DOI: 10.31038/IDT.2022312
Abstract
Objective: To explore weight gain in regular practice involving naïve patients, those who continue the same treatment for at least 6 months or those who changed their antiretroviral treatment.
Methods: We performed a retrospective analysis of patients followed-up between 2013 and 2019. This study included 3 groups of participants (naïve patients, those who had been on viral suppression for more than 6 months, and those with a treatment change).
Results: 317 people living with HIV (PLHIV) participated. The proportion of participants in the overweight and obese categories increased over time, from 40 to 43% and from 9.46% to 12.43% respectively. Proportion of metabolic syndrome increased overtime from 3.79 to 6.22%. Stratification by both sex and ethnicity, showed the greatest weight gain among Latin male. Considering the risk factors for HIV infection, men that had sex with men (MSM) and heterosexual patients gained 2.03 (95% CI, 0.42-3.65; p=0.013) and 1.57 (95% CI 0.12-3.02; p=0.034) kg more than those who were former intravenous drug users (IDU). Patients taking boosted protease inhibitors (PI) experienced more weight gain 1.94 kg [95% CI, 0.13-3.75; p=0.036], than those taking integrase strand transfer inhibitors (INSTI). Globally and in decreasing order, rilpivirine [RPV] (+4 kg (IQR: -3.30,5.40]), Lopinavir/ritonavir [LPV/r] (+2.6 kg [IQR 2.40-3]) and Elvitegravir [EVG/c] (+2.20 kg [IQR 0-4.60]) were the “third” drugs most commonly associated with weight gain. Raltegravir [RAL] (-0.40 kg [IQR: -3.20, 3.40]) and nevirapine [NVP] (0.40 kg [-0.80, 0.50]) were the least. cART (combined antiretroviral treatment) based on tenofovir alafenamide (TAF) (5.87 kg [95% CI, 2.65-9.09; p<0.0001]; abacavir (ABV) [3.79 kg (95%CI, 0.83-6.75; p=0.012] and tenofovir disoproxil fumarate (TDF) [3.02 kg (95%CI, 0.24-5.80; p=0.033], gained more weight compared to monotherapy with boosted PI.
Conclusions: Our results suggest that there are demographic, HIV and treatment related contributors to weight gain in PLHIV. Latin-American ethnic race was associated with weight gain, particularly in male sex. We could not find any association of weight gain with sex, age or group of treatment (naïve, treatment continued for six months or change of it). We found boosted PI-based regimens, LPV/r, EVG/c and RPV, and TAF among nucleoside reverse transcriptase inhibitors (NRTI) pairs, associated with the greatest weight gain. We need to improve clinical attention to the maintenance of a healthy weight and implement lifestyle modifications and exercise not only for patients starting treatment but also for those with a long experience in antiretroviral treatment.
Keywords
Antiretroviral treatment, HIV, Latin American men, Weight change
Introduction
The current obesogenic environment is the result of an imbalance between caloric intake and energy expenditure that started in the 1960s-1970s [1]. Disruptive chemical sources have contributed to an inappropriate weight gain altering lipid homeostasis, fat accumulation, energy balance and modifying appetite and satiety regulation [2]. It is important to understand factors related to obesity in PLHIV (people living with HIV) the analysis and understanding of fat changes is gaining importance. Their relationship with HIV and cART (combined antiretroviral treatment), although not yet fully elucidated, seem to be a challenge in this era of long-life antiretroviral treatment. White adipose tissue composed of both innate and adapted immunity cells, is an extremely complex system that allows us to defend ourselves against foreign agents by identifying and eliminating viruses and other pathogens. This way, adipose tissue regulates processes against infection. A characteristic change in HIV infection is the shift towards a predominance of CD8+ T subpopulations which are particularly important in adipose tissue in the context of obesity [3,4]. Infiltration of CD8+ T cells is a necessary factor for recruiting macrophages which, in the context of obesity, produce TNF-α, IL-6 and IL-12 [5]. In obese people, the level of CD4 regulating T-cells (Tregs) is lower, making it easier the arrival of pro-inflammatory and macrophage T cells also [6]. Therefore, both adipose tissue related factors and metabolic dysfunction from HIV infection contribute to tissue inflammation and therefore, immune cell disfunction in obese PLHIV. There is a lot of data suggesting that integrase strand transfer inhibitor (INSTI) based antiretroviral treatment (ART) is associated with increased weight gain. In cell cultures, elvitegravir (EVG) has been shown to inhibit adipocyte differentiation and expression of genes that control adipogenesis. So, cART, modulated by features such as race, female sex and intestinal integrity would enhance the weight gain effect of a high-fat diet in PLHIV [7,8]. Cohort analyses have suggested that integrase inhibitors may increase weight gain, being higher in dolutegravir (DTV) and elvitegravir/cobicistat (EVG/c) than in raltegravir (RAL) treated patients. Regarding to the genetic aspects that influence weight gain, we have to mention the melanocortin-4 receptor (MC4R) gene and the fat mass and obesity-associated gene. Several studies show an statistically significant relationship between some mutations of these genes and an increased adiposity, higher if both mutations are present [9]. MCR4 plays a very important role in regulating energy homeostasis and intake. Deficiency of this receptor is associated with monogenic obesity. In vitro studies, a 64% inhibitory effect of dolutegravir (DTG) on the binding of radiolabeled melanocyte-stimulating hormone (MSH) to MC4R has been demonstrated [10]. Other studies seem to deny the possibility of a direct interference of the MC4R receptor by INSTI at therapeutic doses, inhibiting it only at much higher doses [11]. This paper purpose is to explore the weight gain process in our usual practice, involving three groups of patients, naïve patients who initiated treatment, a second group who were six months on it and a third one of patients changing treatment. We try to explore the possible clinical factors and factors related to the combination of antiretroviral treatment on the weigh changes of our patients in our current clinical practice.
