 40°C for 3 to 12 days. The ammonium hydrogen carbonate had completely sublimated after 2 to 3 h, and the ammonia gas concentration in the chamber reached 1350 ppm.
Transmission Electron Microscopy (TEM) Observations
The cultured cells on a small patch of agar medium were fixed with 2.0% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) at 4°C for 120 min. The samples were subsequently washed three times with 0.1 M phosphate buffer (pH 7.4), then incubated in 1% OsO4 at 4°C for 120 min. The samples were dehydrated in a series of increasing ethanol concentrations as follows: 10 min in 50% ethanol at 4°C; 10 min in 70% ethanol at 4°C; 10 min in 80% ethanol at 4°C; 10 min in 90% ethanol at room temperature (RT), 10 min in 95% ethanol at RT, 10 min in 99.5% ethanol at RT twice, 10 min in 100% ethanol at RT twice, and 15 min in propylene oxide at RT twice. Then, the cells were incubated for 60 min in a 1:2 mixture of EPON:propylene oxide, 60 min in a 1:1 mixture of EPON:propylene oxide, 60 min in a 2:1 mixture of EPON:propylene oxide, and overnight in 100% EPON. Ultrathin sections (60 to 80 nm) were cut parallel to the bacterial layer, collected on single-slot Formvar-coated copper grids, and counterstained with 0.5% uranyl acetate at 20°C for 30 min, and 3% lead citrate at 20°C for 7 min [14,15]. Bacteria were imaged using a HT7700 transmission electron microscope (Hitachi, Tokyo, Japan) at an electron voltage of 80 kV.
Optimal Gaseous Ammonia Concentration for Mixotrophic and Autotrophic Growth
The NH33 suspension (50 μL/plate) was spread onto MM or MMG agar plates without lids, placed in an airtight plastic chamber (7.0 L) with ammonium hydrogen carbonate powder (0 to 0.337 g), and incubated at 40°C for 72 h. The ammonium hydrogen carbonate had completely sublimated after 2 to 3 h, and the ammonia gas concentration in the chamber reached 0 to 2560 ppm. The gaseous ammonia concentration was measured with an ammonia gas sensor (New Cosmos Electric Co. Ltd., Osake, Japan). The amount of gaseous ammonia was calculated using the ideal gas law [14].
Usable Nitrogen Sources in Mixotrophic Culture
The NH33 suspension was spread onto MMG agar plate containing different nitrogen sources (10 mM of (NH4)SO4, NaNO2, or KNO3), and cultured under air at 40°C for 72 h. To investigate the usefulness of gaseous ammonia as a nitrogen source, NH33 was inoculated onto MMG agar plate without lids, then the plate was placed in an airtight plastic chamber (7.0 L) with ammonium hydrogen carbonate powder (0.178 g; initial gaseous ammonia concentration of 1350 ppm), and incubated at 40°C for 72 h.
Usable Energy Sources in Autotrophic Culture
The NH33 suspension was spread onto MM agar plate containing ammonium (10 mM of (NH4)2SO4 or NH4Cl), and incubated at 40°C for 72 h under air. In addition, NH33 was inoculated onto MM agar plate, and incubated at 40°C for 72 h under 1350 ppm of gaseous ammonia.
Rate of Stable Nitrogen Isotope Uptake in NH33 Cells
A lidless MM agar plate inoculated with NH33 was placed in the 7-L chamber in the presence of 0.35 mL of 3.4% 15N-labeled ammonia solution (Kao Co. Ltd., Tokyo, Japan) or unlabeled ammonia solution (3.4%), and incubated at 40°C for 72 h. Initially, the concentration of the gaseous ammonia that had vaporized from the ammonia solution was 170 ppm. Cultured cells were recovered with distilled water and centrifuged. The recovered cells were dried at 100°C for 6 h. The rate of 15N stable isotope uptake (calculated as the ratio of 15N/total N) in the dried samples was measured by a combined system with a mass spectrometer (DELTAplus Advantage, Thermo Fisher Scientific, Bremen, Germany) and an elemental analyzer (FLASH 2000, Thermo Fisher Scientific, Bremen, Germany). The accuracy of this analysis system is ±0.3% of the measured value. As the standard samples, International Atomic Energy Agency (IAEA)-N-1 and IAEA-N-2 were used [16].
