DOI: 10.31038/CST.2022731
Abstract
Objectives: To compare aspects related to the care of patients with terminal cancer during the 30 days prior to death between usual care units (UCUs) and a palliative care unit (PCU).
Methods: A retrospective cohort, for the last 30 days preceding the death of patients with terminal cancer, followed in UCUs and PCU. Demographic, clinical and nutritional (baseline) data were collected; also, performance of medical examinations and procedures, prescription of nutritional therapy (referring to 30, 7 and 3 days before death); and prescription of drugs and administration routes (relating to the last 3 days of life).
Results: We evaluated 239 patients, of which 131 (54.8%) have been assisted in UCUs and 108 (45.2%) in the PCU. Prescription of nutritional therapy, number of laboratory tests, imaging and procedures performed in the UCUs was higher than in the PCU. Regarding the four drugs considered essential for end-of-life care, we found that all were prescribed to patients in the PCU, while in the UCUs there was no prescription of haloperidol and scopolamine in any of the cases.
Conclusion: In the PCU, there was a better use of health resources, as clinical guidelines recommend limiting the use of disproportionate resources to the advancement of the disease in patients with limited life expectancy.
Keywords
Advanced cancer, Terminal cancer, Health care, Oncology, Usual care units, Palliative care unit
Introduction
The elaboration of the care plan for patients with terminal cancer must be based on a careful evaluation of clinical, bioethical and prognostic elements. The prognostic assessment can lead to the improvement of treatment strategies and support the planning of care and the efficient use of available resources, helping to minimize the risks of under treatment or excessive and futile treatments, especially in the phase close to death [1]. In the hospital setting, it is common for patients with terminal cancer to receive inadequate and ineffective care, with no provision for palliative care and pain relief. Even in a reality of scarce resources, there is an unnecessary use of invasive and high-tech methods, focused on trying to cure, which are unable to treat the most prevalent symptoms of the disease, prolonging suffering and pain [2].
Furthermore, the World Health Organization (WHO) [3] points out that palliative care with quality requires access to essential medicines (basic basket of medications) able to treat the most prevalent symptoms in terminal disease, rather than the use of measures and futile drugs. In 2013, a study published in the Journal of Palliative Medicine carried out through an international consensus of specialist physicians and practitioners in large Palliative Care centers described the relevance of four essential drugs (Morphine, Midazolam, Haloperidol and Scopolamine) for the relief of the most prevalent symptoms in patients with terminal cancer in the days before death. Therefore, physicians caring for patients with terminal cancer must be familiar with these medications to prescribe them and achieve their benefits [4].
In Brazil, the possibility of a patient with chronic illness in a terminal stage of disease, including oncological disease, not having access to basic medications to control symptoms and also remaining without access to the team and palliative care, is very large [5]. It is necessary to improve care for this group so that their real demands are met. In the end-of-life care (EOLC) phase, the patient may present different signs, symptoms and suffering that demand a reorganization of the therapeutic plan. Thus, this study proposes the comparison of aspects related to the care of patients with terminal cancer during the last 30 days prior to death between usual care units (UCUs) and a palliative care unit (PCU) of an oncological center of national reference.
Methods
This is a clinical, observational, retrospective cohort study, referring to the last 30 days of life of patients with terminal cancer, followed up in the different care units of an oncological center of national reference, located in Brazil. The study was approved by the Research Ethics Committee. The oncological center of national reference is composed UCU where treatments are carried out aimed at cytoreduction, whether by chemotherapy, surgery or radiotherapy. It also has the exclusive PCU, where patients from UCUs are referred to control symptoms and promote quality of life and death, at the end of the possibilities of treatment lines and failure to cure, disease progression during treatment or worsening of their clinical condition.
All patients who died of any reason in the period of interest defined in the research proposal (06/01/2019 to 07/31/2019) were identified through an electronic system and selected according to the criteria of inclusion, namely: ≥20 years of age; confirmed diagnosis of advanced-stage malignant tumor (locally advanced and/or with distant metastasis); having died between June and July 2019; having been enrolled at least 30 days before the date of death for follow-up at INCA; and having been admitted to INCA in at least one of the last three days of life. Patients with missing or inconsistent data on the date of death were considered losses.
Data Collection
The thirtieth day before death was considered the baseline and the day of death was the study deadline. The data were extracted from medical records and recorded in a specific form, as shown in Figure 1.