Material and Methods
Design and Population of This Study
This retrospective observational study was carried out between January 2013 and January 2019 in a cohort of HIV-infected patients followed at Severo Ochoa University Hospital, in the southwest of Madrid (Leganés). Severo Ochoa University Hospital has a urban population of 180,000 inhabitants. The patients analyzed in this study are included in the COMESEM cohort, a larger cohort of HIV-1 infected patients followed at five different hospitals (Metropolitan Crown of southeastern Madrid, including Leganés, Alcorcón, Getafe, Móstoles and Alcalá de Henares hospitals). It is an open and dynamic cohort with data collected both in a retrospective and prospective way. The COMESEM cohort organization and functioning as well as the written informed consent of the patients were approved by the Clinical Research and Ethics Committee as required [12]. Patients gave their informed consent to be included in the cohort and their data to be used for this and other research purposes. They were verbally informed of the information that was going to be obtained in the study. From the 550 patients of the COMESEM cohort followed in our hospital, 317 whose weight and height had been recorded in the clinical history for at least 6 months were included in this analysis. We report 3-year data. Exclusion criteria were pregnancy and recent opportunistic infection. Three groups of patients were considered depending on their treatment status at the initial visit: group 1, patients who started antiretroviral treatment (naïve), group 2, those who had been on viral suppression for more than 6 months and continue with their treatment and finally, group 3, those whose treatment was changed in that visit (treatment switch). There was no subject on the new integrase inhibitor bictegravir.
Variables and Laboratory Measurements
Age, gender, ethnicity, clinical data including weight and height, the history of HIV infection and cART were collected in each clinical visit. These data included risk practice for HIV acquisition, smoking habits, alcohol consumption, methadone therapy, current CD4 cell count, CD4:CD8 ratio, current and previous therapy and HIV RNA level. No patient was a current illicit drug injection user. Blood samples were collected to analyze HIV related parameters (current CD4 cell count and current HIV viral load). As a rule, blood samples were obtained within one month of clinical visits.
Statistical Analysis
The study objective was to analyze the change of weight adjusted by ethnicity, gender, antiretroviral treatment, risk practice for HIV acquisition and other factors related to HIV infection. Description of variables was done showing frequencies and proportions for categorical and mean, median, and range for continuous variables respectively. A linear regression model was created with the change of weight considered as a continuous dependent variable. Analyses were processed using statistical package Stata/IC 14.2 for Mac (64-bit Intel). In order to estimate the predictive model of all the possible equations, we used user-command “all sets”. A p value less than 0.05 was considered statistically significant. For statistical calculation only differences at 2 years were considered, as data on weight gain was available for 88% as compared with only for 60.88% at 3 years.
Results
Population, Demographics, and Baseline Disease Characteristics
At basal visit, median body max index (BMI) was 24.87 kg/m2; 9.46% were obese (BMI6 ≥30 kg/m2) 40.06% overweight (BMI 25-29.9 kg/m2) and 50.47% normal (18.5-24.9kg/m2) or underweight (<18.5 kg/m2). Additional baseline weight and demographic data are summarized in Table 1, and baseline disease characteristics are summarized in Table 2. In the naïve patients, contrary to age and viral load which were significantly different, we could not find statistically significant differences with respect to BMI or immune system parameters.