Detection of Nitrite in NH33 Cells
The NH33 suspension was spread onto MM agar plate and incubated at 40°C for 72 h in the presence of gaseous ammonia at 170 ppm. Cultured cells were recovered with distilled water, washed with a 0.8% NaCl solution, and centrifuged. The cells were then resuspended in 0.8% NaCl solution, and grounded by a bead grinder at 3200 rpm for 30 s. The nitrite concentration in the supernatant after centrifugation was measured by the naphthylethylenediamine hydrochloride spectrophotometric method [17]. The reaction time and temperature was 120 s and 30°C, respectively. The absorbance of the reaction solution was immediately measured by a spectrophotometer at 540 nm.
Biomass Estimation
Biomass production in wet weight was estimated for agar plate cultures by collecting 2.0 ml sterilized distilled water and streak bar. The collected cells were centrifuged (7,740 x g, 10 min) in micro tubes. The cell pellet was then gently washed with 1.5 mL of sterilized distilled water to remove culture medium salts. The sample was again pelleted (7,740 x g, 10 min), the supernatant was carefully removed and 1.5 mL of sterilized distilled water used to resuspend the pellet into pre-weighed 1.5 mL micro tubes. Cells were again pelleted at 9,000 x g for 5 min, and the supernatant discarded. Samples were semi-dried at 40˚C until visible water evaporated. Tubes were weighed on a precision balance (Sartorius CP324S) to estimate the weight of biomass. The wet weight of cells was quantified by subtraction the weight of tube from the total weight.
Results
Mixotrophic Growth
NH33 was inoculated onto MMG agar plate (mixotrophic medium), and incubated at 40°C for 6 days in the presence of 1350 ppm of gaseous ammonia (Figure 1). As shown in Figure 2A, opal colonies with a mucoid morphology were observed [18,19]. Compared to the growth on the MM agar plate, there was more growth on the MMG agar plate, and the colonies had larger diameters that reached over 10 mm. This suggested that active motility [20] or swarming behavior [21] occurred when NH33 was grown on mixotrophic medium. NH33 is an obligate that specifically requires gaseous ammonia for growth, as demonstrated by its inability to grow on MMG agar medium without gaseous ammonia.
Autotrophic Growth
NH33 was inoculated onto MM agar plate (autotrophic medium), and incubated at 40°C for 6 days in the presence of 1350 ppm of gaseous ammonia. As shown in Figure 2B, opal single colonies with diameters of 6 to 7 mm formed on the MM agar plate. NH33 was able to grow on MM agar plate, which did not contain carbon and nitrogen sources, indicating that it is a chemolithoautotrophic bacterium that could gain energy by the oxidation of gaseous ammonia [22].
TEM Observations
The TEM images of the mixotrophically and autotrophically grown NH33 cells are shown in the right panel of Figure 2. NH33 had a bacilliform morphology, and measured 5.0 to 6.5 µm in length and 0.5 to 0.7 µm in width. Under the two different culture conditions, the cells had a cell wall, and a previously unknown white substance which shows low electron dense was observed in the cytoplasm. In particular, the low electron dense area filled most of the cytoplasm in the autotrophically grown cells. Many of the cells grown on the autotrophic medium had an irregular shape, but this was not observed in the cells grown on the mixotrophic medium.
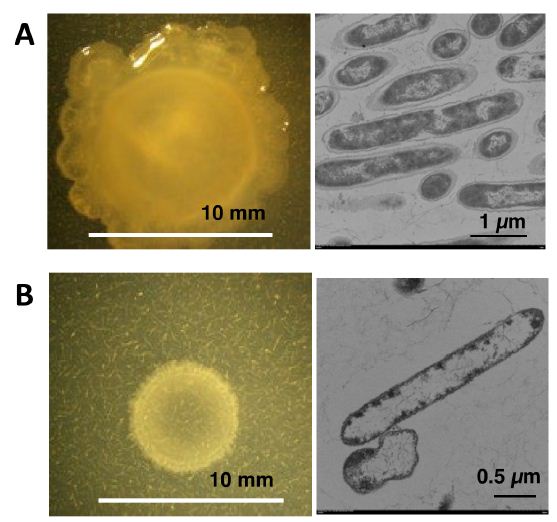
Figure 2: The colony morphology (left) and a transmission electron microscopic image (right) of Paenibacillus lentus NH33. P. lentus NH33 was cultured for 6 days at 40oC on MMG agar plate in the presence of gaseous ammonia at 1350 ppm for mixotrophic growth (A), and MM agar plate in the presence of gaseous ammonia at 1350 ppm for autotrophic growth (B).
