Abstract
Uvaria comperei is a plant of the Annonaceae family listed as one of the most diversified plant families in the tropical environment. The present study was carried out to investigate the anti-inflammatory activity of a methanolic extract and fractions of stems of Uvaria comperei. The crude extract was obtained by maceration of the powder in methanol and fractions by vacuum chromatography from the methanolic extract. To study the anti-inflammatory activity in vitro, red blood cell lysis inhibition assay and albumin denaturation inhibition were performed, while in vivo measurements of carrageenan-induced paw oedema and formalin-induced pain in albino mice were performed. Acute toxicity and cytotoxicity studies of the fraction F2 were performed and some biochemical parameters were measured. The Uvaria comperei crude extract (UCCE) at 250 and 500 µg/mL completely inhibited albumin denaturation, while decreasing 75.5% heat blood cell lysis at 500 µg/mL. The fractions 128-136 (F3), 10-11 (F1), and 56-62 (F2) at 500 µg/mL displayed a significant anti-inflammatory activity with a percentage of inhibition of 60.5, 67.4, and 100%, respectively. Pre-treatment with fraction F2 produced a dose-dependent (p<0.05) inhibition of formalin-induced pain in both neurogenic and inflammatory phases. Similarly, the time-dependent increase in carrageenan-induced paw circumference was inhibited by pre-treatment with F2: 50% of inhibition at 25 mg/kg after 30 min (p<0.05), and 96.5% inhibition at 25 mg/kg after 6 h (p<0.05). In this research, the fraction F2 presented its best analgesic property at 50 mg/kg, while producing its highest anti-inflammatory property at 25 mg/kg. The oral lethal dose 50 (LD50) of F2 was determined to be greater than 2000 mg/kg. Overall, this work shows that the methanolic crude extract and fractions, mainly F2, of Uvaria comperei stem have noticeable anti-inflammatory properties, and could be a potential source of anti-inflammatory compounds.
Keywords
Uvaria comperei, HPLC, Antinociceptive, Anti-inflammatory, Toxicity
Introduction
SeveralspeciesbelongingtotheAnnonaceaefamilyarewidelyknown and used in folk medicine and commercialized as phytomedicines. Among them, plants of the genus Uvaria are traditionally used for the treatment of dysentery, wounds, abdominal ache, and malaria [1]. However, very few ethnobotanical and pharmacological studies have been conducted on Uvaria comperei species despite its high bioactivity properties [2,3].Uvaria comperei is a liana with a blackish bark. The leaves are long-stalked and long, wide limbs rounded at the base. The yellowish, solitary flowers have a long pedicel, long and wide petals and obtuse at the top, small sepals, broadly ovate, wide, welded at the base, the surface of which is covered with long, soft, crisscrossed, and frizzy hairs. The stamens are very numerous at about 1.5 mm long. Fruits with long pedicels; mericarps smooth, ellipsoid, yellow-green when fresh, and blackish brown when dry. The seeds, wide, are biseriate, in the shape of a flattened ellipsoid and with a brown testa, finely honey-combed [4]. The Uvaria comperei contains a wide variety of secondary metabolites, mainly phenolic compounds [2,3]. Phenolic compounds are a well-known group of secondary metabolites with various pharmacological activities [5]. According to Loomis and Battaile [6] phenols belong to either one of two biochemical groups: (1) flavonoid compounds (including condensed tannins), and (2) the group of compounds where the 6-carbon ring has a 1 or 3 carbon side chain and its derivatives, e.g. caffeic acid, gallic acid, hydrolysable tannins, and lignin. Flavonoids and phenolic acids are the important secondary metabolites and bioactive compounds in plants [7]. Flavonoids are an important coloring component of flowering plants, and are found in several plant-based foods [8]. In nutrients, flavonoids are generally responsible for color, taste, prevention of fat oxidation, and protection of vitamins and enzymes. Furthermore, flavonoids are important for human health due to their pharmacological activities as radical scavengers [9]. Several epidemiological studies also suggested protective effects against cardiovascular diseases, cancer, and other age-related diseases [9]. Some flavonoids have been reported to have a variety of biological activities, including antiallergic, antiviral, antiproliferative, anticarcinogenic, and anti-inflammatory activities [10]. Inflammation is a complex biological response in which vascular tissues respond to harmful stimuli such as irritants, pathogens, and damaged cells [11]. Inflammation is commonly divided into three phases: acute inflammation, immune system response, and finally chronic inflammation [12-14]. The inflammation response implicates macrophages and neutrophils that secrete a number of mediators (eicosanoids, oxidants, cytokines and lytic enzymes) responsible for the initiation, progression and persistence of the acute or chronic state of inflammation [15]. Following the release of these mediators, inflammatory processes cause tissue damage accompanied by pain. Researchers are still battling to develop more effective and less toxic agents to treat signs and symptoms of acute inflammation, as well as the consequences of chronic inflammatory diseases such as pain. The search for new drugs capable of disrupting the inflammatory process is an important issue in scientific research, mainly from natural substances. The purpose of this research was to study the antinociceptive and anti- inflammatory potential of extracts and fractions of Uvaria comperei stems.
Materials and Methods
Animals
Swiss mice (20-30 g) and Wistar rats (100-150 g), raised in the Animal house of the Animal Physiology laboratory, Faculty of Science, University of Yaoundé I (Cameroon), were used. They were fed with a standard laboratory diet and allowed access to tap water ad libitum. Animals were randomly housed in appropriate cages at room temperature and subjected to a natural day/night cycle. Animals were handled following the Guide for the Care and Use of Laboratory Animals, published by the US National Institutes of Health (NIH publication 85-23, revised 1996). The authorization for the use of laboratory animals in this study was obtained from the Cameroon National Ethics Committee (number FWA-IRB00001954).
Plant Collection
The Uvaria comperei plant was harvested in February 2013 in Kalla Mount in the central region of Cameroon and identified by the botanist Dr Nana. A voucher specimen of the plant (52882/HNC) has been deposited at the Cameroon National Herbarium in Yaoundé.
Extract Preparation
Fresh stems were chopped, air dried and ground into powder. Hundred grams of powder were introduced into a conical flask and soaked for three days in 500 mL of methanol at room temperature. The resulting mixture was filtered through a filter paper (Whatman No. 3) and then roto-evaporated until complete alcohol evaporation was obtained.
Fractionation of Methanolic Extract
The stem methanolic extract was fractionated by vacuum chromatography using silica gel 40 (0.2-0.5 mm) and eluted with solvents of increasing polarity: hexane (Hex), ethyl acetate (EtOAc) and methanol (MeOH), leading to several fractions. The elution was done successively with a gradient system of Hex-Hex/EtOAc-EtOAc- EtOAc/MeOH-MeOH (from 100% hexane to 100% methanol). Each elution (400 mL) was evaporated to dryness under reduced pressure and 202 fractions were obtained and grouped according to their thin- layer chromatographic profile using Merk 60 F254 silica gel 60 F254 (Merck, USA) to obtain 28 new fractions. UV light (λmax=254 nm, 366 nm) and 50% aqueous sulfuric acid were used to visualize TLC plates. The three fractions (F1, F2, F3) that had appreciable antioxidant activity [2] were successively tested to detect their antinociceptive and anti-inflammatory activities.