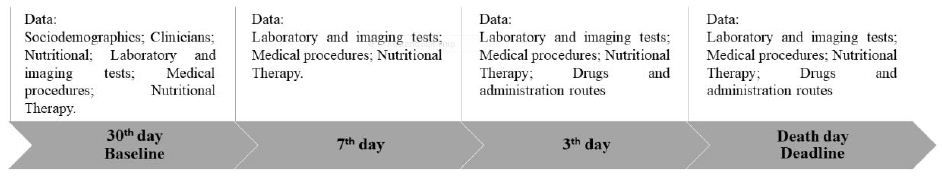
Figure 1: Flowchart of data collection from patients with terminal cancer in the 30 days prior to death
Data Sociodemographic, Clinical, Nutritional and Performance Status (for the Baseline Study)
Age (<60 vs. ≥60 years old); gender (male vs. female); diagnosis [cancer of the gastrointestinal tract (GIT) vs. breast vs. head and neck vs. gynecological vs. lung vs. connective bone tissue vs. others]; disease progression (local vs. local + distance); Previous cancer treatment (yes vs. no)].
Information was collected on the Patient-Generated Subjective Global Assessment short form (PG-SGA SF) (©FD Ottery, 2005, 2006, 2015), available at pt-global.org. The tool is answered by the patient and allows for the assessment of: (1) change in body weight: the score can range from 0 to 5; (2) food intake: with a score from 0 to 4; (3) presence of symptoms of nutritional impact: scoring up to 24; and (4) functional capacity assessment: scoring from 0 to 3. At the end of the assessment, a numerical score is generated based on the sum of each of the items in the questionnaire. The higher the score, the worse the nutritional status. Patients with scores ≥9 were classified as being at nutritional risk [6,7].
The cachexia is defined by the modified Glasgow prognosis score (mGPS) at four different stages: not cachexia, malnutrition, pre-cachectic and refractory cachectic [8] (Table 1).
Table 1: Classification of cachexia using the modified Glasgow Prognostic Score
|
Biomarker |
|||
|
mGPS |
Cachexia Stages |
||
|
CRP (mg/L) |
Albumin (g/dL) |
||
|
0 |
<10 | >3.5 |
Non cachectic |
|
0 |
<10 | <3.5 |
Malnourished |
|
1 |
>10 | >3.5 |
Pre-cachectic |
|
2 |
>10 | <3.5 |
Refratary cachectic |
Note: mGPS= modified Glasgow Prognostic Score; CRP= C-reactive protein. Source: Douglas and McMillan (2014).
The performance status data obtained in the UCU refer to the Performance Status Eastern Cooperative Oncology Group (ECOG-PS) that ranges from 0 (normal activity) to 5 (death) [9]; while in the PCU was used the Karnofsky Performance Status (KPS) that ranging from 100 (normally active) to 0 (dead) [10]. These scales were converted and categorized as PS < 3 or KPS ≥ 40% (yes or no), as proposed by Ma et al. [11]
Laboratory Tests and Procedures (Referring to the Period of 30, 7 and 3 Days before Death)
Total number of laboratory tests performed; total number of full images of examinations and the most frequent types [e.g.: computed radiography (CR), computed tomography (CT), endoscopy, ultrasound and magnetic resonance image (MRI)]; and the total number of procedures performed and the most frequent types [e.g., chemotherapy (QT), radiotherapy, blood and platelet transfusion, biopsy, and gastrostomy].
Nutritional Therapy Prescription (Referring to the Period of 30, 7 and 3 Days before Death)
Prescription of oral (ONT), enteral (ENT) and parenteral (PNT) nutritional therapy.
Prescription and Administration Routes (Referring to the Period of 3 Days before Death)
Prescription of medications and routes of administration.
Statistical Analysis
Analyses were performed using Stata Data Analysis and Statistical Software (STATA) version 13.1 (Stata Corp., College Station, Texas, USA). To assess data, the Kolmogorov Smirnov test was applied. For continuous parametric data, averages, standard deviation, Student’s T test and ANOVA were used; for the categorical variables, number of observations and frequency were used, and the Chi-square test was used for proportions. The p-value <0.05 was considered statistically significant.
Results
The study included 239 patients who, in the majority, were >60 years old (63.2%), female (61.1%) and had the primary tumor site located in the breast (20.1%), followed by GIT (19.7%). The prevalence of nutritional risk was 70.3% and most patients were cachectic (35.4%) or refractory cachectic (46.9%). One hundred and thirty-one (54.8%) were assisted in the UCUs and 108 (45.2%) in the PCU (Table 2). In most patients, the reason for hospitalization was a decline in their general condition, with no statistically significant difference between the units (data not shown in tables).