Optimal Gaseous Ammonia Concentration for Mixotrophic and Autotrophic Growth
We investigated the optimal gaseous ammonia concentration for the growth of NH33 on MMG agar plate. As shown in Figure 3A, the gaseous ammonia concentration at 1350 ppm (initial concentration) was optimal for cell growth (relative growth rate: 100). Gaseous ammonia at 2560 ppm repressed the growth of NH33 to a relative growth rate of 10, indicating that an excessively high concentration of gaseous ammonia had a toxic effect on NH33 growth. No growth of NH33 was observed on MMG agar plate without gaseous ammonia.
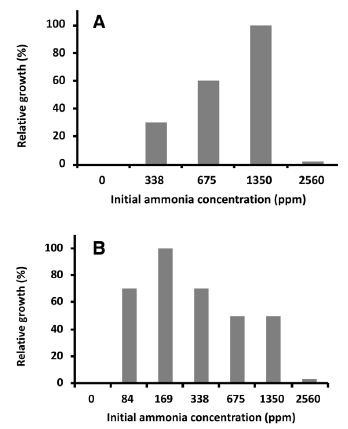
Figure 3: Optimal gaseous ammonia concentration for mixotrophic and autotrophic growth. Paenibacillus lentus NH33 was cultured on MMG agar plate (A) and MM agar plate (B) in the presence of different concentrations of gaseous ammonia. The values indicate the relative wet weight of the cells.
With the initial gaseous ammonia concentration set to 1350 ppm, we measured the changes in the gaseous ammonia concentration and the growth of NH33 on MMG agar plate (Figure 4A). When no bacteria were inoculated, no change was seen in the gaseous ammonia concentration in the chamber during 170 h of incubation. On inoculated plates, NH33 continued to grow until the 80-h time point, when the gaseous ammonia concentration had decreased to 900 ppm, and the cells stopped growing. These results demonstrated that NH33 assimilated gaseous ammonia into the cells. A plot of the wet weight of cells versus the amount of assimilated gaseous ammonia (Figure 4a) indicated that mixotrophically grown NH33 required 0.62 mmol of gaseous ammonia to produce a biomass of 1.0 g (wet weight). The optimal gaseous ammonia concentration for the autotrophic growth of NH33 on MM agar plate (autotrophic medium) was investigated (Figure 3B). The optimal growth of NH33 on MM agar plate was observed (relative value: 100) when incubated in the presence of gaseous ammonia at 170 ppm. Gaseous ammonia at 2560 ppm decreased the growth rate to a relative value of 5. NH33 was more sensitive to gaseous ammonia when grown under the autotrophic growth condition than under the mixotrophic growth condition. No growth of NH33 was observed under the autotrophic condition without gaseous ammonia. These results indicated that NH33 used gaseous ammonia as an energy source. The increase in the biomass of NH33 grown on MM agar plate and the change in the gaseous ammonia concentration from the initial concentration of 150 ppm in the closed chamber were evaluated (Figure 4B). No change in the gaseous ammonia concentration was observed in the closed chamber during 100 h of incubation of MM agar plate without bacterial inoculation. The biomass continuously increased during 75 h of incubation. During this incubation period, the gaseous ammonia concentration decreased from 150 ppm to 90 ppm. This result demonstrated that NH33 assimilated gaseous ammonia to grow. Figure 4b shows a plot of the amount of assimilated gaseous ammonia versus the amount of biomass (wet weight). Autotrophically growing NH33 cells required 0.64 mmol of gaseous ammonia to gain 1.0 g of biomass (wet weight).
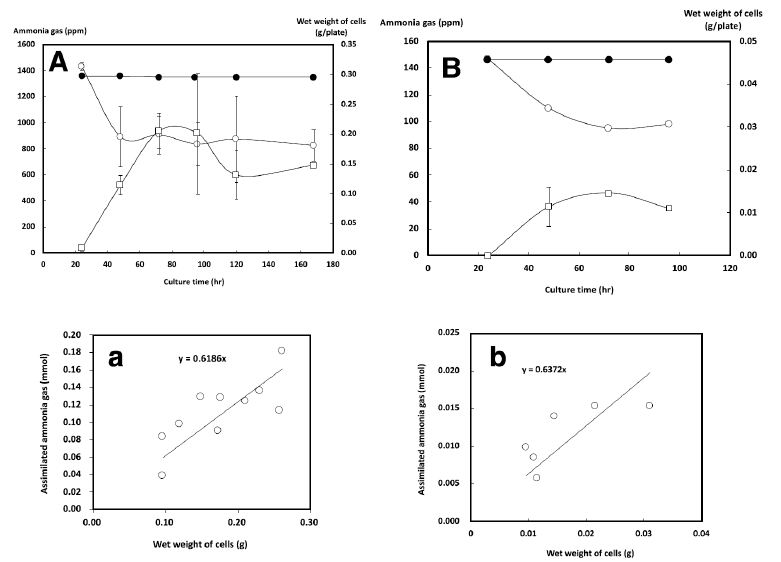
Figure 4: Growth curves of Paenibacillus lentus NH33 on mixotrophic (A) and autotrophic (B) growth media and the changes in the gaseous ammonia concentration. The wet weight of P. lentus NH33 cells and the changes in the gaseous ammonia concentration are indicated by empty squares and circles, respectively. The changes in the gaseous ammonia concentration when using agar medium without P. lentus NH33 inoculation are indicated by black circles. The values indicate the means ± standard deviation of three independent experiments. The cell yield of P. lentus NH33 versus the amount of assimilated gaseous ammonia was plotted for the mixotrophic (a) and autotrophic (b) growth conditions.