In vitro Assays
Inhibition of Albumin Denaturation
The method of Vidhu et al. [16] and Sangita et al. [17] was used for the denaturation protein assay. A solution of BSA (10 mg/ mL) was prepared in phosphate buffer at pH 7.4. Stock solutions of crude extract, fractions, and diclofenac (the reference standard) were prepared at 1 mg/mL. From these stock solutions, five different concentrations (31.25, 62.50, 125, 250 and 500 µg/mL) were obtained. A volume of 0.2 mL of BSA was transferred to Eppendorf tubes, then 2 mL of extract or standard at different concentrations was added, and then 2.8 mL of PBS were added for a total volume of 5 mL. Controls were prepared without extracts (instead of 2 mL of extract were added 2 mL of PBS). The solutions were incubated at 37°C for 30 min and heated at 70°C for 15 min. The absorbance (Abs) was determined at 660 nm. The percentage of inhibition of protein denaturation was determined on a percentage basis relative to the control, using the following formula.
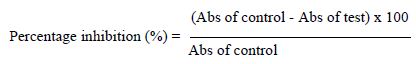
Antihemolytic Activity
Red Blood Cell Suspension
The method of Azeen et al. [18] was used for red blood cell lysis with some modifications. Rat blood was obtained by puncture, collected in heparinized tubes, and centrifuged at 3000 rpm for 15 min. Then plasma was removed and red blood cells (RBCs) were washed three successive times using saline solution.
Heat-induced Haemolysis
Stock solutions of crude extract, fractions and standards (diclofenac and ibuprofen) were prepared at 1 mg/mL. From these stock solutions, five different concentrations (31.25, 62.50, 125, 250 and 500 µg/mL) were obtained. In each test tube, 500 µL of NaCl were added consecutively with 500 µL of extract or each fraction, 500 µL of buffer solution, and finally 500 µL of RBC suspension. The test tubes were homogenized. The reaction mixture was incubated in a 56°C water bath for 30 min. After incubation, the tubes were cooled under running tap water, then centrifuged at 3000 rpm for 10 min and the absorbance of the supernatants was assessed at 560 nm. The controls were prepared without extract or fractions. The percentage of protection against heat-induced haemolysis was calculated using the following formula.
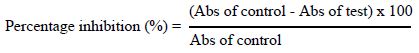
Based on these data, only the fraction F2 that had the highest activity was selected for in vivo studies.
In vivo Assays
Formalin Assay
To assess the antinociceptive effect, the method described by Hunskaar and Hole [19] was used. A volume of 20 μL of 1% formalin solution was injected into the subplantar left hind paw of mice. Mice were observed and the amount of time (seconds) spent licking and biting the injected paw was measured as an indicator of pain. Responses were measured for 5 min after formalin injection (first phase, neurogenic phase), and 15-30 min after formalin injection (second phase, inflammatory phase). Treatments with saline (p.o.), fraction F2 (25, 50, and 100 mg/kg, p.o.), indomethacin (10 mg/ kg, p.o.) were administered 30 min before formalin injection (n = 6 for each group). The percentage of antinociceptive activity was determined using the following formula [20].
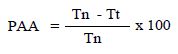
PAA: percentage of antinociceptive activity, Tn: licking time of the control group, and Tt: licking time of each tested group.
Carrageenan-induced Hind Rat Paw Edema Assay
Anti-inflammatory activity was studied using the model of 1% carrageenan-induced paw edema induced by a 1% carrageenan solution, injected at a volume of 100 μL/animal into the subplantar region of the right hind paw of the rats [21]. The rats were divided into six groups, each of six animals. Rats were pretreated with fraction F2 (25, 50, and 100 mg/kg, p.o.), saline (p.o.) or indomethacin (10 mg/kg, p.o.) 30 min before carrageenan injection. The volume of the rat pedal was measured at 0.5, 1, 2, 3, 4, 5, and 6 h after carrageenan injection. The inhibition of the edema paw was calculated using the formula:
VB-VA/VA, where VA is the volume of the right hind paw before carrageenan injection, and VB is the volume of the right hind paw after carrageenan injection.
Biochemical Parameter Analysis
After the carrageenan assay, the animals were sacrificed and blood was collected in heparinized tubes and centrifuged at 3000 rpm for 30 min to obtain serum. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl tra n sp ep tid as e (γ GT) and catalase (CAT), as well as reduced glutathione (GSH) and reactive protein (CRP) were determined using commercial assay kits (Randox, UK) according to the manufacturer’s protocol.
Cytotoxicity Assay
Cell Culture
African green monkey kidney cells (Vero) (ATCC CRL 1586) and macrophages (RAW 264.7) (ATCC TIB-71) were cultured in DMEM medium containing 10% fetal bovine serum (FBS) and 1% penicillin- streptomycin. Complete DMEM medium (500 µL) was prepared with 50 µL of FBS, 5 µL of antibiotic, and 445 µL of simple DMEM medium.
Resazurin Reduction Assay
The cytotoxicity study was performed by a resazurin reduction assay on Vero and RAW cell lines according to the protocol of kuete et al. [22] and O’Brien et al. [23]. This assay is based on the reduction of the indicator dye, resazurin, to highly fluorescent resorufin by viable cells. Non-viable cells rapidly lose metabolic capacity to reduce resazurin and thus produce no fluorescent signal. Briefly, adherent cells were removed by trypsin treatment, incubated at 37°C for 5 min. Then, trypsin was deactivated by adding complete DMEM and the solution was centrifuged. An aliquot of 10,000 cells was placed in each well of a 96-well cell culture plate (100 μL). The microplates were incubated at 37°C overnight. After incubation, the medium was removed from each well and 90 µL of fresh, complete DMEM medium and 10 µL of fraction solution were added. The plates were then incubated at 37°C for 44 h. Fluorescence was measured on an Infinite M2000 Pro™ plate reader (Tecan, Germany) using an excitation wavelength of 530 nm and an emission wavelength of 590 nm. Each assay was performed at least three times, with six replicates each. Viability was evaluated on a comparison with untreated cells. IC50 values represent the concentration required to inhibit 50% of cell proliferation.
Acute Toxicity Study
The estimation of the oral median lethal dose (LD50) of the fraction was determined in female mice using the OECD Guideline 425 [24]. A limit toxicity test of a single dose of 2000 mg/kg body weight was used. Four hours before the toxicity tests, the animals were deprived of food and water. After weighing the mice, three groups of three mice were constituted as follows: Group 1 control lot received only the dissolution solvent, Group 2 mice received the fraction F2 from Uvaria comperei extract at 2000 mg/kg, and Group 3 mice received only food and water. After administration of a unique dose of F2, mice were monitored and individually observed every 30 minutes, during the day, and then daily for 14 days.