Table 2: Sociodemographic, clinical and nutritional characterization of patients with terminal cancer according to health care units (N=239)
|
Variables |
Total
N=239 |
UCU
N=131 (54.8%) |
PCU
N=108 (45.2%) |
p-valuea |
| Age (years) | ||||
|
<60 |
88 (36.8%) |
54 (41.2%) |
34 (31.5%) |
0.120 |
|
>60 |
151 (63.2%) | 77 (58.8%) |
74 (68.5%) |
|
|
Sex |
||||
|
Male |
93 (38.9%) |
52 (39.7%) |
41 (38.0%) |
0.785 |
|
Female |
146 (61.1%) | 79 (60.3%) |
67 (62.0%) |
|
|
Diagnostic |
||||
|
GITb |
47 (19.7%) | 21 (16.0%) | 26 (24.3%) | 0.062 |
|
Breast |
48 (20.2%) | 28 (21.4%) |
20 (18.7%) |
|
|
Head and neck |
31 (13.0%) | 13 (9.9%) |
18 (16.8%) |
|
|
Gynecologicalc |
39 (16.4%) | 27 (20.6%) | 12 (11.2%) | |
|
Lung |
25 (10.5%) | 14 (10.7%) |
11 (10.3%) |
|
|
CBT |
13 (5.5%) | 5 (3.8%) | 8 (7.5%) | |
|
Othersd |
36 (14.7%) | 23 (17.6%) |
13 (11.2%) |
|
|
Metastasis |
||||
|
Local |
62 (26.0%) |
39 (30.0%) |
23 (21.3%) |
0.311 |
|
Local + distant |
177 (74.0%) | 92 (70.0%) |
85 (78.7%) |
|
|
Previous cancer treatment |
||||
|
No (virgin) |
41 (17.2%) |
22 (16.9%) | 19 (17.6%) |
0.206 |
Note: UCU= Usual Care Units; PCU= Palliative Care Unit; N= number of observations; %= frequency; GIT= gastrointestinal tract; CBT= connective bone tissue; PG-SGA SF= Patient-Generated Subjective Global Assessment short form; PS= Performance Status; KPS= Karnofsky Performance Status.
ap-value refers to the chi-square test for proportions; bupper and lower GIT; ccutter, endometrium, ovary and vulva;
dcentral nervous system, kidney and urinary tract, male genitals, peritoneum, mediastinum, haematological and unknown primary; evariables with missing data.
The number of laboratory, imaging tests and procedure performed throughout the follow-up period was greater in patients assisted in the UCUs than in the PCU (Figure 2). The most frequently performed imaging tests were X-ray and CT, and the procedures were QT and blood/platelet transfusion. The frequencies of CT (UCU=4.6% vs. PCU=4.6%) and QT (UCU=0.9% vs. PCU=0) were only similar between units in the last three days of life. Patients followed in the PCU were less likely to prescribe ONT compared to those followed in the UCU during the entire evaluation period (p-value <0.001). The prescription of ENT was lower in the PCU only in the last 3 days before death (p-value <0.050) (Table 3).
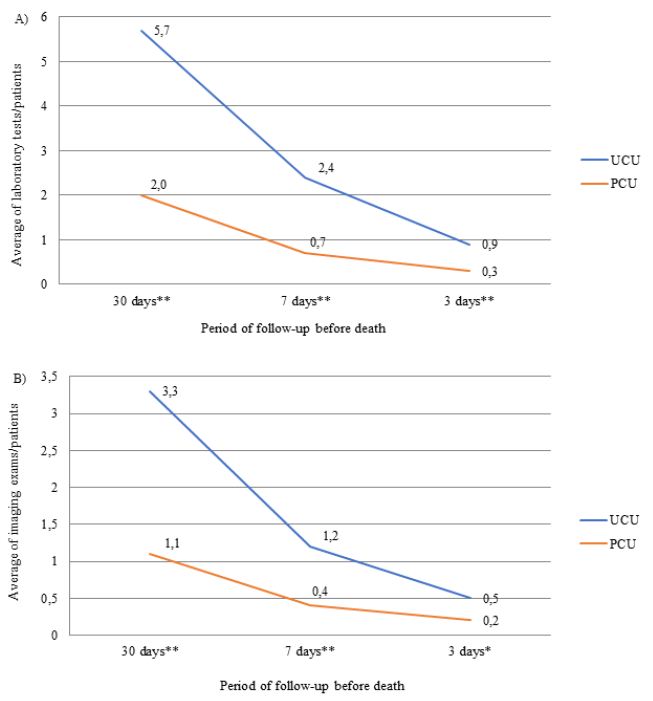
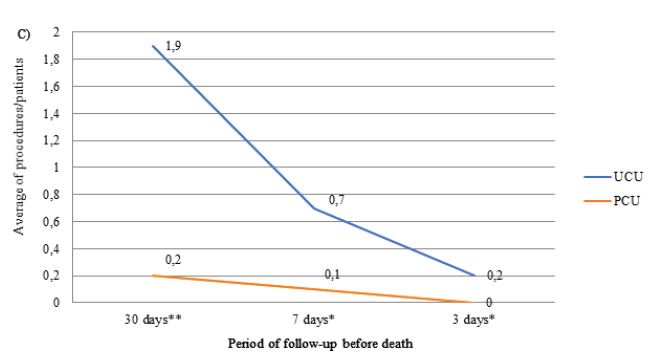
Figure 2: Average number of prescriptions for laboratory tests (A), imaging tests (B) and procedures (C) in the last month of life by patients with terminal cancer according to health care units (N=239).