Table 1: Baseline and demographic characteristics
|
Overall |
Naïve | On viral suppression for more than 6 months | Treatment switch |
p |
|
| N |
317 |
7 | 61 |
249 |
|
| Age (years) |
0.0222* |
||||
| Media (SD) |
53.22 (9.72) |
43.91 (11.86) | 52.31 (9.13) |
53.7 (9.69) |
|
| Median(Q1,Q3) |
54.41(49.27-58.07) |
50.29(32.31-53.95) | 53.95(49.27-58.4) |
54.54(49.28-58.4) |
|
| Sex |
0.528 |
||||
| Men |
217 (69.09%) |
6 (85.71%) | 40 (65.57%) |
173 (69.48%) |
|
| Women |
98 (30.91%) |
1 (14.29%) | 21 (34.43%) |
76 (30.52%) |
|
| Ethnicity |
0.228 |
||||
| Spanish |
272 (85.74%) |
6 (85.71%) | 53 (86.89%) |
215 (86.34%) |
|
| Black |
15 (4.73%) |
1 (14.29%) | 4 (6.56%) |
10 (4.02%) |
|
| Latin-American |
27 (8.52%) |
27 (8.52%) |
24 (9.64%) |
||
| Asian |
1 (0.32%) |
1 (1.64%) |
|||
| Sex and ethnicity |
0.487 |
||||
| Spanish Men |
200 (63.09%) |
5 (71.43%) | 36 (59.02%) |
159 (63.86%) |
|
| Spanish Women |
74 (23.34%) |
1 (14.29%) | 17 (27.87%) |
56 (22.49%) |
|
| Black Men |
9 (2.84%) |
1 (14.29%) | 2 (3.28%) |
6 (2.41%) |
|
| Black Women |
6 (1.89%) |
0 | 2 (3.28%) |
4 (1.61%) |
|
| Latin Men |
9 (2.84%) |
0 | 1 ( 1.64%) |
8 (3.21%) |
|
| Latin Women. |
18 (5.68%) |
0 | 2 (3.28%) |
16 (6.43%) |
|
| Weight |
0.8151 |
||||
| Media (SD) |
72.36 (14.40) |
73.37 (10.08) | 71.33 (15.72) |
72.59 (14.20) |
|
| Median (Q1,Q3) |
71.4 (62.6-80.8) |
73.8 (64.2-80.8) | 70.4 (59.4- 81.6) |
72.1 (63-80.6) |
|
| Baseline BMI kg/m2 |
0.4752 |
||||
| Media (SD) |
25.05 (4.25) |
23.78 (3.22) | 24.62 (4.31) |
25.19 (4.27) |
|
| Median (Q1,Q3) |
24.87(22.26-27.39) |
23.99(22.04-24.98) | 24.63(22.36-26.70) |
25.14(22.21-27.8) |
|
| Underweight <18.5 |
21 (6.62%) |
0 | 6 (9.84%) |
15 (6.02%) |
|
| Normal Weight 18-5-24.99 |
139 (43.85%) |
6 (85.71%) | 26 (42.62%) |
107 (42.97%) |
|
| Overweight 25-25.9 |
127 (40.06%) |
1 (14.29%) | 24 (39.34%) |
102 (40.96%) |
|
| Obesity >30 |
30 (9.46%) |
0 | 5 (8.20%) |
25 (10.04%) |
BMI: body mass index. *p<0.05 categorical variables are expressed as number of cases (percentage of the total); continuous variables are expressed as median (interquartile range) and media (standard deviation); Q1 percentile 25%; Q3 percentile 75%; SD standard deviation.
Table 2: Baseline disease characteristics
|
Overall |
Naïve | On viral suppression for more than 6 months | Treatment switch |
p |
|
| N |
317 |
7 | 61 |
249 |
|
| HIV-1 RNA, log10
copies/mL |
0.0001* |
||||
| Media (SD) |
0.69 (1.31) |
5.17 (0.40) | 0.62 (0.98) |
0.58 (1.17) |
|
| Median (Q1,Q3) |
0 (0-1.4) |
5.3 ( 4.7-5.53) | 0 (0-1.4) |
0 (0-0) |
|
| CD4 count, cells/µL |
0.0983 |
||||
| Media (SD) |
563.89 (333.86) |
772 (545.47) | 507.0328 (304.25) |
571.96 ( 332.19) |
|
| Median (Q1,Q3) |
502 (318-764) |
839 (295-1106) | 454 (30- 682) | 520 (335-766) | |
| CD4 count category, cells/µL |
0.715 |
||||
| < 200 |
42 (13.25%) |
1 (14.29%) | 10 (16.39%) |
31 (12.45%) |
|
| >200 |
275 (86.75%) |
6 (85.71%) | 51 (83.61%) |
218 (87.55 %) |
|
| CD8 count, cells/µL |
0.0737 |
||||
| Media (SD) |
1027.43 (559.51) |
1426.14 (841.83) | 1098.87 (716.2) |
998.72 (501.08) |
|
| Median (Q1,Q3) |
923 (692-1281) |
1300 (728-1670) | 974 (800-1205) |
900 (642-1278) |
|
| Ratio CD4/CD8 |
0.1399 |
||||
| Media (SD) |
0.68 (0.55) |
0.62 (0.54) | 0.56 (0.43) |
0.71 (0.57) |
|
| Median (Q1,Q3) |
0.542(0.32-0.89) |
0.35 (0.20-1.24) | 0.53 (0.27-0.76) |
0.56 (0.33-0.97) |
|
| Ratio CD4/CD8
(category) |
0.541 |
||||
| <0.5 |
141 (44.48%) |
4 (57.14%) | 30 (49.18%) |
107 (42.97%) |
|
| >0.5 |
176 (55.52%) |
3 (42.86%) | 31 (50.82%) |
102 (57.03%) |
Weight Gain in Participants Receiving Treatment
Although median weight gain was 1.0 kg (interquartile range [IQR], −1.4 , 3.8) at 36-month, the proportion of participants in overweight and obese BMI categories increased over time, from 40.06 to 43% and from 9.46 to 12.43% in overweight and obese BMI categories respectively (Figure 1). The proportion of participants that met the International Diabetes Federation definition of metabolic syndrome (central obesity (BMI ≥ 30) plus any two of the following: hypertriglyceridemia, low HDL-cholesterol, hypertension, or hyperglycemia) increased overtime from 3.79% to 6.22%. Participants gained respectively 2.59 (IQR 0.80, 3), 0.70 (IQR-1.80, 3.70) and 1 (IQR: -1.40, 3.80) Kg in group 1, 2 and 3 without statistically significant differences between groups 1 and 2 (-0.70 (95% CI: -5.17, 3.78), p=0.759) nor 1 and 3 (-0.50, (95% CI: -4.84, 3.83); p=0.819).