High-Performance Liquid Chromatography with Diode Array Detection Analysis
High performance liquid chromatography (HPLC) analysis was performed using a Waters Corporation, USA, HPLC system consisting of a Waters 1525 binary HPLC pump and a Waters 2998 photodiode array detector. Separation was carried out on an XTerra RP 18 column (4.6 mmx 15 mmx 3.5 μm, Waters, USA). Gradient elution was performed at 25°C with solution A (water and 1% acetic acid) and solution B (methanol and 5% acetic acid) in the following gradient from 0% to 100% solution B in 50 min. The flow rate was 1 mL/min and the injection volume was 20 μL. The peaks were detected at the wavelengths of 240, 340 and 380 nm. Before injection, each sample (1 mg/mL) was filtered through a 0.45 μm membrane filter. The identification of compounds was performed on the basis of retention time, co-injection, and spectral matching with standard compounds. For this purpose, standard stock solutions of caffeic acid, catechin, chlorogenic acid, gallic acid, herniarin, imperatorin and quercetin were prepared in methanol at 1 mg/mL.
Statistical Analysis
Results were expressed as mean ± SEM and analyzed with SPSS 22.0 software using one-way analysis of variance (ANOVA) followed by Fisher´s LSD test. Values of p<0.05 were considered statistically significant.
Results
In Vitro Assays
Inhibition of Albumin Denaturation
The methanolic extract of Uvaria comperei and fractions showed a strong inhibitory effect on the heat-induced denaturation of albumin (Table 1). The maximum effect was presented by the crude extract (UCCE) and the F2 fraction employed at 500 µg/mL, obtaining the maximal inhibition, whereas at 500 µg/mL diclofenac (the standard anti- inflammatory agent) showed an inhibition of 46%. The crude extract showed the highest inhibition (IC50 = 66.05 ± 0.30 µg/mL, Table 1).
Table 1: Effects of Uvaria comperei stem crude extract and fractions on albumin denaturation
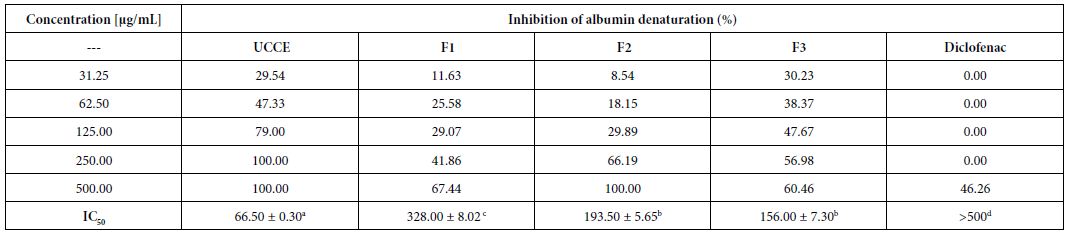
a,b,c,dDifferent letters in the same row indicate a significant difference (p<0.05). UCCE: crude extract of Uvaria comperei stems; F1, F2 and F3: fractions from UCCE.
Heat-induced Haemolysis
The protective effect of the extract and fractions against heat- induced haemolysis was studied showing a concentration-dependent inhibition (Table 2). In fact, the haemolysis ratio gradually decreased with increasing amount of the substances. Protection was slightly manifested using 31.25 µg/mL crude extract, inhibition of 10.5%, while maximum protection of 95.4% was observed using 500 µg/mL F3 fraction, followed by F2 fraction (79.8%). F3 showed the highest protection for red blood cells, with an IC50 < 31.25 µg/mL. This high activity of F3 was similar to that of the reference compound ibuprofen (IC50 < 31.25 µg/mL).
Table 2: Effect of Uvaria comperei stem crude extract and fractions on haemolytic activity
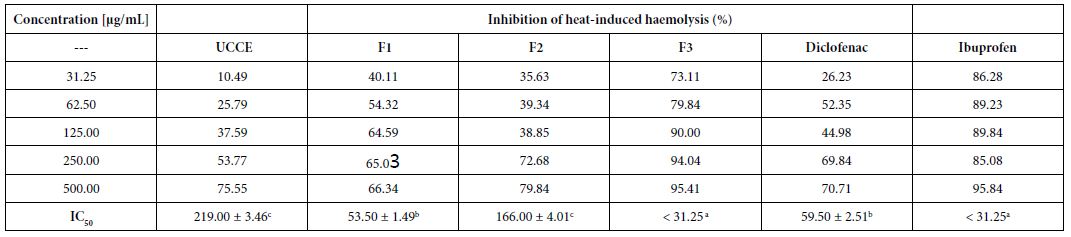
a,b,cDifferent letters in the same row indicate a significant difference (p<0.05); UCCE: crude extract of Uvaria comperei stems; F1, F2 and F3: fractions of UCCE.
In vivo Anti-inflammatory Assays
Inhibition of Formalin Assay
Treatment with fraction F2 produced significant antinociceptive activity compared to controls in both the early and late phases (Table 3). F2 fraction tested at 25, 50 and 100 mg/kg (p.o.) decreased the paw licking time to 54.4, 60.2 and 14.6%, respectively, in the neurogenic phase (first phase), as well as to 70.2, 68.7 and 38.8%, respectively, in the inflammatory phase (second phase). Indomethacin (a reference drug) exhibited a higher inhibition in the second phase.
Indomethacin was used at 10 mg/kg; a,b,cDifferent letters in the same row indicate a significant difference (p<0.05).
Table 3: Effect of fraction F2 on formalin-induced paw licking
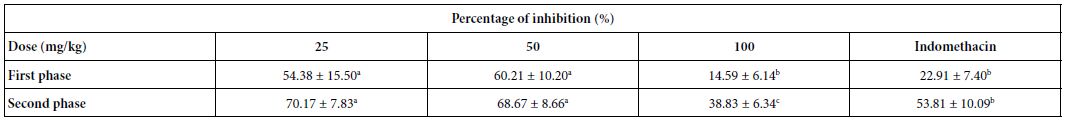
Indomethacin was used at 10 mg/kg; a,b,cDifferent letters in the same row indicate a significant difference (p<0.05)
Inhibition of Carrageenan-induced Hind Paw Oedema
Oral administration of the F2 fraction (25, 50, and 100 mg/kg, p.o.) induced the highest anti-inflammatory activity by reducing the volume of paw oedema induced by carrageenan (Table 4). In detail, 25 mg/kg F2 caused 50% inhibition after 30 min of inflammatory stimulus, and 96% after 6 h, while with 50 mg/kg the inhibition was of 87% after 6 h. Furthermore, a 93% inhibition was observed after 3 h with F2 50 mg/kg. F2 showed high anti-inflammatory activity compared to indomethacin used as a standard drug. No significant differences in the inhibition were observed between 25 and 50 mg/ kg doses.