Note: N=number of observations; UCU=Usual Care Units; PCU= Palliative Care Unit; *p-value<0.050 and **p-value<0.001 of the Student t test.
Table 3: Types of imaging tests and most prescribed procedures in the last month of life for patients with advanced cancer according to health care units (N=239)
|
Period of follow up before death |
|||||||||
| Variables |
30 days |
7 days |
3 days |
||||||
| Total | UCU | PCU | Total | UCU | PCU | Total | UCU |
PCU |
|
| Imaging exams | |||||||||
|
X-ray |
135 (56.5%) |
88 (67.2%) | 47 (43.5%)** | 78 (32.6%) | 57 (53.5%) | 21 (19.4%)** | 50 (21.0%) | 36 (27.5%) |
14 (13.0%)* |
|
CT |
80 (33.5%) |
54 (41.2%) | 26 (24.0%)* | 29 (12.1%) | 22 (16.8%) | 7 (6.5%)* | 11 (4.6%) | 6 (4.6%) |
4 (4.6%) |
|
Ecodoppler |
15 (6.3%) |
15 (11.4%) | 0** | 3 (1.3%) | 3 (2.3%) | 0 | 0 | 0 |
0 |
|
Endoscopy |
10 (4.2%) |
8 (6.1%) | 2 (1.8%) | 5 (2.1%) | 5 (3.8%) | 0 | 0 | 0 |
0 |
|
Ultrasonography |
7 (2.9%) |
7 (5.3%) | 0 | 3 (1.3%) | 3 (2.3%) | 0 | 0 | 0 |
0 |
|
Outrosa |
14 (5.8%) |
12 (9.2%) | 1 (0.9%)* | 5 (2.1%) | 2 (1.5%) | 0 | 3 (1.3%) | 3 (2.3%) |
0 |
| Procedure | |||||||||
|
Quimiotherapy |
31 (13.0%) |
29 (22.1%) | 2 (1.8%)** | 8 (3.3%) | 7 (5.3%) | 1 (0.9%)* | 1 (0.4%) | 1 (0.9%) |
0 |
|
Transfusion |
29 (12.1%) |
29 (22.1%) | 0** | 19 (8.0%) | 19 (14.5%) | 0** | 13 (5.4%) | 13 (9.9%) |
0* |
|
Biopsy |
16 (6.7%) |
16 (12.2%) | 0* | 3 (1.3%) | 3 (2.3%) | 0 | 0 | 0 |
0 |
|
Radiotherapy |
13 (6.4%) |
11 (8.4%) | 2 (1.8%)* | 5 (2.1%) | 5 (3.8%) | 0 | 0 | 0 |
0 |
Note: UCU= Usual Care Units; PCU= Palliative Care Unit; CT=computed tomography; acystoscopy, colonoscopy, cholangio, resonance; bcatheter bi-implantation, lumbar puncture, thoracentesis, nephrostomy, arterial embolization, tracheostomy, gastrostomy, paracentesis, biliary and percutaneous drainage; *p-value<0.050 and **p-value<0.001 of the chi-square test.
Dipyrone remained as the drug with the highest proportion (average of 85%) of prescriptions in the last three days of life in the UCUs, followed by morphine (average of 70%). It should be noted that enoxaparin appeared in the sixth position (average of 38%) and insulin appeared in the ninth position (average of 21%) during the period (Figure 3). The three most prescribed medications in the last three days of life in the PCU were morphine (average of 92%), dipyrone (average of 88%), and midazolam (average of 71%) (Figure 4).