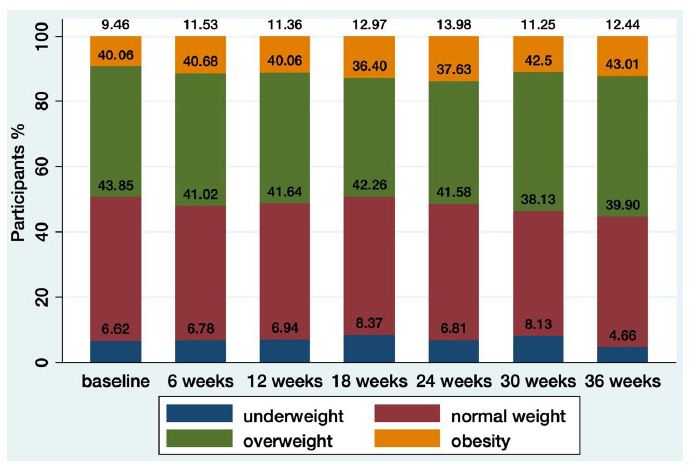
Figure 1: Distribution of BMI over time
Risk Factors for Weight Gain
Considering the risk factors for HIV infection, men that had sex with men (MSM) and heterosexual patients gained 2.03 (95% CI 0.42, 3.65); p=0.013 and 1.57 (95% CI 0.12, 3.02); p=0.034, kg more than those who were former intravenous drug users (IDU) respectively (Table 3) Female sex and age > 50 years had not statistically significant correlations with weight gain (0.44, (95% CI: -0.78, 1.67) p=0.478 and -1.27 (95% CI: -3.20, 0.67) p=0.199 respectively. We further explored these findings by using longitudinal models to assess the relationship between sex, ethnicity, and weight gain. Latin-American gained significantly more weight (2.83 kg (95% CI, 0.80-4.85); p=0.006) than non-Latin-Americans participants (Figure 2). Stratification by both sex and race showed the greatest weight gain among Latin-male participants. Compared to Spanish men and to African women, they gained 5.37 (95% CI 1.66-9.08); p=0.005 and 7.18 (95% CI 2.20-12.35); p=0.007 Kg more respectively (Table 3).
Table 3: Risk factors associated with weight change
|
Variables |
Difference in kg | 95%CI |
p |
| Risk group of infection (ref former IDU) | |||
| MSM |
2.03 |
0.42-3.65 |
0.013* |
| HTX |
1.57 |
0.12 3.02 |
0.034* |
| Ethnicity adjusted by sex (ref. Latin Men) | |||
| Spanish Men |
-5.37 |
-9.08-(-1.66) |
0.005** |
| Spanish Women |
-5.17 |
-8.94-(-1.40) |
0.007** |
| Black Men |
-5.32 |
-10.29-(-.35) |
0.032* |
| Black women |
-7.18 |
-12.35-(-2.02) |
0.007** |
| Latin women |
-3.99 |
-8.19-0.20 |
0.062 |
| cART type (ref. INSTI) | |||
| Boosted PI |
1.94 |
0.13- 3.75 |
0.036* |
| NNRTI |
1.45 |
-0.23-3.12 |
0.090 |
| Backbone (Ref monotherapy with boosted PI) | |||
| TAF |
5.87 |
2.65-9.09 |
<0.0001** |
| TDF |
3.02 |
0.24-5.80 |
0.033* |
| ABV |
3.79 |
0.83-6.75 |
0.012* |
IDU: Intravenous drug user; MSM: Men who have sex with men; HTX: Sex among men and women; cART: Combined antiretroviral therapy; INSTI: Integrase Strand Transfer Inhibitor; PI protease inhibitor; NNRTI: Nonnucleoside reverse transcriptase inhibitor; NRTI: nucleoside reverse transcriptase inhibitor; TAF: Tenofovir Alafenamide Fumarato; TDF: Tenofovir Disoproxil Fumarate; ABV: Abacavir.
*p<0.05; **p<0.01.
