Abstract
Background: Oral carcinogenesis involves genetic changes affecting proto-oncogenes and tumor suppressor genes like P16, P53, H-ras, cyclinD1, and EGFR. Podoplanin, a transmembrane glycoprotein, is a biomarker linked to tumor progression and prognosis in oral squamous cell carcinoma (OSCC). This study aimed to assess podoplanin expression across OSCC grades and its relationship with clinicopathological factors such as age, gender, habits, tumor site, lymphovascular invasion, perineural invasion, and tumor-infiltrating lymphocytes.
Methods: A cross-sectional study at BIRDEM Hospital, Dhaka, analyzed 56 OSCC cases over two years using hematoxylin and eosin-stained slides and tissue blocks. Podoplanin expression was evaluated immunohistochemically and classified as high or low based on staining intensity and extent.
Results: High podoplanin expression was observed in 33.90% of cases, predominantly in moderately and poorly differentiated OSCC, compared to well-differentiated tumors. A significant association was found between podoplanin expression and histological grade (p<0.05). However, no significant relationships were observed with age, gender, habits, anatomical site, lymphovascular or perineural invasion, or tumor-infiltrating lymphocytes.
Conclusion: Podoplanin expression correlates significantly with OSCC histological grade, suggesting its potential as a prognostic marker. High expression may indicate aggressive tumors and poorer outcomes, highlighting its relevance in evaluating and managing OSCC.
Keywords
Podoplanin, Oral Squamous Cell Carcinoma, Immunohistochemistry, Histological Grade, Prognosis, Biomarker
Introduction
Oral cancer is the sixth most common malignancy worldwide and is one of the major public health problems [1]. Oral squamous cell carcinoma (OSCC) is the most common type of malignancy, representing up to 90 percent of all oral neoplasms [2]. Oral cancer ranks 3rd, 2nd, and 3rd in five-year prevalence, incidence, and mortality rate respectively in Bangladesh [3]. Common risk factors for developing OSCC are tobacco smoking, betel nut chewing, and alcohol consumption. Other predisposing factors are viral infection, chronic irritation, oral candidiasis, poor oral hygiene, ionizing radiation, etc [4].
Podoplanin (PDPN) is known to be expressed in numerous human tumors, including squamous cell carcinoma of the oral cavity, mesothelioma, testicular seminoma, soft tissue tumors, thymoma, and brain tumors [5]. PDPN seems to be expressed in aggressive tumors, with higher invasive and metastatic potential [6].
PDPN consists of an extracellular or transmembrane domain and a cytoplasmic domain. The extracellular domain is longer and carries four platelet aggregation-stimulating (PLAG) domains with plenty of potential O-glycosylation sites, crucial for interaction with platelets. The interaction between PDPN and c-type lectin (CLEC2) may regulate tumor invasion and metastasis [7]. The shorter cytoplasmic domain is associated with ezrin/radixin/moesin (ERM) protein that bridges plasma membrane proteins and the actin cytoskeleton [8]. PDPN overexpression causes marked phosphorylation of ERM proteins. It causes adhesion and cancer cell migration through modulating the actin cytoskeleton, Rho A, and epithelial-mesenchymal transition. PDPN causes down-regulation of E-cadherin and overexpression of matrix metalloproteinase [6].
It is upregulated in the invasive front of OSCC. It causes upregulation of TGF-β1 secretion and increases Epidermal Growth Factor Receptor phosphorylation as well as downstream effectors of AKT and ERK. PDPN also causes OSCC progression by interacting with matrix metalloproteinase and induces cytoskeletal remodelling, extracellular matrix degradation, and invasion [9].
PDPN in oral SCC causes tumor cell motility and metastasis by activation of endogenous lectin that binds to extracellular carbohydrate moieties. These cancer cells are remarkably resistant to currently available chemotherapy. Thus, PDPN becomes a chemotherapeutic target for oral SCC [10]. Anti-human Podoplanin antibody (NZ-1) would be an important target for the prevention of metastasis in the future for OSCC [6].