Table 4: Effect of the fraction F2 on carrageenan-induced hind paw edema in rats
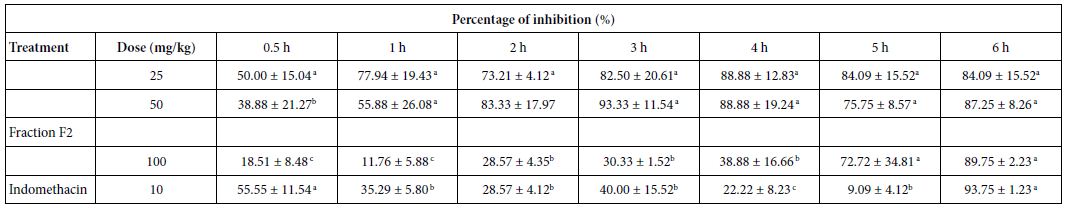
a,b,cDifferent letters in the same column indicate a significant difference (p≤0.05)
Serum Biochemical Analysis
Several biochemical parameters (Table 5) and indicators of oxidative stress (Table 6) were evaluated, as well as C reactive protein (CRP). The F2 fraction showed a significant protective effect on ALT, AST, γ GT, CAT, and reduced glutathione (GSH) levels. Indeed, the administration of the F2 fraction in the test group had significantly decreased the enzymatic activity of these enzymes compared to the control group (p<0.05), the values were 44.6, 196.8 and 3.5 IU/L for ALT, AST and γ-GT, respectively.
Regarding oxidative stress parameters, GSH and catalase levels were significantly higher in the test group compared to the control group, showing the ability of F2 to increase antioxidant defences (Table 6).
Table 5: Effect of F2 fraction administered at 25 mg/kg on serum biochemical parameters
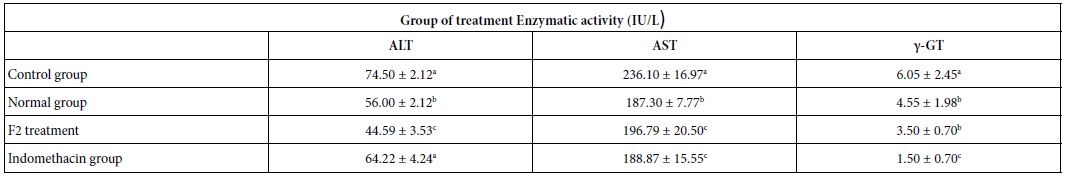
ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase; γ-GT: γ-Glutamyl Transpeptidase. Normal group: rats which did not receive any administration; Control group: rats which received the solvent of dissolution of F2. a,b,cDifferent letters in the same column indicate a significant difference (p<0.05).
Cytotoxicity
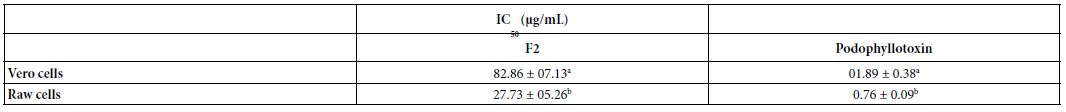
The F2 fraction showed concentration-dependent cytotoxicity activity tested in two cell lines (Table 7). The IC values of F2 were 27.73 and 82.86 µg/mL, respectively, in Raw and50Vero cells. The F2 fraction showed lower cytotoxicity in Vero and Raw cells compared to the standard cytotoxic agent podophyllotoxin.
Table 7: Cytotoxicity of the F2 fraction detected in two cell lines
a,b,cDifferent letters in the same column indicate a significant difference (p<0.05)
Acute Toxicity
The F2 fraction did not induce death of any treated mice, therefore, the lethal dose (LD50) of F2 was found to be greater than 2000 mg/kg body weight.
HPLC Profile
Several flavonoids were detected in F2 fraction with HPLC-DAD analysis at 280, 340, and 380 nm and compared with the chromatograms of standard flavonoids. Figures 1-3 show the HPLC chromatograms of standard phenols (caffeic acid, catechin, chlorogenic acid, epicatechin, gallic acid, herniarin, imperatorin and quercetin) at 280 nm, 340 nm and 380 nm, respectively. Figures 4-6 show the HPLC chromatograms of F2 fraction at 280 nm, 340 nm and 380 nm, respectively. The chromatograms of the standards combined with F2 fraction have been presented in Figures 7-9 while Figure 10 presents the chromatogram of eight standard flavonoids identified at 280 nm and Table 8 shows the different phenols identified in fraction F2.