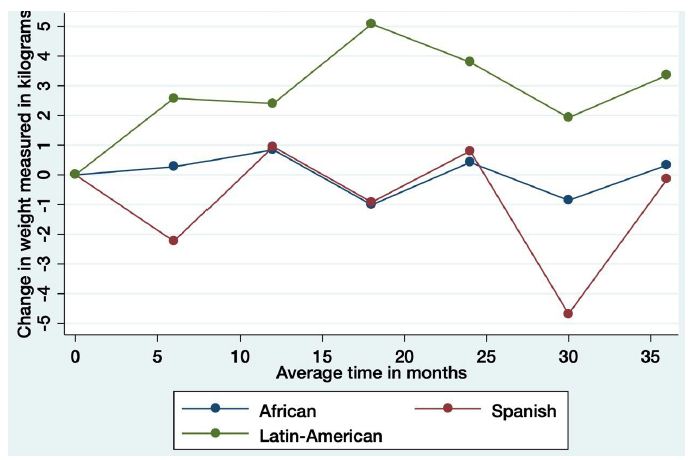
Figure 2: Change in weight in relation to ethnicity
Association of Antiretroviral Regimen Components with Weight Gain
The longitudinal model of weight gain and treatment showed that participants taking boosted PI experienced more weight gain (1.94 kg [95% CI, 0.13-3.75], p=0.036) than those taking INSTI. Weight gain was similar between the NNRTI and INSTI treatment groups (Table 3 and Figure 3). We studied the effect of changing treatment on weight changes with no difference between them. Those patients who changed from INSTI to NNRTI were those in whom we observed the greatest decrease, although not statistically significant: -5.39 (95% CI: -19.12, 7.66) p=0.399. Globally and in decreasing order, rilpivirine [RPV] (+4 kg (IQR: -3.30,5.40]), Lopinavir/ritonavir [LPV/r] (+2.6 kg (IQR 2.40-3) and Elvitegravir [EVG/c] (+2.20 kg (IQR 0-4.60) were the most commonly associated with weight gain, whilst raltegravir [RAL] (-0.40 kg (IQR: -3.20, 3.40) and nevirapine [NVP] (0.40 kg (-0.80, 0.50) were the least (Figure 4). We assessed the association between weight gain and the specific INSTI used. Participants taking EVG/c or DTG demonstrated greater weight gain than those taking RAL (3.00 (95% CI, 0.97, 5.07); p=0.004) and 1.89 (95% CI: -0.034, 3.82); p=0.054) kg respectively. Among participants taking NNRTI, there were no statistically significant differences, although the greatest difference was between rilpivirine and efavirenz (2.46 (95% CI: -0.30, 5.24), p=0.081). Among participants taking boosted PI-containing regimens, those taking lopinavir/ritonavir gained more weight compared to those taking ritonavir and cobicistat-boosted atazanavir (2.57 kg (95% CI 0.80, 4.35) p=0.005) and those taking cobicistat-boosted darunavir (DRV/p) (1.83 kg (95% CI: -0.52, 3.72), p=0.057). Finally, we assessed whether specific nucleoside reverse transcriptase inhibitors (NRTIs) were associated with weight gain compared to boosted PI. At 96 weeks, patients with tenofovir alafenamide (TAF) (5.87 kg (95% CI, 2.65,-9.09; p<0.0001)); abacavir (ABV) (3.79 kg (95%CI, 0.83, 6.75; p=0.012)) and tenofovir disoproxil fumarate (TDF), gained more weight (3.02 kg (95%CI, 0.24, 5.80; p=0.033)) than those in monotherapy with boosted PI.
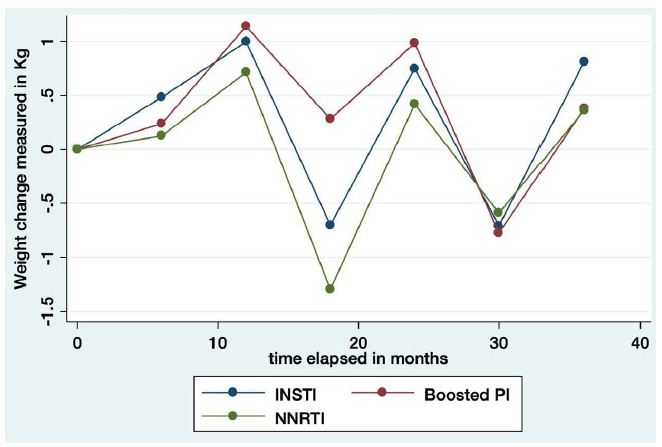
Figure 3: Weight change by the third agent-class
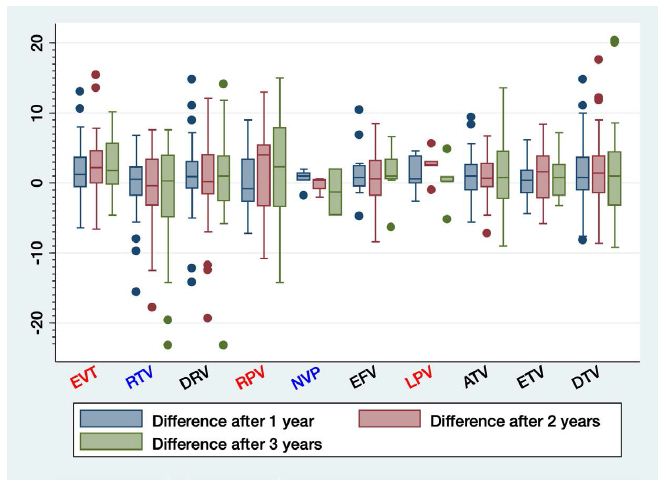
Figure 4: Weight change by the third agent
Discussion
Several authors have postulated many factors that would drive the weight gain in PLHIV on treatment with cART. Mainly, we can define them as HIV-related, traditional risk factors and factors related to antiretroviral therapy. An increase in the weight related to the “return to health” itself is observed as the patient improves. An increased survival has been demonstrated in PLHIV who are underweight when gaining weight [13]. On the other hand, the obesogenic environment is increasing obesity and its associated risks in the general population. Scientific evidence was published in 2016 indicating a 39% and 13% of adults in the general population being overweight and obese respectively [14]. This is especially important when we consider that about 50% of patients who start antiretroviral therapy are overweight [15,16]. In the case of our sample the obesity percentage was 9.46% at the start of the study and the mean BMI was 24.87 kg/m2. These values are similar to those of general population. There is increasing evidence of the effects in weight gain owed to lipoatrophy and lipohypertrophy induced by current treatments [17]. Those patients treated with old cART regimens presented lipodystrophy, defined by central obesity and peripheral lipoatrophy, as well as an increased cardiovascular risk. In contrast, those treated with modern cART regimens experienced modest or minimal weight gain Patients exposed to the actual obesogenic environment will have two different outcomes. Whilst patients treated with older cART regimens will have worsening central obesity but persistence of peripheral lipoatrophy, those treated with modern cART regimens will be overweight or obese, with augmented risk of metabolic disease in both cases [2]. Several authors have presented the results of PLHIV cohort studies in different regions of the world in order to demonstrate the relevance of the weight gaining phenomenon with cART and its impact. It is important to keep in mind that environmental factors determine population differences. We have analyzed some of those factors in our cohort of PLHIV who are treated with cART living in Leganés (a village in the South of Madrid, Spain) to clarify which of them determine the weight gain in our population. In our analysis of PLHIV ranging from the years 2013 to 2019, we found that independently of initiating, changing treatment or maintaining viral suppression, all of them had an increase in overweight, obesity and metabolic syndrome, although absolute weight gain was not significant during the 3 years of observation and was independent of the reason for receiving treatment. A study was conducted with the VACS