Materials and Methods
Study Design and Subjects
This cross-sectional observational study was conducted at the Department of Pathology, Bangladesh Institute of Research and Rehabilitation in Diabetes, Endocrine and Metabolic Disorder (BIRDEM), during the period of March 2022 to February 2024. This study was approved by the Institutional Review Board (IRB) of BIRDEM Hospital, Dhaka, Bangladesh. Written informed consent was obtained from all participants, and confidentiality of patient data was maintained in compliance with ethical standards. A total of 56 histologically diagnosed cases of oral squamous cell carcinoma (OSCC) were included in this study, meeting the following inclusion criteria: small and resected biopsy cases of OSCC. Exclusion criteria included carcinoma in situ, dysplastic lesions of the oral cavity, and patients who received neoadjuvant therapy or radiotherapy before surgery.
Demographic and clinical information, such as age, sex, clinical presentation, and histopathological diagnosis (including grade), were collected from the departmental records of the pathology department.
Sample Collection
The 56 cases of oral SCC were retrieved from the Department of Pathology, BIRDEM, including H&E stained slides and paraffin- embedded blocks. All slides were reviewed to assess tumor grade and other prognostic factors, including tumor-infiltrating lymphocytes (TILs), and the presence of lymphovascular and perineural invasion.
Immunohistochemical Staining for Podoplanin
After confirming the diagnosis of OSCC, immunohistochemical (IHC) staining for podoplanin was performed at Bangabandhu Sheikh Mujib Medical University (BSMMU), using an appropriate positive control. Paraffin-embedded tissue blocks were sectioned at 3 µm thickness. The slides were gently lowered onto a water bath set to 45°C, spread without wrinkles onto slides coated with 0.1% poly-L- lysine, and air-dried. Slides were then baked at 60°C for 30 minutes on a hot plate. Dewaxing was performed by treating the slides in xylene, followed by rehydration through a graded series of alcohols.
For antigen retrieval, the slides were immersed in preheated citrate buffer in a pressure cooker, boiled, and allowed to cool naturally. To block endogenous enzyme activity, hydrogen peroxide was added in a moist chamber at room temperature.
Primary and Secondary Antibodies
- Primary antibody: Monoclonal Rabbit d2-40 (pre-diluted, ready-to-use).
- Secondary antibody: DAKO REAL™ Envision™ (HRP Rabbit/ Mouse) (ENV).
- Positive control: Appedicular tissue was used as a positive
-
Chromogen: 3,3’-diaminobenzidine (DAB) was used for visualizing the antigen-antibody Finally, slides were counterstained with hematoxylin.
Scoring of Podoplanin Expression
Podoplanin expression was assessed by reviewing 10 representative areas from each slide. For each area, 100 tumor cells were counted, and both the percentage of positive cells and the intensity of podoplanin expression were evaluated. The following scoring system, as introduced by Yuan et al. (2006), was applied:
Quantitative Score
- 0: 0% positive cells
- 1: 1-10% positive cells
- 2: 11-30% positive cells
- 3: 31-50% positive cells
- 4: 51-80% positive cells
- 5: 81-100% positive cells
Intensity Score
- 0: Negative (no staining)
- 1: Weak (faint staining)
- 2: Moderate (staining between weak and dark brown)
- 3: Strong (dark brown staining)
The immunoreactive score (IRS) for podoplanin expression was calculated by multiplying the quantitative score by the intensity score, yielding a final score ranging from 0 to 15. According to this score, the expression was classified as follows:
- 0-7: Low expression
- ≥8: High expression
Statistical Analysis
All collected data were compiled and analyzed using SPSS (Statistical Package for Social Sciences), version 25. The Chi-square test was used to assess statistical significance between different clinicopathologic variables. A p-value of <0.05 was considered statistically significant.