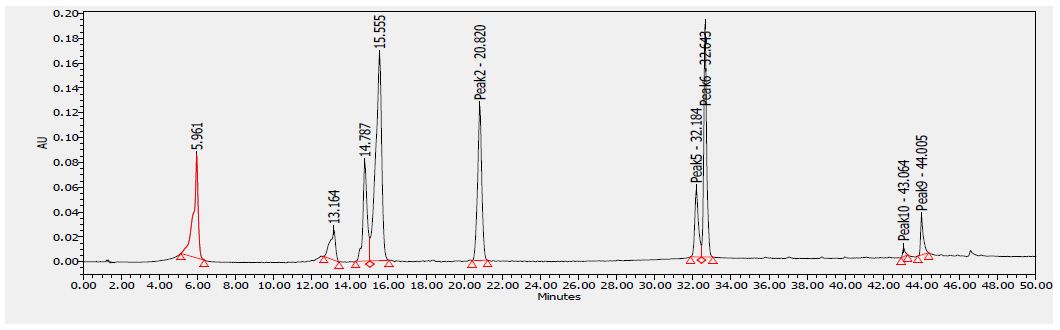
Figure 1: HPLC chromatogram of several standard phenols detected at 280 nm
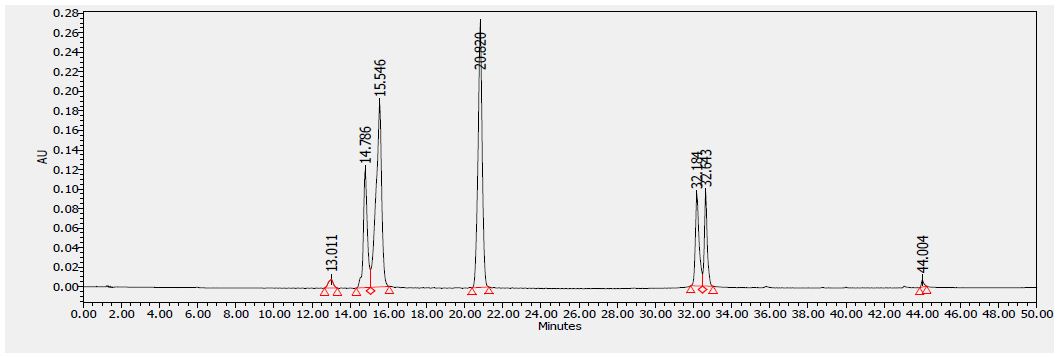
Figure 2: HPLC chromatogram of several standard phenols detected at 340 nm
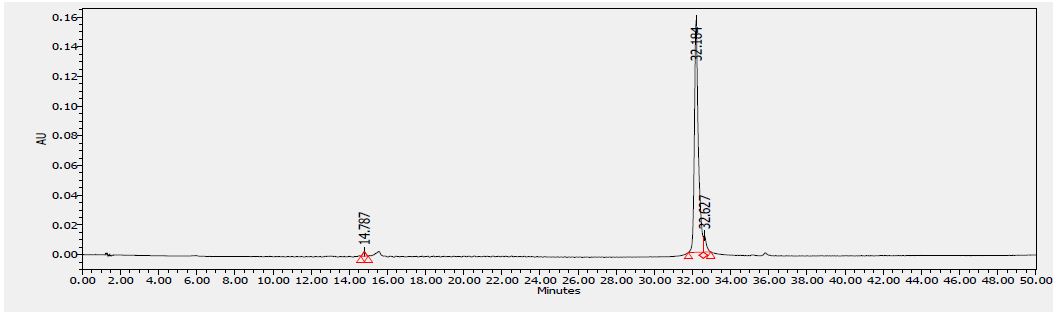
Figure 3: HPLC chromatogram of several standard phenols detected at 380 nm
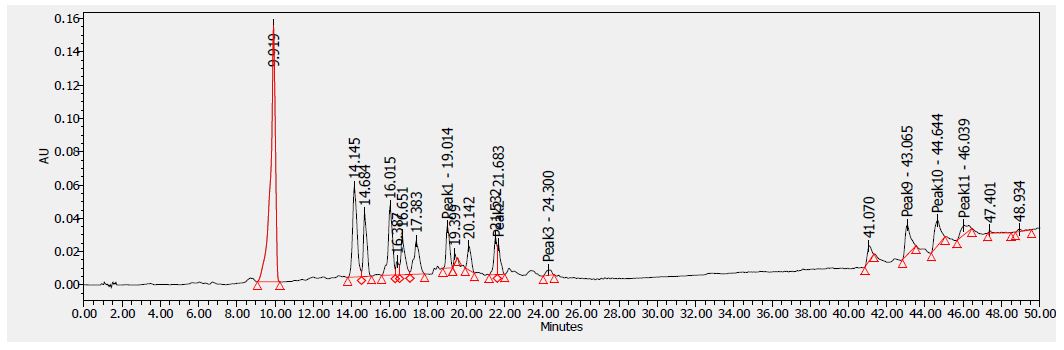
Figure 4: HPLC chromatogram of F2 fraction of Uvaria comperei stem extract at 280 nm
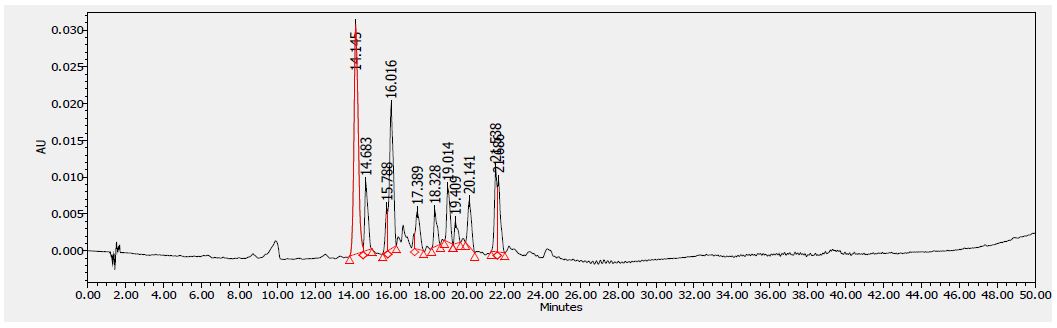
Figure 5: HPLC chromatogram of F2 fraction of Uvaria comperei stem extract at 340 nm
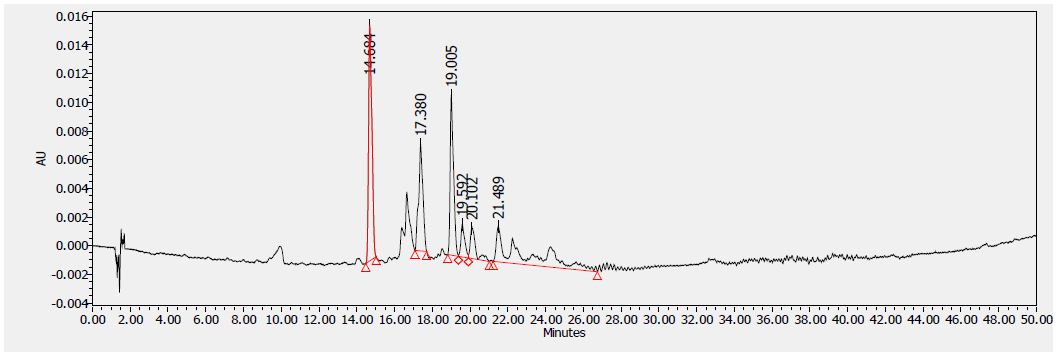
Figure 6: HPLC chromatogram of F2 fraction of Uvaria comperei stem extract at 380 nm
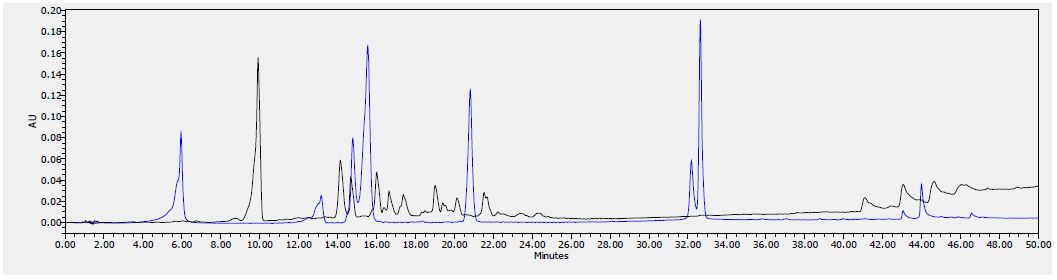
Figure 7: HPLC chromatogram of F2 fraction combined with the standards at 280 nm
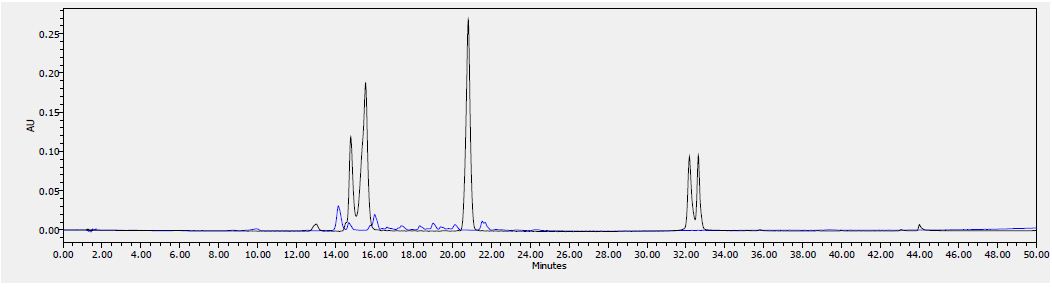
Figure 8: HPLC chromatogram of F2 fraction combined with the standards at 340 nm
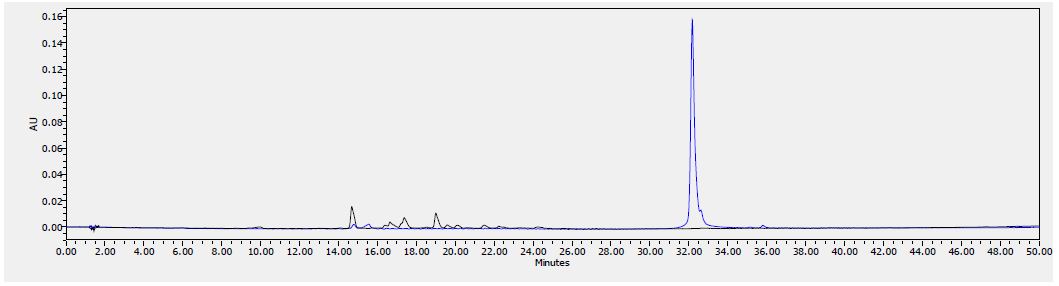
Figure 9: HPLC chromatogram of F2 fraction combined with the standards at 380 nm
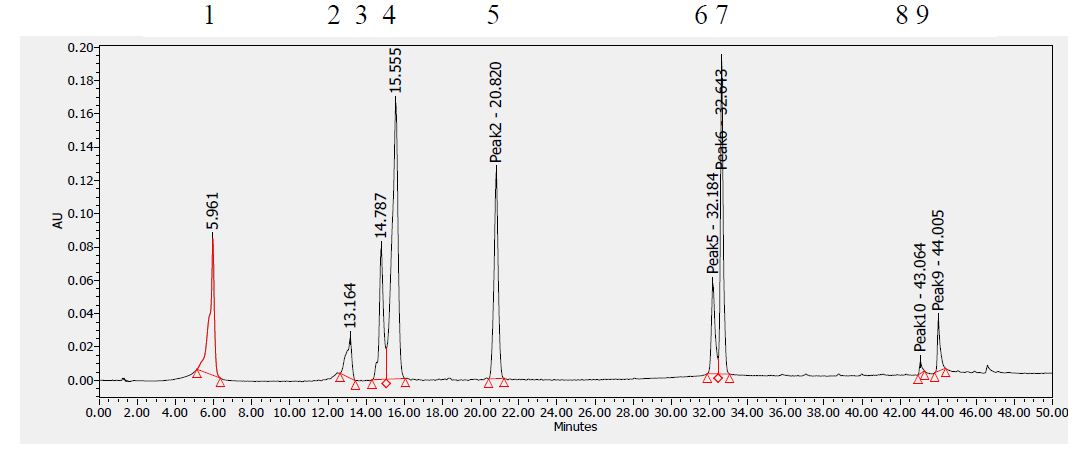
Figure 10: HPLC chromatogram of standards at 280 nm. Peaks: (1) Gallic acid, (2) Epicatechin, (3) Chlorogenic acid, (4) Caffeic acid, (5) Herniarin, (6) Quercetin (7) Imperatorin, (8 and 9) Catechins.
Table 8 shows the flavonoids identified in F2 fraction. The chromatogram analyses showed that F2 fraction contains numerous phenols; moreover, chlorogenic acid and catechins were identified in the present phytochemical analysis.
Table 8: Compounds identified by HPLC in F2 fraction of Uvaria comperei stem extract

1: Gallic Acid, 2: Epicatechin, 3: Chlorogenic Acid, 4: Caffeic Acid, 5: Heniarin, 6: Quercetin 7: Imperatorin, 8: Catechin
Discussion
A previous analysis of the crude stem methanol extract of stems (UCCE) and the F2 fraction of Uvaria comperei demonstrated its high antioxidant activity and the characteristic presence of phenols, flavonoids, tannins and anthraquinones [2,3]. The high number of polyphenols in these extracts can explain their scavenging and antioxidant activities. Knowing that radical species are involved in the inflammation process as inductors, previous results suggest the potential anti-inflammatory activity of the UCCE and F2 fraction. Effectively, for the first time, the present research shows the antinociceptive and anti-inflammatory properties of the crude methanol extract of the stem and F2 fraction of Uvaria comperei in several in vitro and in vivo models. In detail, albumin denaturation, heat