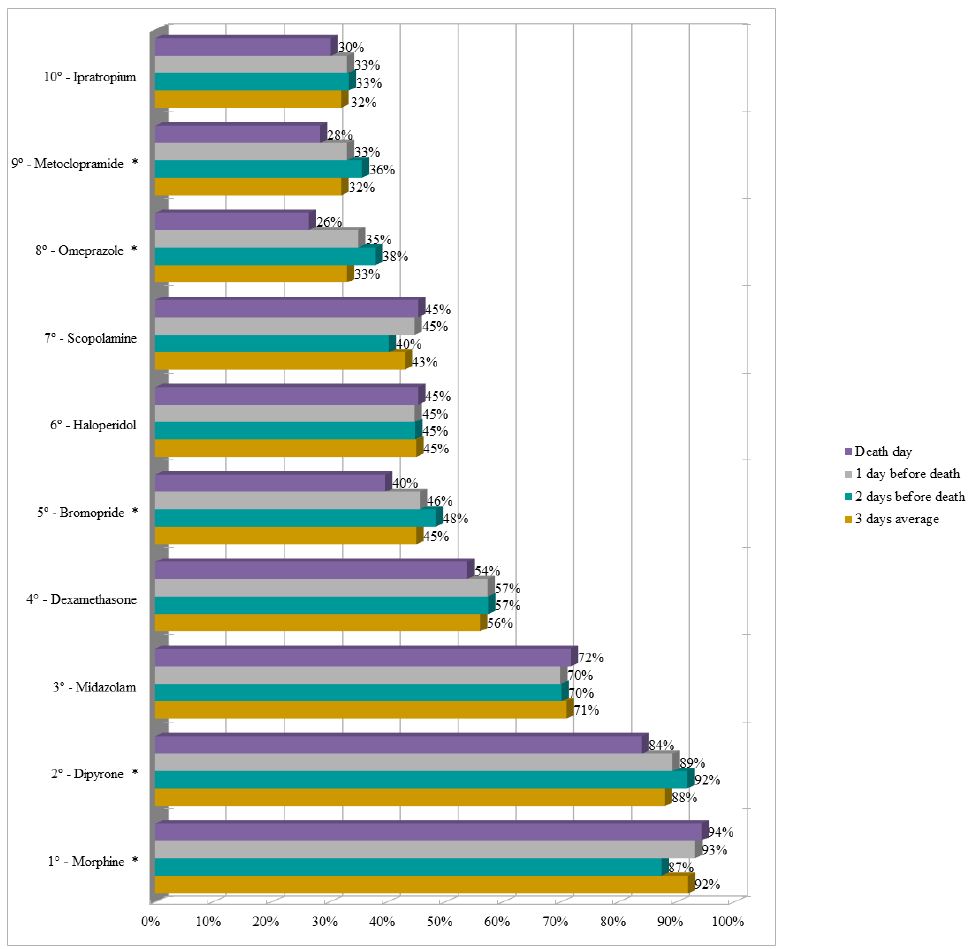
Figure 3: Ranking of the ten most prescribed drugs in the Usual Care Units in the last three days of life of patients with terminal cancer (N=131).
Note: N=number of observations. *p-value<0.050 of the Chi-square test for proportions.
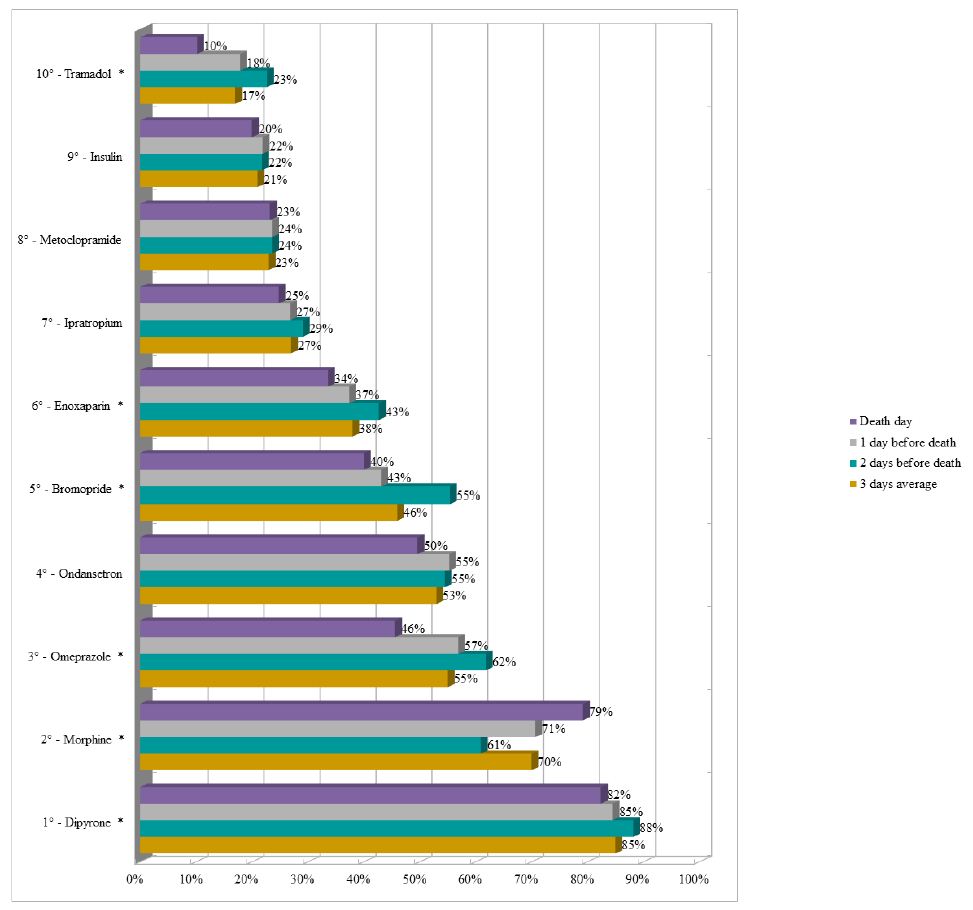
Figure 4: Ranking of the ten most prescribed drugs in the Palliative Care Unit in the last three days of life of patients with terminal cancer (N=108).