Results
A total of 56 oral squamous cell carcinoma (OSCC) patients were included in the study, and their clinicopathologic characteristics along with Podoplanin expression were analyzed. The association between Podoplanin expression and various clinicopathologic parameters, as well as histological grading, was evaluated (Tables 1 and 2).
Table 1: Clinicopathologic parameters of OSCC patients and their association with Podoplanin expression (n =56).
|
Clinicopathologic Parameter |
Podoplanin Expression | P value | ||
| High |
Low |
|||
| Age distribution | ||||
| Up to 40 years |
2(33.3) |
4(66.7) |
0.58 |
|
| 41-50 years |
3 (42.9) |
4(57.1) |
||
| 51-60 years |
4(25.0) |
12(75.0) |
||
| 61-70 years |
7(50.0) |
7(50.0) |
||
| Above 70 years |
3(23.1) |
10(76.9) |
||
| Gender | ||||
| Female |
8(29.6) |
19(70.4) |
0.58 |
|
| Male |
11(37.9) |
18(62.1) |
||
| Clinical Presentation | ||||
| Ulcer |
15(35.7) |
27(64.3) |
0.58 |
|
| Red patches |
2(33.3) |
4(66.7) |
||
| White patches |
1 (33.3) |
2(66.7) |
||
| Exophytic growth |
1 (20.0) |
4(66.1) |
||
| Characteristics | ||||
| Tobacco | Yes |
9(39.1) |
14(60.9) |
0.57 |
| No |
6(46.2) |
7(53.8) |
||
| Betel quid | Yes |
12 (34.3) |
23(65.7) |
1 |
| No |
7(33.3) |
14(63.8) |
||
| Tobacco & betel quid both | Yes |
3 (30.0) |
7(70.0) |
1 |
| No |
16(34.8) |
30(66.1) |
||
| Anatomic Location | ||||
| Tongue |
7(46.7) |
8(53.3) |
0.24 |
|
| Buccal mucosa |
11(34.4) |
21(65.6) |
||
| Others |
1 (11.1) |
8(88.9) |
||
The table summarizes the distribution of Podoplanin expression (high vs. low) based on various clinicopathologic parameters, including age, gender, clinical presentation, tobacco use, betel quid use, and anatomic location of the tumor. The P values indicate the statistical significance of the associations between Podoplanin expression and each parameter. No significant associations were observed between Podoplanin expression and age, gender, clinical presentation, or personal habits. The only significant association found was between Podoplanin expression and histological grading (not shown in this table).
Podoplanin Expression and Clinicopathologic Parameters
Podoplanin expression was classified as high or low based on immunohistochemical staining. High Podoplanin expression was observed in 19 cases (33.90%) of OSCC. The distribution of high and low Podoplanin expression did not show any statistically significant association with age, gender, clinical presentation, or personal habits (tobacco use, betel quid use). Specifically, no significant differences in Podoplanin expression were noted across different age groups (P = 0.58), between genders (P = 0.58), or based on the clinical presentation (P = 0.58). Furthermore, Podoplanin expression did not correlate with tobacco use (P = 0.57), betel quid use (P = 1.0), or both tobacco and betel quid use (P = 1.0). Similarly, no significant association was found between Podoplanin expression and the anatomic location of the tumors (P = 0.24) (Table 1 & 2).
Podoplanin Expression and Histological Grading
The association between Podoplanin expression and the histological grading of OSCC was found to be statistically significant (P = 0.00) (Table 2). Podoplanin expression varied considerably across different grades of differentiation. In well-differentiated OSCC (n = 25), no cases exhibited high Podoplanin expression, with all tumors showing low expression. In moderately differentiated OSCC (n = 27), 17 cases (63.0%) exhibited high Podoplanin expression, while 10 cases (37.0%) showed low expression. In poorly differentiated OSCC (n = 4), high Podoplanin expression was observed in 2 cases (50.0%) and low expression in the remaining 2 cases (50.0%) (Table 2).