cohort where it was shown that a 5 lb [18]. Weight gain resulted in a 14% increased risk of diabetes in PLHIV vs. 8% in HIV negative controls. Likewise, other authors from the D:A:D cohort showed a 13% increased risk of diabetes for every unit of BMI gained [19]. An observational study called SCOLTA with a cohort followed at least for one year showed significant evidence of INSTI producing weight gain [20]. The study NA-ACCORD compared the weight gain between patients treated with INSTI, PI and NNRTI-based combinations. Patients treated with INSTI-based combinations had greater weight gain, and within this group of drugs, especially combinations with DTV [21]. We did not observe these differences as our patients exposed to boosted PI gained the greatest weight. Among the NNRTIs, efavirenz was associated with the least weight gain and rilpivirine the greatest. Among the INSTIs, RAL had the least and EVG the greatest weight gain probably because this last one was used co-formulated with TAF. In another retrospective cohort study, they analyzed the effect of treatment change on weight gain in patients receiving EFV/TDF or TAF /emtricitabine (FTC) combinations who switched to INSTI or boosted PI. They were followed for 18 months and a significant weight gain was observed in those treated with INSTI and, above all, in those treated with DTV [22]. We did not find these differences. This can be related to the fact that there are multiple combinations in our study patients, following a real life situation, preventing it from having statistical power. Pre-exposure prophylaxis allows direct comparisons face-to face. The iPrEx study showed weight reduction in those patients treated with TDF (-0.3 kg) versus those who received placebo (+0.5 kg) at 48 weeks [23]. On the other hand, the DISCOVER study [24,25] compared two groups of patients, one with TDF and the other with TAF. Weight loss with TDF was observed up to week 24, as in the iPrEx study, reaching the least weight at 48 weeks but with weight gain at week 96 (+0.5 kg). On the other hand, those treated with TAF showed a sustained increase which reached 1 kg at week 48 and 1.7 kg at week 96. Other double-blind clinical trials on Hepatitis B virus (HBV) mono-infection support this evidence by demonstrating a weight gain of 0.8 kg with TAF and a lost of 0.7 kg with TDF (difference of 1.5 kg) at week 48 [26]. In the AMBER double-blind clinical trial, they compared face to face TAF vs. TDF with a weight gain at 48 weeks 1 kg higher in the former group [27]. These data are consistent with the results of our study in which we found a 2.85 kg weight difference at 96 weeks. The least weight gain was with nucleoside analog-free therapy (monotherapy based on boosted PI). The first clinical trial to report the largest increase in weight in naïve patients treated with TAF and DTV was the ADVANCE study. It was carried out in Johannesburg, South Africa. During 96 weeks 3 groups of patients were randomized to receive treatment TAF/FTC+DTG, TDF/FTC+DTG or TDF/FTC/EFV. Obesity in terms of BMI increase was significantly higher in TAF/FTC+DTG [28]. The baseline characteristics of the study were very different from ours so the conclusions of that study cannot be extrapolated to our population. 59% were women (twice as many as in our sample) and 100% were African subjects (no white or Latin American individuals were studied). As in the ADVANCE study, we were able to show that the main treatment factor associated with obesity was the use of TAF/FTC+DTG. Other study made in Africa is the NAMSAL clinical trial [29]. It was conducted at three sites in Yaoundé, Cameroon. The population characteristics were different from those of our cohort (66% were women, 100% were African subjects) and this was a randomized phase III study in which they compared the combination of DTG+TDF/3TC with EFV+TDF/3TC. The combination based on DTG had a statistically significant greater weight gain (5 vs. 3kg). In our cohort the mean weight gain in the African ethnicity was 4.66 kg at 96 weeks and it was higher in African women. Unlike our sample, they could not analyze ethnic differences because only African subjects were studied. Paul Sax analyzed factors related to weight gain in a pooled analysis of eight randomized clinical trials with a control group of untreated PLHIV [30]. The biological factors associated with greater weight gain were female sex, African ethnicity, and non-being IDU. Factors related to basal HIV were a decreased CD4 count, a higher viral load, a low or normal weight, and being symptomatic HIV. ART-related factors that resulted in the greatest weight gain were DTG/BIC versus EVG and RPV versus VTE use in the INSTI and the NNRTIs family respectively. Within the ITINNs family TAF was the one that increased more the weight. In our sample, the biggest increase in weight was not seen in the African ethnicity (although they augmented weight as well) but in Latin American ethnicity (3.97 kg more than in whites of Spanish nationality). Participants who had no history of consumption of intravenous drugs at baseline had more weight