hemolysis, formalin-induced paw licking, and carrageenan-induced hind paw oedema tests were used to evaluate antinociceptive and anti-inflammatory activities of the UCCE and F2 fraction. The crude extract and the F2 fraction at 500 mg/mL completely inhibited heat- induced albumin denaturation; Furthermore, UCCE showed greater activity than the fractions. It is well known that protein denaturation is a process by which tertiary and secondary structures change, causing loss of protein biological function of proteins. Moreover, one of the main features of inflammation is protein denaturation [25]. In fact, many disorders such as serum disease, rheumatoid arthritis, glomerulonephritis, and systemic lupus erythematosus are the result from hypersensitive reaction, which, in turn, is related to the antigens produced during protein denaturation [26]. Data from the literature suggest that the anti-denaturation property of BSA is due to the presence of binding sites in the aromatic tyrosine rich function and aliphatic regions of the threonine and lysine residues of BSA [27]. According to Verma [28], inhibition of the protein denaturation process by plant-derived extracts can be due to the presence of flavonoids. Several studies have shown that interaction with polyphenolic compounds improved protein thermal stability [5,29]. The Uvaria comperei products effectively inhibited heat-induced haemolysis with a 75% inhibition by using 500 µg/mL UCCE extract, while the 500 µg/mL F1, F2 and F3 fractions exhibited a inhibition of 66,80 and 95%, respectively. These results suggest that these may inhibit the release of the neutrophil lysosomal content of neutrophils at the inflammation sites. Indeed, the erythrocyte membrane is analogous to the lysosomal membrane, and its stabilization implies that the extract could stabilize the lysosomal membranes, which is important in limiting the inflammatory response by preventing the release of activated neutrophil lysosomal components causing further tissue inflammation and damage. Nonsteroidal drugs act by inhibiting these lysosomal enzymes or stabilizing the lysosomal membrane [30]. The membrane stabilizing effect of UCCE and F2 could be due to the quality and quantity of phenolic compounds, as UCCE and fraction F2 have a high tenor of polyphenols [2,3]. Consistent with this idea, Bouhlali et al. [29] showed high correlations between the stabilizing effect of the membrane and phenol contents. The authors suggested that flavonoids may interact at the water-lipid interface with the polar head of phospholipids increasing membrane rigidity, reducing fluidity, and increasing the stability of the mechanical lipid bilayer [31].