Usable Nitrogen Sources in Mixotrophic Growth
NH33 could grow well on MMG agar plate in the presence of 1350 ppm of gaseous ammonia (specific growth rate: 2.21 ± 1.18), but not in the absence of gaseous ammonia (Table 1). Replacing gaseous ammonia with ammonium sulfate, sodium nitrite, or potassium nitrate did not enable the growth of NH33 (Table 1). These results indicated that NH33 could not use dissociated ammonia (i.e., ammonium ions), nitrite, or nitrate as a nitrogen source. NH33 required only undissociated ammonia (gaseous ammonia) as a nitrogen source.
Table 1: Usable nitrogen sources for Paenibacillus lentus NH33 growth on MMG agar plate (mixotrophic medium).
|
Medium |
Nitrogen source |
Cell yield (mg wet weight/h/plate) |
|
| MMG agar | Gaseous ammonia |
1350 ppm |
2.21 ± 1.18 |
| MMG agar | None |
– |
|
| MMG agar | (NH4)2SO4 |
10 mM |
– |
| MMG agar | NaNO2 |
10 mM |
– |
| MMG agar | KNO3 |
10 mM |
– |
-, no growth
Usable Energy Sources in Autotrophic Culture
NH33 could grow on MM agar plate in the presence of 1350 ppm of gaseous ammonia (specific growth rate: 0.11 ± 0.01; Figure 2B, Table 2), but it could not grow on MM agar plate containing dissociated ammonia, i.e., 10 mM (NH4)2SO4 or NH4Cl, instead of gaseous ammonia (Table 2). These results suggested that NH33 could specifically use undissociated ammonia, i.e., gaseous ammonia, but not dissociated ammonia as an energy source. In addition, NH33 did not grow on MM agar medium without carbon and nitrogen sources (Table 2).
Table 2: Usable energy sources for Paenibacillus lentus NH33 growth on MM agar plate (autotrophic medium)
|
Medium |
Energy source |
Cell yield (mg wet weight/h/plate) |
|
| MM agar | Gaseous ammonia |
1350 ppm |
0.11 ± 0.01 |
| MM agar | (NH4)2SO4 |
10 mM |
– |
| MM agar | NH4Cl |
10 mM |
– |
| MM agar | None |
– |
|
-, no growth
Rate of Stable Nitrogen Isotope Uptake in NH33 Cells
NH33 was cultured in the presence of stable nitrogen isotope-labeled [15N] gaseous ammonia (170 ppm) or conventional gaseous ammonia (170 ppm) at 40°C for 72 h. No difference in the growth rate was observed between the two cultures. The rate of stable nitrogen isotope uptake (calculated as the ratio of 15N/total N) in the recovered cells was analyzed. As shown in Table 3, all of the nitrogen molecules in the cells grown in the presence of the stable nitrogen isotope-labeled [15N] gaseous ammonia were replaced by the stable nitrogen isotope. The rate of stable nitrogen isotope uptake (15N/total N) in the cells grown in the presence of the conventional gaseous ammonia was 0.0036, which is similar to the value of the natural content of 15N [23,24]. These results confirmed that NH33 took up and assimilated nitrogen derived from the gaseous ammonia. Thus, NH33 is a novel organism that uses gaseous ammonia as both an energy source and nitrogen source.
