Abstract
Introduction: Rhinitis and rhinosinusitis are common in the general population and Intranasal corticosteroid (INCS) sprays are generally safe and effective in the treatment of these conditions. However, they are often burdened by side effects that can reduce compliance with therapy, one of the most common of which is epistaxis.
Objective: To review the current literature about the most common adverse events of beclometasone dipropionato aqueous nasal sprays therapy in chronic rhinosinusitis and allergic rhinitis, focusing on epistaxis.
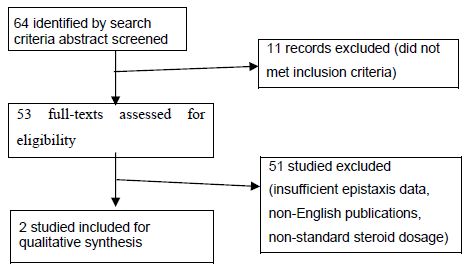
Material and Methods: Using different search engines, the most common adverse events were reviewed and a total of 64 full-length articles were examined for eligibility. After applying inclusion and exclusion criteria, a total of 2 articles were reviewed.
Results: BDP is counted among the group of INCS with the lowest frequency of epistaxis reported as a side effect in the studies analyzed.
Conclusion: BDP aqueous nasal spray is one of the most frequently prescribed INC for rhinitis and rhinosinusitis, with a low frequency of epistaxis. The otolaryngologist and the general physicians should therefore consider prescribing this active principle, particularly to a target group of patients at increased risk of epistaxis.
Introduction
INCS are supported by level-1 evidence for medical management of numerous chronic nasal diseases such allergic rhinitis (AR) and chronic rhinosinusitis (CRS) reducing airway inflammation and improving symptom control [1]. The ability to undergo multiple formulations, such as nasal sprays, aerosols, dry powder inhalers, and ointments means that they can deliver a powerful local anti-inflammatory effect [2].
The intranasal administration of drugs, used for many centuries, has been increasing widespread in recent years, both due to the availability of molecules with specific activity on the airways and the numerous technological innovations that have increased the efficiency of devices available in clinical practice.
The success of inflammatory disease management with intranasal medications depends on the activity of the drug, its pharmacokinetic and pharmacodynamic properties [2]. However, the clinical efficacy of topic INCS is conditioned by some limitations related to possible side effects, due to the bioavailability of the drug. For INCS, these adverse events (AEs) are certainly less frequent and less serious than those observed with oral steroids, but they can considerably limit adherence to treatment, especially in pediatric patients, adolescents and the elderly [3]. The most common AE of INCS treatment is epistaxis [4]. Regardless of the cause, epistaxis is a common emergency encountered by primary care physicians. Up to 60% of the general population experience epistaxis, and 6% seek medical attention for it [5]. Possible causes are factors that damage the lining of the nasal mucosa, affect the walls of the vessels or alter the coagulability of the blood and related drugs such as nasal steroids [6].
Among the various molecules available for the treatment of CRS and AR, beclometasone dipropionato aqueous (BDP) nasal spray represents a possible “first choice”, since this molecule has an excellent efficacy and safety profile boasting decades of use experience [7]. The potential drug interaction risk of beclomethasone dipropionate is low as the drug has limited systemic bioavailability: Paul H. et al. confirmed this showing lower systemic exposure with intranasal administration than with oral inhalation [8]. Patients with nasal chronic inflammatory diseases often require long-term strategies to control symptoms: although the efficacy and safety of INCs are well established, concerns remain regarding systemic AEs including epistaxis, headache, anosmia, ageusia/dysgeusia, among others [9]. The aim of this review is to evaluate the BDP nasal spray adverse event reported in the literature, focusing on epistaxis.
Materials and Methods
To evaluate the studies that analyzed epistaxis as a side effect of BDP in the treatment of inflammatory sinonasal disease, a Pubmed research was conducted searching for articles written by 2010 and 2022, exclusively in English language, including randomized clinical trials, cohort studies, meta-analyses, case reports, and case series and excluding non- English studies, abstract and articles about non nasal Inhalation corticosteroids.
Search criteria included all occurrences of the following terms in the title or abstract: beclometasone dipropionato aqueous; one between “epistaxis”, “adverse event”, “adverse effect” and “complications”.
The corresponding results in the literature dating back to the last 10 years were examined for eligibility and 64 articles were identified: 53 articles were assessed for eligibility. Finally, after applying the above-mentioned inclusion/exclusion criteria, 2 reviews were analyzed [9,10].
Results
The first article analyzed was published by Salma Ahsanuddin and addressed the Proportional Reporting Ratios (PRR) and Reporting Odds Ratios (ROR) for different AEs related to different drugs used to treat CRS and AR, referring to the “Adverse Event Food and Drug Administration Reporting System” and evaluating the relationship between AEs and 10 different INCSs.