The antinociceptive effects of the F2 fraction showed a dose- dependent reduction in pain, which was greater compared to the indomethacin-induced effect. The behavioral response to formalin follows a biphasic pattern composed of an initial acute phase (first phase) and then of a longer period (second phase), while the period between phases is called the quiescent interval. Phase I consists of neurogenic nociception by direct stimulation of nociceptors via C fibers to the dorsal horn of the spinal cord after substance P is secreted and acts as a neurotransmitter. The second phase consists of inflammatory-induced pain due to the release of serotonin, histamine, bradykinin, and prostaglandins from formalin-damaged tissue [32]. The pain response in both phases is processed at the spinal level. The spinal cord contains mechanisms that inhibit the activity of neurons that receive and transmit nociceptive information. The primary afferent fibers of the spinal cord utilize excitatory amino acids (EAs) such as glutamate and aspartate as their neurotransmitters. There is evidence that selective EA receptor antagonists produce antinociception [33]. Hence, the antinociceptive activity of the F2 fraction may be due to the capacity to act on the EA receptors or to inhibit phospholipase or cyclooxygenase that participate in the synthesis of prostaglandins. The first phase is reported to be inhibited by opioid analgesics, and the second phase is inhibited by both nonsteroidal anti-inflammatory drugs (NSAIDs) and opioid analgesics. The antinociceptive activity of the F2 fraction could be due to the presence of flavonoids and phenols detected by HPLC analysis and also shown in previous data [2]. However, the cellular mechanism involved in the antinociception of the F2 fraction needs further research, as it was not investigated here. The F2 fraction had the highest anti-inflammatory activity by reducing the carrageenan-induced paw oedema. It was showed 50% of inhibition just 30 min after carrageenan-induced reaction, and 96% after 6 h using 25 mg/kg of F2. Its activity was higher than that of indomethacin, used as standard drug. Previous studies have shown that carrageenan-induced paw oedema is usually a biphasic process. The early stage (0-1 h) is characterised by the secretion of histamine, serotonin, bradykinin, and overproduction of prostaglandins in the surrounding damaged tissue. The later stage (1-6 h) is the target of the most clinically effective anti-inflammatory drugs due to an overproduction of pro-inflammatory mediators such as bradykinin, leukotrienes, prostaglandins, platelet activating factor, nitric oxide, and proteolytic enzymes by neutrophils in the inflamed tissues [29]. The present study revealed that the F2 fraction decreased the paw oedema in both phases, differently to indomethacin. The low activity of indomethacin in the early stage is as expected because non-steroidal anti-inflammatory drugs such as aspirin or indomethacin are unable to inhibit the early stage of swelling [34]. The inhibition of the paw oedema during the two phases of inflammation suggests that F2 fraction could inhibit various chemical mediators of inflammation. Therefore, it can be speculated that F2 fraction contains phytoconstituents that might be acting through the inhibition of various mediators implicated in the inflammatory damage. Based on the well-known involvement of free radicals in inflammation, it seems that at least a part of the anti-inflammatory effects of F2 fraction may also be attributed to its antioxidant activity [3]. Among the constituents, phenols and flavonoids as chlorogenic acid and catechin were identified in F2 fraction. According to Kimura et al. [35], chlorogenic and caffeic acids also inhibited the histamine and leukotriene production. Other authors reported that luteolin and quercetin inhibited the release of histamine, prostaglandin and leukotrienes [36], while ferulic and caffeic acids inhibited the enzymes COX-1 and COX-2 [37]. Gallic acid inhibited the production of histamine and proinflammatory cytokines such as TNF-α and IL-6 from human activated mast cells [38]. Biochemical parameters and oxidative stress markers were also evaluated. Administration of fraction F2 in the test group significantly decreased ALT, AST and γ-GT levels compared to the control group. Furthermore, GSH and catalase levels were significantly decreased in the control group compared to the normal group, while the treatment with the F2 fraction (tested group) brings the levels close to the normal group. These observations converge with those of [39], who observed that aqueous and ethanolic leaf and root extracts of Uvaria chamae were not significant in rats at the level of uremia and creatinemia, but a sharp increase of AST and ALT levels was observed compared to the control. The results suggest that the fraction F2 could have hepatoprotective properties. The decrease in GSH and catalase activity in the control group compared to the normal and test groups suggest that the fraction F2 may have antioxidant activity. This property could be due to the bioactive substances in fraction F2, such as flavonoids, which are the main antioxidant metabolites [5].
The oral median lethal dose (LD50) of fraction F2 in mice was found to be greater than 2000 mg/kg body weight. This meant that the extract was practically non-toxic according to the acute toxicity classification standard, thereby validating the ethnomedicinal use of the plant. Reduction in body weight and relative organ weight is generally considered a toxic effect of the extract on the animal, resulting in reduced food and water intake. There was no visible difference between mice in the tested group (G2) and mice in the normal group (G3). The estimated value of LD50 was in line with Legba et al. [39] findings, who reported that ethanol and aqueous extracts of the leaves and roots of Uvaria chamae were not toxic at 2000 mg/ kg in rats. No mortality and no renal histological perturbations were recorded in the treated rats. The fraction F2 showed concentration- dependent cytotoxicity activity against the tested cells. The IC values shown by F2 were 27.73 and 82.86 µg/mL, in Raw and Ver50o cells, respectively. According to the American National Cancer Institute (NCI), the criteria of cytotoxicity for crude extracts were IC50<30 μg/ mL after an exposure time of 72 h in a preliminary assay [40]. Fraction F2 met this criterion with an IC50 value less than 30 μg/mL on raw cells, but was slightly cytotoxic in Vero cells.
Conclusion
This research revealed for the first time that the crude extract and the F2 fraction of Uvaria comperei possess and interesting anti- inflammatory activity. Furthermore, a dose-dependent antinociceptive effect of the F2 fraction has also been observed. In vivo hepatoprotective properties could also be suggested. Furthermore, the F2 fraction was not toxic up to 2000 mg/kg. The results support the traditional use of Uvaria comperei, and give credence to the ethnopharmacological approach for the selection of specific plant species for the discovery of new anti-inflammatory agents from natural sources.
Conflict of Interest
We declare that we have no conflict of interest.
Acknowledgment
The authors thank the Coimbra Group Program and the Department of Pharmaceutical and Pharmacological Sciences, University of Padua, Italy, for their financial and technical support. These funders had no involvement in study design, data collection, analysis and interpretation, writing, and the decision to submit the paper for publication.