Table 3: Stable 15N isotope content in Paenibacillus lentus NH33 grown in 170 ppm of 15N ammonia gas
|
Growth type |
15N atoms/total N atoms |
| Cells grown in 15NH3 gas |
0.99999 |
| Cells grown in NH3 gas |
0.00367 |
| Natural content of 15N |
0.00366 |
Detection of Nitrite in NH33 Cells
NH33 cells that were cultured on MM agar plate in the presence of 170 ppm of gaseous ammonia at 40°C for 72 h (autotrophic growth cells) were collected and physically ground. The naphthylethylenediamine visual colorimetric method was used to detect nitrite. Figure 5 shows the absorbance spectrum of nitrite solution. The single peak at 540 nm indicates the presence of nitrite, and the maximum absorbance value of the peak reflects the amount of nitrite. As shown in Figure 5, line C, the absorbance peak at 540 nm was observed in the supernatant of the ground cells. The absorbance value at 540 nm indicated that 0.1 µg of nitrite was extracted from 100 mg (wet weight) of NH33 cells, which suggested that gaseous ammonia was oxidized to nitrite by NH33. No nitrite was detected in the agar medium, indicating that NH33 accumulates nitrite intracellularly, and does not eliminate it.
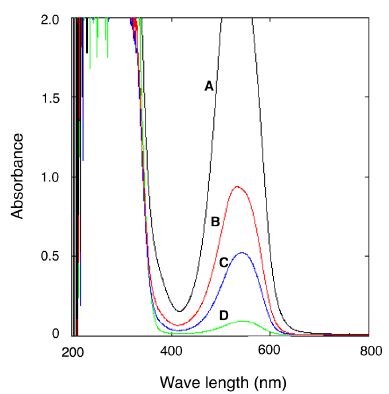
Figure 5: Detection of nitrite in the cell-free extract from autotrophically grown Paenibacillus lentus NH33 cells. The absorbance spectra of 0.5 mg/L (line A), 0.2 mg/L (line B), and 0.05 mg/L (line D) nitrite standard solutions were determined by a spectrophotometer. The spectral peak indicates the nitrite content. The specific peak (line C) was also detected in the cell-free extract.
Discussion
NH33 was first isolated from soil using minimal medium (MM) agar plate containing 28 mM glucose in the presence of gaseous ammonia at 1350 ppm [4]. This bacterium could not grow on MM agar plate containing glucose without gaseous ammonia, indicating that it cannot carry out aerobic nitrogen fixation. Bacillus subtilis str. 168 [25,26] and Escherichia coli K-12 [27] could not grow on nutrient agar plate in the presence of gaseous ammonia at 1350 ppm. NH33 is not only a gaseous ammonia-requiring microbe, but also a gaseous ammonia-tolerant microbe. This report showed for the first time that NH33 is capable of growing on MM agar plate in the presence of gaseous ammonia. This growth property indicated chemolithoautotrophic growth, in which energy is gained through ammonia oxidization. NH33 could grow on MM with or without organic substrates in the presence of gaseous ammonia, indicating mixotrophic growth. Up until now, many researchers have thought that ammonia-oxidizing bacteria do not require an organic carbon source, and that autotrophic growth using ammonia is inhibited by the presence of organic carbons [28]. However, that notion was put into question when a pure culture of a mixotrophic ammonia-oxidizing bacterium (NH33) was established. Ammonia usually has a dual nutritional role: it serves simultaneously as a source of nitrogen and energy [29] for some chemolithoautotrophic bacterial species. It was suggested that NH33 uses glucose as an energy and carbon source, and gaseous ammonia mainly as a nitrogen source, when grown on MMG agar plate. When NH33 is grown on MM agar plate, it uses gaseous ammonia as both an energy and nitrogen source, and carbon dioxide as a carbon source. Microorganisms take up extracellular ammonia, nitrate, urea, and amino acids as nitrogen substrates, and assimilate them [30]. The most reductive form of nitrogen (ammonia) is a favorable nitrogen source as it is energetically efficient. When NH33 was grown on MMG agar plate in the presence of gaseous ammonia, it used gaseous ammonia (undissociated ammonia) as the main nitrogen source. However, NH33 grown on MMG was unable to use ammonium (protonated ammonia), nitrite, and nitrate as a substitute for gaseous ammonia. To introduce ionic substrates intracellularly, organisms require the active transporter (channel protein) system [31,32]. NH33 may lack gene sets involved in the active transporter system for nitrogen species uptake or express them at extremely low levels. Undissociated substances, such as gaseous ammonia, permeate through the cell membrane to the cytoplasm by passive diffusion [33]. The cell surface of NH33 has a high affinity for gaseous ammonia, and can directly assimilate gaseous ammonia intracellularly. NH33 likely gains energy by the oxidation of gaseous ammonia during chemolithoautotrophic growth (growth on MM agar plate in the presence of gaseous ammonia). Ammonia is a stable compound consisting of a nitrogen atom and three hydrogen atoms. It is well-known that the unshared electron pair in the ammonia molecule readily undergoes protonation for the formation of an ammonium ion, as indicated by the following formula: NH3 + H+ ⇄ NH4+. Ammonia in solution is present as NH3 and NH4+, and the ratio of NH3/NH4+ depends on the pH, as defined by the Henderson-Hasselbalch equation [34]. For example, under physiological conditions with a blood pH of 7.4, more than 98% of ammonia is found in the NH4+ form. NH33 may use dissociated ammonia (ammonium ion) as an energy source by dissolving gaseous ammonia in the agar medium. However, NH33 was incapable of growth on MM agar plate (pH 7.0) containing ammonium ion (ammonium sulfate or ammonium chloride). NH33 could be defined as a nitrifying bacterium that uses only undissociated ammonia, but not dissociated ammonia as an energy source. Therefore, NH33 is an obligate that requires gaseous ammonia as both a nitrogen source and an energy source to support its chemolithoautotrophic metabolism.