Note: N=number of observations.*p-value<0.050 of the Chi-square test for proportions.
According to the analysis of the average frequency of prescription of the four essential drugs, in the last three days of life of patients with advanced cancer, according to the health care units, it was verified that in the UCU there was no prescription of haloperidol and scopolamine. Morphine and midazolam were prescribed in the UCUs, but in a much lower quantity than the PCU (p-value <0.050) (Figure 5).
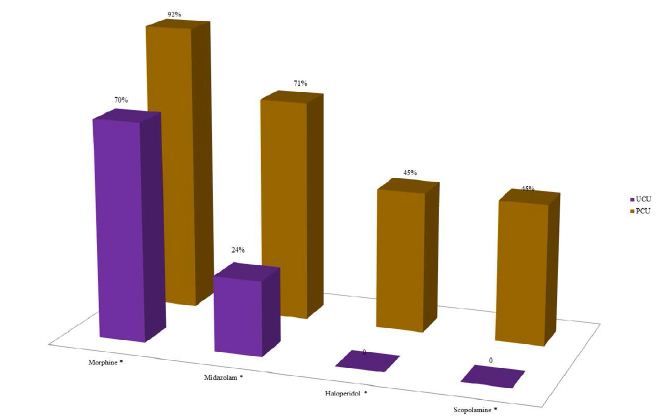
Figure 5: Analysis of the average frequency of prescription of the four essential drugs, according to Lindqvist et al. 2013, in the last three days of life of patients with terminal cancer according to the health care units (N=239).
Note: N=number of observations; UCU=Usual Care Units; PCU=Palliative Care Unit. *p-value<0.050 of the Chi-square test for proportions.
Discussion
The present study, about care provided to patients with terminal cancer in a national cancer treatment center, brings some main results. Patients followed-up in the last 30 days prior to death in the PCU underwent fewer laboratory, imaging and procedural tests, had fewer prescriptions for nutritional therapy and more prescriptions for essential drugs for end-of-life care, when compared to those in treatment in the UCUs. Therefore, as was to be expected, at PCU there was a limitation of the use of futile therapies and incapable of meeting the most relevant demands of terminally ill patients. This approach may be related to the fact that teams specialized in palliative care have greater technical knowledge about prognosis and a careful look at the management of symptoms, promotion of quality of life and death [12].
Even in follow-up at a national referral center for cancer treatment, most patients (54.8%) did not receive assistance from a team specialized in palliative care during the terminal process. World estimates by WHO5 indicate that more than 56.8 million people demand palliative care but only 12% of this need is met. Brazil has one of the worst offers of palliative care services, accessed by only about 0.3% of people who die annually in the country [13]. In addition to the incipient offer of this type of service, the referral of patients to exclusive palliative care is a difficult task that permeates different barriers, such as those related to health professionals, among which we can mention those related to oncologists. They find referring a patient with advanced cancer to exclusive palliative care a complex task, causing patients to be referred late or never be referred [14].
As expected, we found a high prevalence of nutritional impairment (nutritional risk: 70.3%; cachexia: 82.3%), regardless of the type of care unit. It is irrefutable that the impairment of nutritional status increases as cancer progresses [15]. Previous studies show that nutritional risk may be present in 71% to 100% [16,17] and cachexia in 13.8% to 53.9% of patients with advanced cancer [18].
The high prevalence of laboratory tests, imaging and procedures (chemotherapy and blood/platelet transfusion) performed in the UCUs reflect the therapeutic futility often present in care provided by professionals who are not specialized in palliative care for patients in the process of finitude. Receiving the last dose of chemotherapy within 14 days before death can be defined as an aggressive intervention [19]. Blood transfusion, in turn, involves the expenditure of a finite resource and requires careful evaluation for indication in patients with advanced cancer. However, scientific evidence has shown that patients with terminal cancer admitted to UCUs are likely to receive treatments with questionable benefits, such as chemotherapy and blood transfusion, towards the end of life, differently from those seen in PCUs [20,21].