increase. We postulate we could not find an association between weight gain and HIV disease characteristics because baseline median of CD4 count, viral load copies and CD4/CD8 ratio were respectively 502 cel/mm3, < 20 copies/ml y > 0.5, in line with immune reconstitution and so, the return-to-health phenomenon did not take place. Among the comparisons by third agent-class we observed that those who had the greatest weight gain were those who received LPV/r, EVT/c and RPV. We could not find statistically significant differences between the 3 groups of treatment. Probably the small number of “naïve” patients and the absence of immunologic differences in their group with respect to the others, prevented us to see the weight gain expected for the effect of “the return to health”. Although methodologically this is a lower quality study than the clinical trials as it is a retrospective study, we are confident of its great utility because of being a real life study. There are several limitations to our analyses. It did not evaluate aspects such as psychiatric comorbidities, concomitant medications, diet, physical activity, or smoking. In the study, third agents were generally co-administered with NRTIs, with the exception of those regimes based on boosted PI monotherapy where no analogs were used. This makes it difficult to find a link between weight gain and an individual agent. Two or three year’s follow-up does not allow conclusions to be drawn about the long term metabolic disturbances because of the usual clinical practice of addition of new drugs and frequent changes in therapy. Additional important areas for investigation include the magnitude, clinical significance, and biologic mechanisms of ART-related weight gain.
Conclusions
In our study a mix of demographic, HIV disease-specific and ART-specific factors were associated with weight increase during follow-up. Latin-American ethnicity was associated with weight gain. This association was particularly important among Latin-American male, who gained more weight than males of other ethnics. The mechanism underlying this observation is unknown, but it´s probably related to dietary habits and not genetic issues. These findings highlight the need for increased obesity awareness, monitoring and clinical intervention in this population. We could not find any association of weight gain with sex or group of treatment (naïve, treatment continued for six months or change of it). We found PI-based regimens and among NRTI pairs, TAF, associated with the greatest weight gain. Our findings show us that we need to improve clinical attention to the maintenance of a healthy body weight and implement lifestyle modifications and exercise not only for patients starting treatment but also for those with a long experience in antiretroviral treatment.
Acknowledgement
FUNDACIÓN PARA LA INVESTIGACIÓN BIOMÉDICA DEL H.U.PUERTA DE HIERRO had participated in the expenses for the publication in the journal.
Funding
The authors received no funding for this work.
Conflict of Interest
The authors declare that they have no conflicts of interest.
References
- Finkelstein EA, Khavjou OA, Thompson H, Trogdon JG, Pan L, et al. (2012) Obesity and Severe Obesity Forecasts Through 2030. Am J Prev Med 42: 563-570. [crossref]
- Veiga-Lopez A, Pu Y, Gingrich J, Padmanabhan V (2018) Obesogenic Endocrine Disrupting Chemicals: Identifying Knowledge Gaps. Trends Endocrinol Metabol 29:607-625. [crossref]
- Damouche A, Pourcher G, Pourcher V, Benoist S, Busson E, et al. (2017) High proportion of PD-1-expressing CD4 +T cells in adipose tissue constitutes an immunomodulatory microenvironment that may support HIV persistence. Eur J Immunol 47: 2113-2123. [crossref]
- Koethe JR, McDonnell W, Kennedy A, Abana CO, Pilkinton M, et al. (2018) Adipose Tissue is Enriched for Activated and Late-Differentiated CD8+T Cells and Shows Distinct CD8+ Receptor Usage, Compared With Blood in HIV-Infected Persons. JAIDS 77:e14-e21. [crossref]
- Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, et al. (2009) CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nature Medicine 15: 914-920. [crossref]
- Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, et al. (2009) Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nature Medicine 15: 930-939. [crossref]
- Vrouenraets SM, Wit FW, Fernandez Garcia E, Moyle GJ, Jackson AG, et al. (2011) Randomized comparison of metabolic and renal effects of saquinavir/r or atazanavir/r plus tenofovir/emtricitabine in treatment-naïve HIV-1-infected patients. HIV Med 12: 620-631. [crossref]
- El Kamari V, Moser C, Hileman CO, Currier JS, Brown TT, Johnston L, et al. (2018) Lower Pretreatment Gut Integrity Is Independently Associated With Fat Gain on Antiretroviral Therapy. Clinical Infectious Diseases 68: 1394-1401.
- Hetherington MM, Cecil JE (2010) Gene-Environment Interactions in Obesity. Forum Nutr 63:195-203.
- International non-proprietary name: dolutegravir. Assesment report. London, United Kingdom European Medicines Agency, Committee for Medicinal Products for Human Use CHMP. 2014;Report No.:EMA/CHMP/772068/2013.