This significant association between Podoplanin expression and histological grading suggests that Podoplanin expression is higher in less differentiated OSCC, with well-differentiated tumors showing predominantly low expression. The findings suggest that Podoplanin may serve as a useful marker of tumor differentiation, with higher expression being indicative of greater aggressiveness and poorer differentiation (Figures 4-7).
Table 2: Histological grading of OSCC and immune-histochemical expression of podoplanin (n=56).
|
Histological grading |
Expression of Podoplanin |
P Value* |
|
|
High |
Low |
||
| Well differentiated |
0 (0.0) |
25 (100) |
0.00 |
| Moderately differentiated |
17 (63.0) |
10 (37) |
|
| Poorly differentiated |
2 (50.0) |
2 (50.0) |
|
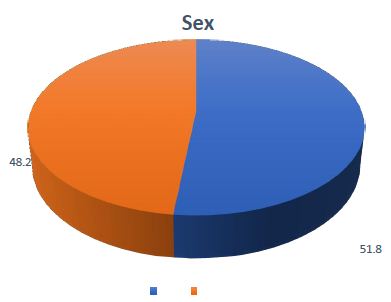
Figure 1: Gender distribution of the patients with Oral SCC (n =56). The pie chart illustrates the gender distribution among the study participants. The results indicate a higher prevalence of oral SCC in males, with a male-to-female ratio of 1.07: 1.

Figure 2: Locations of SCC in the oral cavity (n=56). The figure shows the distribution of oral squamous cell carcinoma (SCC) cases based on their anatomical location within the oral cavity. Among the 56 patients, the majority of biopsies (57.1%) were taken from the buccal mucosa, followed by the tongue. The remaining cases were found in the retromolar trigone (16.1%), alveolus (10.7%), and gingiva (3.6%). A small percentage of cases (1.8%) were located in other areas of the oral cavity.
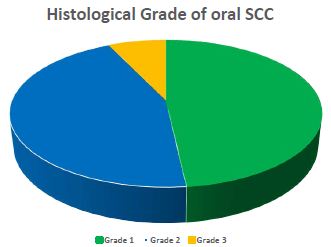
Figure 3: Histological grade of oral SCC (n=56). The histological grade of oral SCC was assessed as per criteria set by WHO 2017. Among 56 patients, 48.2% were moderately differentiated (grade 2) followed by 44.6% of well differentiated (grade 1). Only 7.1% showed poorly differentiated (grade 3).
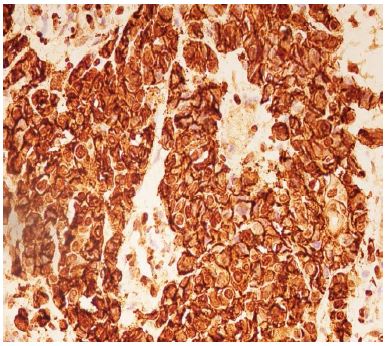
Figure 4: High expression of podoplanin IRS – in Moderately Differentiated Squamous Cell Carcinoma.
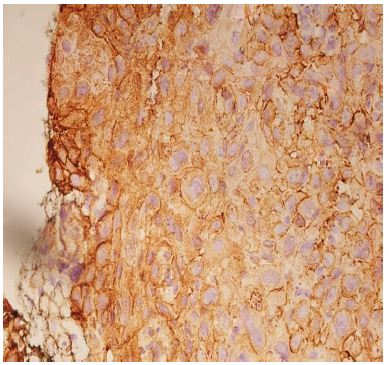
Figure 5: High expression of podoplanin IRS – in Moderately Differentiated Squamous Cell Carcinoma.
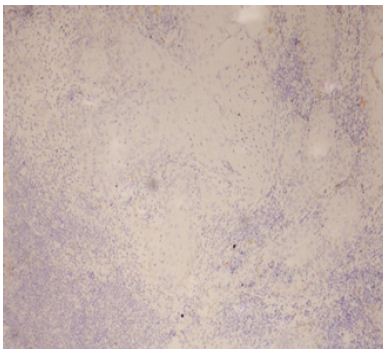
Figure 6: Low expression of Podoplanin IRS – in well Differentiated Squamous Cell Carcinoma.