The highest prevalence of ONT, ENT and PNT prescription occurred in UCUs. The decision to initiate and maintain Nutritional Therapy in patients with advanced cancer involves prognostic and bioethical issues, as an inadequate prescription can increase discomfort and suffering [22-24]. Kempf et al.20, in a study carried out in France, demonstrated that more than 15% of patients with advanced cancer received ENT and PNT in the last weeks of life, most of them (75.3%) in non-specialized hospitals. It is likely that palliative care providers are more conservative in their conduct related to Nutritional Therapy prescription in the last weeks of life, which may be related to the experience of patient-centered care [25,26].
The quantity and quality of medications used by patients with advanced cancer during their last days of life reflect the quality of care provided. In this context, we observed, for example, the presence of enoxaparin (advised in the institutional protocol for prevention of venous thromboembolism in prolonged hospitalization) and the absence of haloperidol (indicated in cases of hyperactive delirium) [27] among the 10 drugs. These data suggest the absence of medication reconciliation practice among non-palliative professionals, through the continuity of the prescription of futile medications [28,29].
Another relevant fact regarding the ranking of the 10 most prescribed drugs in UCUs is the absence of sedative drugs such as midazolam. We hypothesize that, this fact, linked to the prescription of morphine, and may indicate the use of opioids to sedate at the end of life, to the detriment of the use of appropriate sedatives, making it difficult to achieve safe sedation. Morphine is a strong opioid indicated for the treatment of pain and terminal dyspnea, which has a decreased level of consciousness as an adverse effect, characteristic of drug intoxication. Therefore, its use for the purpose of sedation is considered an inappropriate conduct [30]. In addition, the high prescription of omeprazole, ondansetron and bromopride found in UCUs may be related to the increase in symptoms of nausea and vomiting, common in intoxication conditions [31].
According to an international consensus of specialist physicians working in large Palliative Care centers, morphine, midazolam, haloperidol and scopolamine were considered the four essential drugs to control the symptoms prevalent in patients with terminal cancer, especially in the last 48 hours of life. Therefore, they must be available and prescribed in all care units for cancer patients. However, our results showed the absence of prescription of haloperidol and scopolamine and the reduced prescription of morphine and midazolam for patients followed in the UCUs during the last three days of life, when compared to those in the PCU.
Considering, therefore, the high prevalence of distressing symptoms at the end of life of cancer patients [32] and that appropriate drug interventions are essential to reduce suffering, we assume that terminal patients not assisted by a team specialized in palliative care are unlikely to receive adequate comfort for a good death. The development of institutional protocols for terminal patients, whether in the PCU or in the UCUs, could contribute to reversing this reality.
Despite all the evidence brought by this study, some methodological limitations need to be highlighted. Due to the retrospective design, it was not possible to assess the comfort and quality of life and death of the patients who made up the study group. Despite not having been the objective of the proposal, such an evaluation would enrich our findings. In addition, data collection from medical records can be a source of bias derived from potentially inadequate or insufficient records of information about the care provided to patients in the source document. It is necessary to develop further studies, with an appropriate design to assess other important variables such as symptom control based on the interventions performed.
Conclusion
The present study demonstrated that the use of health resources in the care of patients with terminal cancer differ between the assessed care units. The assistance provided at the PCU involved a better use of health resources, reflected in the limitation of the use of futile therapies in the context of limited life expectancy, as well as in the prescription of drugs potentially capable of contributing to reduce the burden of symptoms inherent in the terminal phase.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not for profit sectors.
Declaration of Conflicts of Interest
The Authors declares that there is no conflict of interest
References
- Hui D, Mori M, Watanabe SM, et al. (2016) Referral criteria for outpatient specialty palliative cancer care: an international consensus. Lancet Oncol 17: 552-559. [crossref]
- Carvalho RCT, Parsons HA (2012) Academia Nacional de Cuidados Paliativos (ANCP). Manual de Cuidados Paliativos.
- World Health Organization (WHO) (2014) Global atlas of palliative care at the end of life.
- Lindqvist O, Lundquist G, Dickman A, et al. (2013) Four essential drugs needed for quality care of the dying: a Delphi-study based international expert consensus opinion. J Palliat Med 16: 38-43. [crossref]
- World Health Organization WHO (2020) WHO report on cancer: setting priorities, investing wisely and providing care for all.