- McMahon C, Trevaskis JL, Carter C, Holsapple K, White K, et al. (2020) Lack of an association between clinical INSTI- related body weight gain and direct interference with MC4 receptor (MC4R), a key central regulator of body weight. PLoS One 15: e0229617. [crossref]
- Castilla V, Alberdi JC, Barros C, Gómez J, Gaspar G, et al. (2003) [Multicenter cohort of patients with HIV infection in the Madrid south-eastern metropolitan crown (COMESEM): basis, organization and initial results]. Rev Clín Esp 203: 170-177. [crossref]
- Yuh B, Tate J, Butt AA, Crothers K, Freiberg M, et al. (2015) Weight Change After Antiretroviral Therapy and Mortality. Clin Infect Dis 60: 1852-1859. [crossref]
- Obesity and Overweight (2018) Fact sheet WHO.
- Koethe JR, Jenkins CA, Lau B, Shepherd BE, Justice AC, et al. (2016) Rising Obesity Prevalence and Weight Gain Among Adults Starting Antiretroviral Therapy in the United States and Canada. AIDS Res Hum Retroviruses 32: 50-58. [crossref]
- Tate T, Willig AL, Willig JH, Raper JL, Moneyham L, et al. (2012) HIV infection and obesity: where did all the wasting go? Antivir Ther 17: 1281-1289. [crossref]
- Finkelstein JL, Gala P, Rochford R, Glesby MJ, Mehta S (2015) HIV/AIDS and lipodystrophy: Implications for clinical management in resource-limited settings. JIAS 18:19033. [crossref]
- Herrin M, Tate JP, Akgün KM, Butt AA, Crothers K, et al. (2016) Weight Gain and Incident Diabetes Among HIV-Infected Veterans Initiating Antiretroviral Therapy Compared With Uninfected Individuals. JAIDS 73: 228-236. [crossref]
- Achhra AC, Mocroft A, Reiss P, Sabin C, Ryom L, et al. (2015) Short-term weight gain after antiretroviral therapy initiation and subsequent risk of cardiovascular disease and diabetes: the D:A:D study. HIV Med 17: 255-268. [crossref]
- Taramasso L, Ricci E, Menzaghi B, Orofino G, Passerini S, et al. (2017) Weight Gain: A Possible Side Effect of All Antiretrovirals. Open Forum Infec Dis 4: 121-123. [crossref]
- Bourgi K, Jenkins CA, Rebeiro PF, Palella F, Moore RD, et al. (2020) Weight gain among treatment-naïve persons with HIV starting integrase inhibitors compared to non-nucleoside reverse transcriptase inhibitors or protease inhibitors in a large observational cohort in the United States and Canada. JIAS 23: e25484. [crossref]
- Norwood J, Turner M, Bofill C, Rebeiro P, Shepherd B, et al. (2017) Weight Gain in Persons with HIV Switched from Efavirenz-based to Integrase Strand Transfer Inhibitor-based Regimens. JAIDS 76: 527-531.
- Glidden DV, Mulligan K, McMahan V, Anderson PL, Guanira J, et al. (2018) Metabolic Effects of Preexposure Prophylaxis With Coformulated Tenofovir Disoproxil Fumarate and Emtricitabine. Clin Infect Dis 67: 411-419. [crossref]
- Spinner CD, Brunetta J, Shalit P, Prins M, Cespedes M, et al. (2019) DISCOVER study for HIV pre-exposure prophylaxis (PrEP): F/TAF has a more rapid onset and longer sustained duration of HIV protection compared with F/TDF. http://programme.ias2019.org. Mexico.
- Ruane P, Clarke A, Post FA, Schembri G, Jessen H, et al. (2019) Phase3 Randomized, Controlled DISCOVER Study of Daily F/TAF or F/TDF for HIV Pre-exposure Prophylaxis: Week 96 Results Basel; https://onlinelibrary.wiley.com /doi/10.1111/hiv.12814 PE3/16.
- Buti M, Gane E, Seto WK, Chan HL, Chuang WL, et al. (2016) Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 1: 196-206. [crossref]
- Eron JJ, Orkin C, Gallant J, Molina JM, Negredo E, et al. (2018) A week-48 randomized phase-3 trial of darunavir/cobicistat/emtricitabine/tenofovir alafenamide in treatment-naïve HIV-1 patients. AIDS 32: 1431-1442. [crossref]
- Venter WDF, Moorhouse M, Sokhela S, Fairlie L, Mashabane N, et al. (2019) Dolutegravir plus Two Different Prodrugs of Tenofovir to Treat HIV. N Engl J Med 381: 803-815. [crossref]
- The NAMSAL ANRS 12313 Study Group, Kouanfack C, Mpoudi-Etame M, Omgba Bassega P, Eymard-Duvernay S, et al. (2019) Dolutegravir-Based or Low-Dose Efavirenz-Based Regimen for the Treatment of HIV-1. N Engl J Med 381: 816-826. [crossref]
- Sax PE, Erlandson KM, Lake JE, McComsey GA, Orkin C, Esser S, et al. Weight Gain Following Initiation of Antiretroviral Therapy: Risk Factors in Randomized Comparative Clinical Trials. Clin Infec Dis 71: 1379-1389. [crossref]