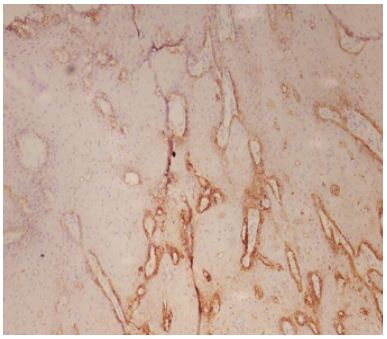
Figure 7: Low expression of Podoplanin IRS – in well Differentiated Squamous Cell Carcinoma.
Discussion
The present study demonstrates that Podoplanin expression is present in nearly all cases of oral squamous cell carcinoma (OSCC), with a statistically significant association observed with histological grading. Specifically, the expression of Podoplanin was found to be higher in moderately and poorly differentiated OSCC cases, while all well-differentiated tumors exhibited low expression. This finding supports the hypothesis that Podoplanin could serve as an important biomarker for the aggressive nature of OSCC, with its expression correlating with poorer histological differentiation.
In this study, 33.9% of the cases showed high Podoplanin expression, while the remaining 66.1% exhibited low expression. These findings are consistent with those of other studies, although variability in the levels of Podoplanin expression has been reported in different populations globally. Previous studies have documented a range of Podoplanin expression, with high expression varying from 20% to 60% and low expression ranging from 21.6% to 80%. For instance, Yuan et al. (2006) reported that 60% of oral SCC cases exhibited high Podoplanin expression, while other investigators, such as Parhar et al. (2015), Patil et al. (2015), Kim et al. (2015), and Sgaramella et al. (2016), reported high expression rates of 56.6%, 20%, 51.3%, and 51%, respectively [12,14-17]. The considerable variability in these findings can be attributed to several factors, including genetic and ethnic diversity, personal habits such as tobacco and betel quid use, and environmental influences that contribute to the development of oral cancer [1,4].
It is important to note that while the current study found no significant association between Podoplanin expression and factors such as age, gender, anatomic site, clinical presentation, or the use of tobacco and betel quid, other studies have suggested that Podoplanin expression correlates with tumor size, nodal involvement, advanced stage, poor treatment response, and reduced survival rates [5,7,19]. These associations indicate the potential of Podoplanin as a prognostic marker in OSCC, which could aid in predicting clinical outcomes and guiding therapeutic decisions.
Furthermore, the observed relationship between Podoplanin expression and histological grading is in line with studies by Kim et al. (2015), Pradhan et al. (2019), and Patil et al. (2015), who also reported significant associations between high Podoplanin expression and poorly differentiated or moderately differentiated tumors [16,18,19]. However, our results differ from studies by Yuan et al. (2006), Aiswariya et al. (2019), and Prasad et al. (2015), who did not observe the same level of correlation between Podoplanin expression and histological grade [12,13,19].
The reasons for these discrepancies can likely be attributed to differences in study design, sample sizes, and the specific characteristics of the populations studied. For instance, genetic and environmental factors, as well as variations in diagnostic and histopathological assessment methods, may contribute to differing findings. Therefore, while the relationship between Podoplanin expression and OSCC is becoming increasingly recognized, further research involving larger and more diverse cohorts is necessary to better understand the complex interplay of factors that influence its expression and its potential as a prognostic biomarker.
In conclusion, the current study reinforces the notion that Podoplanin expression is closely linked to the histological grading of OSCC, particularly with higher expression observed in more aggressive, poorly differentiated tumors. Despite some inconsistencies in the literature, the potential of Podoplanin as a prognostic marker for OSCC, especially in predicting tumor progression and patient outcomes, remains promising and warrants further exploration.