NH33 is a chemolithoautotrophic bacterium that uses gaseous ammonia as the sole energy source. It extracts energy from a single inorganic source (NH3), assimilates inorganic substrates (e.g., CO2 and NH3), and uses them to synthesize all of the biochemicals necessary to support growth. Ammonia-oxidizing bacteria are incapable of growing mixotrophically [35]. In this study, rather than inhibiting growth, organic compounds, such as glucose, enhanced the growth of NH33. It was considered that NH33 preferentially uses glucose before gaseous ammonia when grown in the presence of gaseous ammonia and glucose simultaneously. Lactose and fructose promoted cell growth more than glucose (data not shown). This mixotrophic growth property suggested that NH33 likely expresses beta-galactosidase and glucose isomerase [36,37]. NH33 is the first reported ammonia-oxidizing bacterium to show mixotrophic growth properties. During mixotrophic growth on MMG agar plate, NH33 mainly used ammonia gas as a nitrogen source. The optimum gaseous ammonia concentration for mixotrophic growth was 1350 ppm. During autotrophic growth on MM agar plate, NH33 used gaseous ammonia as both an energy and nitrogen source. The optimum ammonia gas concentration for autotrophic growth was 170 ppm. Organic substances, such as glucose, were thought to promote the formation of mucoid-like colonies [38] and enhance ammonia gas resistance. The amount of gaseous ammonia required to obtain 1.0 g of cells (wet weight) was 0.62 to 0.64 mmol in both the mixotrophic and autotrophic growth conditions.
NH33 was cultured under autotrophic growth conditions in the presence of gaseous ammonia containing stable isotope nitrogen (15N), and the stable isotope nitrogen ratio in the cells was subsequently examined. All of the nitrogen in the cells was 15N, confirming that the gaseous ammonia in the air had been assimilated into NH33 cells. Autotrophic ammonia-oxidizing bacteria are among a specific group of microbes that are able to use ammonia as the sole energy source and reductant for growth. They extract energy from a single inorganic source (NH3), assimilate inorganic substrates (CO2 and NH3), and use them to synthesize all of the biochemicals necessary to support growth. In ammonia-oxidizing bacteria, ammonia is first oxidized to hydroxylamine by ammonia monooxygenase [39]. The hydroxylamine is then oxidized to nitrite by hydroxylamine oxidoreductase. The predominant nitrogen oxide produced in the process of ammonia oxidation is nitrite [40]. Nitrite was detected in the NH33 cells grown autotrophically in the presence of 170 ppm gaseous ammonia. The final substance of gaseous ammonia oxidation in NH33 was nitrite, suggesting that NH33 has an ammonia oxidation mechanism similar to that of conventional ammonia-oxidizing bacteria. However, it remains unclear whether nitrate can be detected in NH33. It is necessary in future studies to determine whether NH33 can completely oxidize ammonia. Chemolithoautotrophic ammonia-oxidizing bacteria are responsible for the rate-limiting step of nitrification in a wide variety of environments, and are thus very important in the global cycling of nitrogen. NH33 is an autotrophic bacterium capable of both mixotrophic and autotrophic growth. Under autotrophic conditions, it uses only gaseous ammonia as an energy source and a nitrogen source. These findings considerably challenge the currently accepted role of microbial communities in global nitrogen cycling. Due to the importance of this functional group of bacteria, understanding of their ecology and physiology has become a subject of intense research in recent years.
Conclusion
Previous studies of ammonia-oxidizing bacteria have reported ammonium ions in a dissociated state as an energy source. Many researchers are unaware of the existence of organisms that can grow in the presence of gaseous ammonia, such as the Paenibacillus lentus NH33 examined in this study. P. lentus NH33 is a previously unknown ammonia-oxidizing bacterium that uses gaseous ammonia as its sole nitrogen source for both assimilation and dissimilation. Understanding and systematizing gaseous ammonia-requiring bacteria in detail will contribute to the elucidation of yet-unknown roles of ammonia in the biosphere and details of the nitrogen cycle.