BDP nasal spray collocates in the group with least adverse event, accounting for only 1,4% of the total AEs founded, contrary to Fluticasone Propionate and Mometasone, which instead represented the majority of the side effects identified in the analysis, representing 47,7% and 16,7% of total AEs, respectively.
Epistaxis was listed among the top 300 AE for each medication studied together with headache.
The PRR value for epistaxis of the INCs analyzed ranged from 1 to 27,2, with an average value of 4,64: the PRR value of epistaxis due to BDP was 1,5.
Similarly, the ROR value for epistaxis ranged from 1 to 30.8, with an average value of 5: the ROR value of epistaxis due to BDP was 1,5 (Table 1, modified).
Table 1: Intranasal corticosteroid Spray and Reported Epistaxis in FAERS
|
Corticosteroid |
N. patients |
PRR (95%CI) |
ROR (95% CI) |
| Fluticasone propionate 50 mcg |
578 |
2.88 (2.65, 3.12) |
2.90(2.67, 3.15) |
| Fluticasone propionate 90 mcg |
211 |
4.66 (4.08, 5.33) |
4.73(4.13, 5.42) |
| Mometasone |
142 |
2.02 (1.72, 2.38) |
2.03(1.72, 2.39) |
| Budesonide |
81 |
1.55 (1.25, 1.93) |
1.56(1.25, 1.94) |
| Triamcinolone |
42 |
1.32 (0.98, 1.79) |
1.33(0.98, 1.79) |
| Fluticasone furoate |
15 |
27.24(16.93,43.82) |
30.76(18.54,51.03) |
| Beclometasone dipropionato |
9 |
1.49(0.78, 2.87) |
1.50(0.78, 2.88) |
From: Adverse Events Associated with Intranasal Sprays: An Analysis of the Food and Drug Administration Database and Literature Review. Ahsanuddin S et al. 2021.
The second study analyzed was written by Wu EL et al and identified randomized control trials of INCs for treatment of AR that reported incidence of epistaxis: 72 articles with 82 distinct INCS-versus-placebo comparisons were included for meta-analysis.
For all the included comparisons, the meta-analysis demonstrated an overall risk ratio of 1.48 (95% CI, 1.32-1.67) for epistaxis.
In the studies analyzed, the INCSs associated with an increased risk of epistaxis after comparison with placebo were beclomethasone HFA, fluticasone furoate, mometasone furoate, and fluticasone propionate, while patients treated with BDP, ciclesonide HFA, and ciclesonide aqueous did not shown an elevated risk of epistaxis compared to patients treated with placebo (Table 2, modified).
Table 2: INCS-Related Epistaxis: Meta-analyses
|
Studies in Review, n |
Epistaxis |
|||
|
INCS |
Quantitative (82) |
RR (1,48) |
95% CI (1.32-1.67) |
P value (<.001) |
| Beclomethasone HFA |
6 |
2,35 |
1,06-5,20 |
.03 |
| Fluticasone furoato |
15 |
1,85 |
1,46-2,34 |
>.001 |
| Mometasone furoato |
14 |
1,48 |
1,06-2,07 |
.02 |
| Fluticasone propionato |
17 |
1,36 |
1,00-1,85 |
.05 |
| Ciclesonide HFA |
4 |
1,26 |
0.87-1.83 |
.22 |
| Beclomethasone acqueous |
8 |
1,24 |
0,84-1,81 |
.28 |
| Ciclesonide acqueous |
10 |
1,16 |
0,83-1,62 |
.39 |
| Budesonide |
5 |
2,49 |
0,91-6,79 |
.07 |
| Triamcinolone |
3 |
1,87 |
0,16-22,88 |
.62 |
| Flunisolide |
0 |
N/A |
N/A |
N/A |
From: Epistaxis Risk Associated with Intranasal Corticosteroid Sprays: A Systematic Review and Meta-analysis. Wu EL et al. 2019.
Discussion
The most frequent adverse events in the treatment of AR and rhinosinusitis in the literature are revealed to be due to intranasal antihistamines and intranasal steroids, even if these AEs seem well tolerated. Many articles in literature report that epistaxis is the most frequent AEs following intranasal corticosteroids therapy. Epistaxis, while often minor and self-limiting, can result in lack of medication compliance, leading to patient and provider’s frustration resulting in additional procedures, medications, or ineffective treatments [11]. The possible causes are to be found among the thinning of the mucosa due to the vasoconstrictor effect or the direct trauma to the tip of the applicator at the level of the Kiesselbach’s plexus [9].