Conclusion
In recent years, numerous studies have sought to elucidate the role of Podoplanin in oral squamous cell carcinoma (OSCC), yet significant controversies remain in the scientific literature. In this study, we observed that Podoplanin expression was present in nearly all OSCC cases in Bangladesh, with high expression predominantly associated with higher-grade tumors. Furthermore, tumors originating from the tongue and those exhibiting lymphovascular and perineural invasion showed elevated levels of Podoplanin expression. While these findings are promising, the study’s interpretation is limited by the small sample size, reliance on small biopsy specimens, and the absence of follow-up data. To solidify these results, further research with a larger cohort, utilizing resected specimens, is essential. Additionally, future studies should explore the role of Podoplanin in tumor staging, nodal metastasis, overall survival, and its potential as a target for novel therapies. By advancing our understanding of Podoplanin’s prognostic value, we can pave the way for more precise diagnostic and therapeutic strategies in OSCC management.
Acknowledgement
The study was guided and supported by Dr. Nazma Afroze, Professor of Pathology, whose expert advice and encouragement were invaluable throughout the research.
Gratitude is extended to the medical technologists and staff at BIRDEM General Hospital, Dhaka, and Bangabandhu Sheikh Mujib Medical University (BSMMU) for their diligent assistance. Special recognition is given to Md. Joynal Abedin, Md. Fazle Elahi, Mrs. Shimu Akhter, Mr. Sojib Sorder, Md. Aorango Jeb Sheikh, and Mr. Ruhul Amin Bhuiyan for their contributions.
Appreciation is also expressed to all the patients and their attendants who participated in this study, and to the colleagues and contemporary MD student Dr. Bilkis for their cooperation and support.
Finally, sincere thanks are given to the families, including first author’s parents, in-laws, husband Md. A. Rahim, and daughter Manha, for their unwavering support and encouragement.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Funding Statement
No specific funding was received for this study.
Conflict of Interest Disclosure
The authors declare that there are no financial, personal, or professional conflicts of interest that could have influenced the work presented in this manuscript. All authors have disclosed any potential conflicts, and no competing interests exist.
Ethics Approval Statement
This study was approved by the institutional ethics committee of Bangladesh Institute of Research and Rehabilitation in Diabetes, Endocrine and Metabolic Disorders (BIRDEM) General Hospital, Dhaka, Bangladesh
Patient Consent Statement
Informed consent was obtained from all patients or their legal guardians prior to participation in the study.
Contribution Statement
The project was designed by the Principal Investigator, Mst. Rubeyatul Jannat. The data collection and article writing were primarily conducted by Mst. Rubeyatul Jannat, Nafisa Abedin, Sayeed Kishwara Kashfi, Shamim Ahamed, and Shahana Sultana. Sayed Kishwara Kashfi also contributed to the literature review. Sadia Shirin provided essential photographic documentation for the study. Shamim Ahamed played a significant role in preparing the manuscript for publication. Shahana Sultana contributed extensively to the discussion section. Prof. Mousumi Ahmed provided crucial intellectual input, offered critical feedback on the manuscript drafts, and guided the team through key methodological and analytical aspects of the study. Prof. Rita Rani Barua offered critical advice regarding the publication process. Nafisa Abedin performed the final proofreading and served as the corresponding author.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request. All relevant data, including patient demographic details, clinicopathological features, and podoplanin immunohistochemical expression levels, have been anonymized to ensure patient confidentiality in compliance with ethical guidelines.
References
- Sah R, Akhter M (2020) Oral Cancer Senario in Multiple Centers of Dhaka, Biomedical Journal of Scientific & Technical Research 32(2).
- Hossain MA, Ahmed MM, Rahman AFM S, Haider MN, Alam AKMS (2015) Epidemiological study of oral squamous cell carcinoma: A hospital based study in Dhaka city. The Journal of Teachers Association RMC (Vol. 28).
- Ferlay J, Ervik M, Lam F, Laversanne M, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F (2024) Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer.
- Hannan MA, Rahman MA, Hossain S, Rahman QB (2018) Role Of Habitual Risk Factors On Oral Squamous Cell Carcinoma. Update Dental College Journal (Vol. 8).