- Vigano AL, Tomasso J, Kilgour RD, et al. (2014) The Abridged Patient-Generated Subjective Global Assessment Is a Useful Tool for Early Detection and Characterization of Cancer Cachexia. J Acad Nutr Diet 114: 1088-98. [crossref]
- Abbott J, Teleni L, McKavanagh D, et al. (2016) Patient-Generated Subjective Global Assessment Short Form (PG-SGA SF) is a valid screening tool in chemotherapy outpatients. Support Care Cancer 24: 3883-7. [crossref]
- Douglas E, McMillan DC (2014) Towards a simple objective framework for investigation and treatment of cancer cachexia: the Glasgow Prognostic Score. Cancer Treat Ver 40: 685-91. [crossref]
- Eastern Cooperative Oncology Group (ECOG-ACRIN) Cancer Research Group. ECOG Performance Status.
- Schag CC, Heinrich RL, Ganz PA (1984) Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol 2: 187-193. [crossref]
- Ma C, Bandukwala S, Burman D, et al. (2010) Interconversion of three measures of performance status: An empirical analysis. Eur J Cancer 46: 3175-3183. [crossref]
- White N, Reid F, Vickerstaff V, et al. (2020) Specialist palliative medicine physicians and nurses accuracy at predicting imminent death (within 72 hours): a short report. BMJ Support Palliat Care 10: 209-212. [crossref]
- Academia Nacional De Cuidados Paliativos. Análise situacional e recomendações da ANCP para estruturação de programas de cuidados paliativos no Brasil. 2018. São Paulo: ANCP.
- Horlait M, Chambaere K, Pardon K, et al. (2016) What are the barriers faced by medical oncologists in initiating discussion of palliative care? A qualitative study in Flanders, Belgium. Support Care Cancer 24: 3873-81. [crossref]
- Fearon K, Strasser F, Anker SD, et al. (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12: 489-95. [crossref]
- Andrew IM, Waterfield K, Hildreth AJ, et al. (2009) Quantifying the impact of standardized assessment and symptom management tools on symptoms associated with cancer-induced anorexia cachexia syndrome. Palliat Med 23: 680-8. [crossref]
- Cunha MS, Wiegert EVM, Calixto-Lima L, et al. (2018) Relationship of nutritional status and inflammation with survival in patients with advanced cancer in palliative care. Nutrition 51: 98-103. [crossref]
- Wiegert EVM, Oliveira LC, Calixto-Lima L, et al. (2020) Cancer cachexia: Comparing diagnostic criteria in patients with incurable cancer. Nutrition 79-80.
- Cheung MC, Earle CC, Rangrej J, et al. (2015) Impact of aggressive management and palliative care on cancer costs in the final month of life. Cancer 121: 3307–15. [crossref]
- Kempf E, Tournigand C, Rochigneux PL, et al. (2017) Discrepancies in the use of chemotherapy and artificial nutrition near the end of life for hospitalised patients with metastatic gastric or oesophageal cancer. A countrywide, register-based study. Eur J Cancer 79: 31-40. [crossref]
- Wachtel TJ, Mor V (1985) The use of transfusion in terminal cancer patients. Hospice versus conventional care setting. Transfusion 25: 278-9. [crossref]
- Druml C, Ballmer PE, Druml W, et al. (2016) ESPEN guideline on ethical aspects of artificial nutrition and hydration. Clin Nutr 35: 545-56. [crossref]
- Bischoff SC, Austin P, Boeykens K, et al. (2020) ESPEN guideline on home enteral nutrition. Clin Nutr 39: 5-22. [crossref]
- Weimann A, Braga M, Carli F, et al. (2017) ESPEN guideline: Clinical nutrition in surgery. Clin Nutr 36: 623-50. [crossref]
- Masuda Y, Noguchi H, Kuzuya M, et al. (2006) Comparison of medical treatments for the dying in a hospice and a geriatric hospital in Japan. J Palliat Med 9: 152-60.
- Hickman SE, Tolle SW, Brummel-Smith K, et al. (2004) Use of the physician orders for life-sustaining treatment program in Oregon nursing facilities: beyond resuscitation status. J Am Geriatr Soc 52: 1424-9. [crossref]
- Friedlander MM, Brayman Y, Breitbart WS (2004) Delirium in palliative care. Oncology 18: 1541-53. [crossref]
- Saito AM, Landrum MB, Neville BA, et al. (2011) The effect on survival of continuing chemotherapy to near death. BMC Palliat Care 10: 1-11. [crossref]
- Marin H, Mayo P, Thai V, et al. (2020) The impact of palliative care consults on deprescribing in palliative cancer patients. Support Care Cancer 28: 4107–13.
- De Graeff A, Van Bommel JMP, Van Deijck RHPD (2010) Palliative care guidelines. Comprehensive cancer center the Netherlands (IKNL): Utrecht
- Pereira J, Bruera E (1997) Emerging neuropsychiatric toxicities of opioids. J Pharmaceut Care Pain Symptom Contr 5: 3-29
- Lichter I, Hunt E (1990) The last 48 hours of life. J Palliat Care 6: 7-15. [crossref]