Conclusions
Intranasal corticosteroids are accepted as a safe and effective first line therapy for allergic rhinitis and rhinosinusitis [12,13], improving to decrease comorbidities and costs. Studies in literature have shown that satisfaction and comfort with an intranasal treatment device are likely to enhance adherence to that treatment among patients with AR and rhinosinusitis. Therapeutic compliance of these drugs depends on several factors, among which there are odor, taste, comfort of delivery, delivery devices (aerosol versus aqueous), patient cost [14] and the possible side effect such as epistaxis, headache, anosmia, ageusia/dysgeusia [9,15]. Waddell AN et al analyzed 16 randomized controlled trials which compared the efficacy of INCs and oral antihistamines in the treatment of allergic rhinitis, and found and incidence of epistaxis due to INCs between 17% and 23% versus an appreciable rate of placebo spray between 10% to 15% [16]. As pointed out by Wu E L, prescribers should be aware of which INCSs may place patients at a higher risk for epistaxis, and they should consider selecting an INCS with a lower risk of this side effect for patients with recurrent or persistent nose bleeding [10]. Only a few articles analyzed the frequency of epistaxis due to BDP, and agree that BDP is among the INCs who cause epistaxis less frequently.
In conclusion, patients with allergic rhinitis and rhinosinusitis represent a high portion of the population and they must chronically continue topical therapy to have optimal symptom control. Since epistaxis is one of the most common side effects of INCs, the otolaryngologist and the general physicians should consider those active principles that are least related to epistaxis, such as BDP.
References
- Eugenio De Corso, Pipolo C, Cantone E, Ottaviano G, Gallo S, et al. (2022) Survey on Use of Local and Systemic Corticosteroids in the Management of Chronic Rhinosinusitis with Nasal Polyps: Identification of Unmet Clinical Needs. Identification of Unmet Clinical Needs. J Pers Med 12: 897. [crossref]
- Peter J (2014) Barnes Glucocorticoids History of Allergy. Chem Immunol Allergy 100: 311-316. [crossref]
- Carlo C, Giovanni AR (2017) Efficacy and safety of beclomethasone dipropionate Suppl. Recenti Prog Med 108: S1-S11.
- Corren J (1999) Intranasal corticosteroids for allergic rhinitis: how do different agents compare? J Allergy Clin Immunol 104: S144-9. [crossref]
- Womack JP, Kropa J, Stabile MJ (2018) Epistaxis: Outpatient Management. Am Fam Physician 98: 240-245. [crossref]
- Paul M (2004) Epistaxis Emerg Med Australas 16: 428-440.
- James Fowler, Brian W Rotenberg, Leigh J Sowerby (2021) The subtle nuances of intranasal corticosteroids. Journal of Otolaryngology – Head and Neck Surgery 50: 18. [crossref]
- Paul HR, Melchior A, Dunbar SA, Tantry SK, Dorinsky PM (2012) Pharmacokinetic Profile of Beclomethasone Dipropionate Hydrofluoroalkane after Intranasal Administration Versus Oral Inhalation in Healthy Subjects: Results of a Single-Dose Randomized, Open-Label, 3-Period Crossover Study. Clinical Therapeutics 4: 1422-1431. [crossref]
- Ahsanuddin S, Povolotskiy R, Tayyab R, Nasser W, Barinsky GL, et al. (2021) Adverse Events Associated with Intranasal Sprays: An Analysis of the Food and Drug Administration Database and Literature Review. Ann Otol Rhinol Laryngol 130: 1292-1301. [crossref]
- Wu EL, Harris WC, Babcock CM, Alexander BH, Riley CA, et al. (2019) Epistaxis Risk Associated with Intranasal Corticosteroid Sprays: A Systematic Review and Meta-analysis. Otolaryngol Head Neck Surg 161: 18-27. [crossref]
- Bridgeman MB (2017) Overcoming barriers to intranasal corticosteroid use in patients with uncontrolled allergic rhinitis. Integr Pharm Res Pract 6: 109-119. [crossref]
- EPOS 2020
- Management of allergic rhinitis and its impact on asthma. ARIA guidelines, 2019.
- Sher ER, Ross JA (2014) Intranasal corticosteroids: the role of patient preference and satisfaction. Allergy Asthma Proc 35: 24-33. [crossref]
- Eli O Meltzer, Greg W, Bensch, William W Storms (2014) New intranasal formulations for the treatment of allergic rhinitis. Allergy Asthma Proc 35: S11-S19. [crossref]
- Waddell AN, Patel SK, Toma AG, Maw AR (2003) Intranasal steroid sprays in the treatment of rhinitis: is one better than another? J Laryngol Otol 117: 843-855. [crossref]