- Lee HY, Yu NY, Lee SH, Tsai HJ, Wu CC, Cheng JC, Chen DP, Wang YR, Tseng CP (2020) Podoplanin promotes cancer-associated thrombosis and contributes to the unfavorable overall survival in an ectopic xenograft mouse model of oral cancer. Biomedical Journal. [crossref]
- MARIUS R, CIMPEAN AM, DOMENICO R (2008) The Role of Podoplanin in Tumor Progression and Metastasis. ANTICANCER RESEARCH. [crossref].
- Hwang BO, Park SY, Cho ES, Zhang X, Lee SK, Ahn HJ, Chun KS, Chung WY, Song NY (2021/15/December) Platelet CLEC2-Podoplanin Axis as a Promising Target for Oral Cancer Frontiers in Immunology. Frontiers Media S.A. [crossref]
- Astarita JL, Acton SE, Turley SJ (2012) Podoplanin: Emerging functions in development, the immune system, and cancer. Frontiers in Immunology. [crossref]
- Li Y yin, Zhou CX, Gao Y (2018) Interaction between oral squamous cell carcinoma cells and fibroblasts through TGF-β1 mediated by podoplanin. Experimental Cell Research. [crossref]
- Ochoa-Alvarez JA, Krishnan H, Pastorino JG, Nevel E, Kephart D, Lee JJ et al (2015) Antibody and lectin target podoplanin to inhibit oral squamous carcinoma cell migration and viability by distinct mechanisms. [crossref]
- Oliveira LR, Ribeiro-Silva A (2011/March) Prognostic significance of immunohistochemical biomarkers in oral squamous cell carcinoma. International Journal of Oral and Maxillofacial Surgery. [crossref]
- Yuan P, Temam S, El-Naggar A, Zhou X, Liu DD, Lee JJ, Mao L (2006) Overexpression of podoplanin in oral cancer and its association with poor clinical Cancer. [crossref]
- Aiswarya A, Suresh R, Janardhanan M, Savithri V, Aravind T, Mathew L (2019) An immunohistochemical evaluation of podoplanin expression in oral leukoplakia and oral squamous cell carcinoma to explore its potential to be used as a predictor for malignant Journal of Oral and Maxillofacial Pathology. [crossref]
- Parhar S, Kaur H, Vashist A, Verma S (2015) Role of podoplanin in potentially malignant disorders and oral squamous cell carcinoma and its correlation with In Indian Journal of Cancer (Vol. 52). Medknow Publications. [crossref]
- Patil A, Patil K, Tupsakhare S, Gabhane M, Sonune S, Kandalgaonkar S (2015) Evaluation of Podoplanin in Oral Leukoplakia and Oral Squamous Cell Scientifica. [crossref]
- Kim HY, Rha KS, Shim GA, Kim JH, Kim JM, Huang SM, Koo BS (2015) Podoplanin is involved in the prognosis of head and neck squamous cell carcinoma through interaction with VEGF-C. Oncology Reports. [crossref]
- Sgaramella N, Jonsson EL, Boldrup L, Califano L, Coates PJ Tartaro G, Muzio L Lo, Fåhraeus R, Colella G, Dell’ G, Orabona A, Loljung L, Santagata M, Rossiello R, Wilms T, Danielsson K, Oran Laurell G, Nylander K (2016) High expression of podoplanin in squamous cell carcinoma of the tongue occurs predominantly in patients £ 40 years but does not correlate with tumour spread. 2: 3–8. [crossref]
- Pradhan S, Guddattu V, Solomon MC (2019) Association of the co-expression of SOX2 and podoplanin in the progression of oral squamous cell carcinomas – An immunohistochemical Journal of Applied Oral Science 27. [crossref]
- Prasad B, Kashyap B, Babu G, Kumar G, Manyam R (2015) Expression of podoplanin in different grades of oral squamous cell carcinoma. Annals of Medical and Health Sciences Research. [crossref]