Acknowledgement
This research was supported by a Grant-in-Aid from the Japan Science and Technology Agency (Grant Number: MP28116808363).
References
- Senanu BM, Boakye P, Oduro-Kwarteng S, Sewu DD, et al. (2021) Inhibition of ammonia and hydrogen sulphide as faecal sludge odour control in dry sanitation toilet facilities using plant waste materials. Scientific Reports 11: 1-13. [crossref]
- Bertone E, Chang C, Thiel P, O’Halloran K (2018) Analysis and modelling of powdered activated carbon dosing for taste and odour removal. Water Res 139: 321-328.
- Kalemba K, Kasperczyk D, Urbaniec K, Kozik V (2017) Biological methods for odor treatment-A review. J Clean Prod 152: 223-241.
- Tada S, Itoh Y, Kiyoshi K, Yoshida N (2021) Isolation of ammonia gas-tolerant extremophilic bacteria and their application to the elimination of malodorous gas emitted from outdoor heat-treated toilets. J Biosci Bioeng 131: 509-517. [crossref]
- Myszograj S, Płuciennik-Koropczuk E (2020) COD and nitrogen compounds balance in mechanical-biological wastewater treatment plant with sludge treatment. Desalination Water Treat 186: 443-449.
- Reitzer L (2003) Nitrogen assimilation and global regulation in Escherichia coli. Annu Rev Microbiol 57: 155-176. [crossref]
- Dasarathy S, Mookerjee RP, Rackayov V, Thrane VR, Balasubramaniyan V, et al. (2017) Ammonia toxicity: from head to toe?. Metab Brain Dis 32: 529-538. [crossref]
- Arp DJ, Stein LY (2003) Metabolism of inorganic N compounds by ammonia-oxidizing bacteria. Crit Rev Biochem Mol Biol 38: 471-495. [crossref]
- Kuenen JG (2008) Anammox bacteria: from discovery to application. Nat Rev Microbiol 6: 320-326.
- Hu HW, He JZ (2017) Comammox-a newly discovered nitrification process in the terrestrial nitrogen cycle. J Soils Sediments 17: 2709-2717.
- Nicol GW, Schleper C (2006) Ammonia-oxidising Crenarchaeota: important players in the nitrogen cycle?. Trends Microbiol 14: 207-212.
- Steinmüller W, Bock E (1976) Growth of Nitrobacter in the presence of organic matter. I. Mixotrophic growth. Arch Microbiol 108: 299-304. [crossref]
- Pflug IJ, Smith GM, Christensen R (1981) Effect of soybean casein digest agar lot on number of Bacillus stearothermophilus spores recovered. Appl Environ Microbiol 42: 226-230. [crossref]
- Haar L, Gallagher JS (1978) Thermodynamic properties of ammonia. J Phys Che Ref Data 7: 635-792.
- Wenzel M, Dekker MP, Wang B, Burggraaf MJ, Wilbert B, et al. (2021) A flat embedding method for transmission electron microscopy reveals an unknown mechanism of tetracycline. Commun Biol 4: 1-13. [crossref]
- Tayasu I, Hirasawa R, Ogawa NO, Ohkouchi N, et al. (2011) New organic reference materials for carbon-and nitrogen-stable isotope ratio measurements provided by Center for Ecological Research, Kyoto University, and Institute of Biogeosciences, Japan Agency for Marine-Earth Science and Technology. Limnology (Tokyo) 12: 261-266.
- Ridnour LA, Sim JE, Hayward MA, Wink DA, et al. (2000) A spectrophotometric method for the direct detection and quantitation of nitric oxide, nitrite, and nitrate in cell culture media. Anal Biochem 281: 223-229. [crossref]
- Payment P, Coffin E, Paquette G (1994) Blood agar to detect virulence factors in tap water heterotrophic bacteria. Appl Environ Microbiol 60: 1179-1183. [crossref]
- Chiarelli A, Cabanel N, Rosinski-Chupin I, Zongo PD, Thierry N, et al. (2020) Diversity of mucoid to non-mucoid switch among carbapenemase-producing Klebsiella pneumoniae. BMC Microbiol 20: 1-14.
- Park SY, Kim R, Ryu CM, Choi SK, Choong HL et al. (2008) Citrinin, a mycotoxin from Penicillium citrinum, plays a role in inducing motility of Paenibacillus polymyxa. FEMS Microbiol Ecol 65: 229-237. [crossref]
- Fünfhaus A, Göbel J, Ebeling J, Knispel H, et al. (2018) Swarming motility and biofilm formation of Paenibacillus larvae, the etiological agent of American Foulbrood of honey bees (Apis mellifera). Sci Rep 8: 1-12.
- Chain P, Lamerdin J, Larimer F, Regala W, et al. (2003) Complete genome sequence of the ammonia-oxidizing bacterium and obligate chemolithoautotroph Nitrosomonas europaea. J Bacteriol 185: 2759-2773. [crossref]
- Naidu K, Maseko S, Kruger G, Lin J (2020) Purification and characterization of α-amylase from Paenibacillus D9 and Escherichia coli recombinants. Biocatal Biotransformation 38: 24-34.
- Pethybridge H, Choy CA, Logan JM, Allain V et al. (2018) A global meta-analysis of marine predator nitrogen stable isotopes: Relationships between trophic structure and environmental conditions. Glob Ecol Biogeogr 27: 1043-1055.
- Zolfaghari Emameh R, Kazokaitė J, Yakhchali B (2021) Bioinformatics analysis of extracellular subtilisin E from Bacillus subtilis. J Biomol Struct Dyn 4: 1-8. [crossref]
- Zeigler DR, Prágai Z, Rodriguez S, Chevreux B, et al. (2008) The origins of 168, W23, and other Bacillus subtilis legacy strains. J Bacteriol 190: 6983-6995. [crossref]
- Sandler SJ, Leroux M, Windgassen TA, Keck JL (2021) Escherichia coli K‐12 has two distinguishable PriA‐PriB replication restart pathways. Mol Microbiol 116: 1140-1150. [crossref]
- Krümmel A, Harms H (1982) Effect of organic matter on growth and cell yield of ammonia-oxidizing bacteria. Microbiol. 133: 50-54.
- Kowalchuk GA, Stephen J (2001) Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Annu Rev Microbiol 55: 485-529. [crossref]
- Ouyang Y, Norton JM, Stark JM, Reeve JR, et al. (2016) Ammonia-oxidizing bacteria are more responsive than archaea to nitrogen source in an agricultural soil. Soil Biol Biochem 96: 4-15.
- Islam S, Islam R. Kandwal P, Khanam S, et al. (2020) Nitrate transport and assimilation in plants: a potential review. Arch Agron Soil Sci 68: 1-18.
- Li C, Ariga I, Mikami K (2019) Difference in nitrogen starvation-inducible expression patterns among phylogenetically diverse ammonium transporter genes in the red seaweed Pyropia yezoensis. Am J Plant Sci 10: 1325-1349.
- Keerio HA, Bae W, Park J, Kim M (2020) Substrate uptake, loss, and reserve in ammonia-oxidizing bacteria (AOB) under different substrate availabilities. Process Biochem 91: 303-310.
- Thurston RV, Russo RC, Vinogradov GA (1981) Ammonia toxicity to fishes. Effect of pH on the toxicity of the unionized ammonia species. Environ Sci Technol 15: 837-840.
- Liu X, Lin J, Tian K, Yan W (2008) Metabolism of organic compounds by extremely acidophilic, obligately chemolithoautotrophic Thiobacilli: a review. Chin J biotechnol 24: 1-7.
- Kim KH, Seo YL, Baek JH, Jin HM, et al. (2021) Paenibacillus agri nov., isolated from soil. Int J Syst Evol Microbiol 71.
- Soni R, Nanjani S, Keharia H (2021) Genome analysis reveals probiotic propensities of Paenibacillus polymyxa Genomics 113: 861-873.
- Daane LL, Harjono I, Barns SM, Launen L, et al. (2002) PAH-degradation by Paenibacillus and description of Paenibacillus naphthalenovorans sp. nov., a naphthalene-degrading bacterium from the rhizosphere of salt marsh plants. Int J Syst Evol Microbiol 52: 131-139. [crossref]
- Hommes NG, Sayavedra-Soto LA, Arp DJ (2001) Transcript analysis of multiple copies of amo (encoding ammonia monooxygenase) and hao (encoding hydroxylamine oxidoreductase) in Nitrosomonas europaea. J Bacteriol 183: 1096-1100. [crossref]
- Sepehri A, Sarrafzadeh MH (2019) Activity enhancement of ammonia-oxidizing bacteria and nitrite-oxidizing bacteria in activated sludge process: metabolite reduction and CO2 mitigation intensification process. Appl Water Sci 9: 1-12.