Authors Contribution
MKS: Conceptualization; Funding acquisition; Investigation; Methodology; Writing – original draft. GTS: Investigation; Methodology, Writing – review and editing. MKayo: Investigation; Formal analysis; Data curation. ZC: Investigation; Formal analysis. MKouamo: Formal analysis; Writing – review and editing. DD: Investigation. PDJ: Writing – review and editing; Validation. MLS: Writing – review and editing; Validation. FBF: Writing – review and editing; Supervision. GF: Resources; Writing – review and editing; Validation; Supervision.
References
- Gina F, Renata B, Souza L, De-Freitas AH, et al. (2014) Plants of the annonaceae traditionally used as A review edição especial 36: 315-336.
- Simo M, Donati M, Siwe G, Majoumouo S, et al. (2020) Antioxidant potential of fractions from the stem methanol extract of Uvaria comperei (Annonaceae). Int J Pharmacogn 7: 76-82.
- Simo KM, Nguepi NM, Sameza M, Toghueo R, et (2017) Cameroonian medicinal plants belonging to Annonaceae family: radical scavenging and antifungal activities. Nat Prod Res 1478-6427.
- Le Thomas A (1969) Mise au point sur deux Annona africains, Muséum National d’histoire Laboratoire de phanérogamie, Paris, Adansonia 9: 95-103.
- Ghasemzadeh A, Ghasemzadeh N (2011) Flavonoids and phenolic acids: Role and biochemical activity in plants and J Med Plant Res 5: 6697-6703.
- Loomis WD, Battaile J (1966) Plant Phenolic compounds and the isolation of plant enzymes. Phytochemistry 51: 423-38.
- Kim D, Jeond S, Lee C (2003) Antioxidant capacity of phenolic phytochemicals from various cultivars of Food Chem 81: 321-326.
- Peterson J, Dwyer J (1998) Flavonoids: Dietary occurrence and biochemical Nutr Res 18: 1995-2018.
- Cook NC, Samman S (1996) Review: Flavonoids chemistry, metabolism, cardioprotective effects, and dietary J Nutr Biochem 7: 66-76.
- Ren W, Qian Z, Wang H, Zhu L, Zhang L (2003) Flavonoids: Promising anticancer agents. Medicinal Res Rev 23: 519-534.
- Phanse MA, Patil MJ, Chaudhari KAP, Patel B (2012) In-vivo and in-vitro screening of medicinal plants for their anti-inflammatory activity: an overview. J Appl Pharm Sci 2: 19-33.
- Alvarez PGA, Barbosa NL, Patipo VM, Petricevich VL (2012) Anti-inflammatory and antinociceptive activities of the ethanolic extract of Bougainvillea xbuttiana. J Ethnopharmacol 144: 712-719. [crossref]
- Mequanint W, Makonnen E, Urga K (2011) In vivo anti-inflammatory activities of leaf extracts of Ocimum lamiifolium in mice J Ethnopharmacol 134: 32-36. [crossref]
- Boominathan R, Parimaladevi B, Mandal SC, Ghoshal SK (2004) Anti-inflammatory evaluation of Ionidium suffruticosam Ging in rats. J Ethnopharmacol 91: 367-370. [crossref]
- Wang D, Wang LJ, Zhu FX, Zhu JY, et al. (2008) In vitro and in vivo studies on the antioxidant activities of the aqueous extracts of Douchi (a traditional Chinese slat- fermented soybean food). Food Chem 107: 1421-1428.
- Vidhu A, Shyam B, and Yashwant (2013) In vitro anti-inflammatory activity of Raupya (Silver) J Chem Pharm Res 5: 194-197.
- Sangita C, Priyanka C, Protapaditya D, Sanjib B (2012) Evaluation of in vitro anti- inflammatory activity of coffee against the denaturation of Asian Pac J Trop Biomed.
- Azeen A, Dilip C, Prasanth S, Shahima H, et (2010) Anti-inflammatory activity of the glandular extracts of Thunnus alalunga. Asian Pac J Trop Med 794-796p.
- Hunskaar H and Hole K (1987) The formalin test in mice dissociation between inflammatory and non-infammatory pain. Pain 30: 103-114.
- Peanna AT, Moretti MD, Vincenza M, Desole G, Pippia P (1997) Anti-inflammatory activities of aqueous extracts and steroidal sapogenins of Agave americana. Planta Med 63: 199-202.
- Lanher M, Fleurentin J, Defnyan P, Mortier F, Pelt J (1991) Analgesic and anti- inflammatory properties of Euphorbia hirta. Planta Med 57: 225-231. [crossref]
- Kuete V, Tchinda CF, Mambe FT, Beng VP, Thomas E (2016) Cytotoxicity of methanol extracts of 10 Cameroonian medicinal plants towards multi-factorial drug-resistant cancer cell lines. Complement Altern Med 16.
- O’Brien J, Wilson I, Orton T, Pognan F (2000) Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell Eur J Biochem 267:5421–6. [crossref]
- OECD guidelines for the testing of chemicals: Acute oral toxicity – up-and-down- procedure (udp). (2008) 425: 1-27.
- Bouhlali EDT, El Hilaly J, Ennassir J, Benlyas M, et al. (2018) Anti-inflammatory properties and phenolic profile of six Moroccan date fruit (Phoenix dactylifera L.) varieties. J King Saud Univ Sci 30: 519-526.
- Elisha IL, Dzoyem JP, Mc Gaw LJ, Botha FS, Eloff JN (2016) The anti-arthritic, anti-inflammatory, antioxidant activity and relationships with total phenolics and total flavonoids of nine South African plants used traditionally to treat arthritis. Complement Altern Med16.
- Williams LAD, Rosner H, Conard J, Moller W, et al. (2002) Selected secondary metabolites from Phytolaccaceae and their biological/pharmaceutical significance, Research Sign Recent Res Devel In Phytoche 6: 13-68.
- Verma MA, Kumar PA, Kavitha D, Anurag KB (2011) Anti-Denaturation and Antioxidant Activities of Annona cherimola In Int J Pharma Bio Sci 2: 1-6.
- Bouhlali EDT, Abdelbasset H, Bouchra B, Tarik K, et al. (2020) Phenolic profile and anti-inflammatory activity of four Moroccan date (Phoenix dactylifera L.) seed varieties. Heliyon 6.
- Rajendran V, Lakshmi KS (2008) In vitro and in vivo anti-inflammatory activity of leaves of Symplocos cochinchinensis (Lour) Moore ssp Laurina. Bangladesh J Pharmacol 3: 121-124.
- Tarahovsky YS, Kim YA, Yagolnik EA, Muzafarov EN (2014) Flavonoid–membrane interactions: involvement of flavonoid–metal complexes in raft signaling. Biochim Biophys Acta Biomembr 1838: 1235-1246.
- Mohammadifard F, Alimohammadi S (2018) Chemical composition and role of opioidergic system in antinociceptive effect of Ziziphora clinopodioides essential oil Basic. Clin. Neurosci J 9.
- Abdollahi M, Sarrafzadeh J, Nikfar S, Roshanzamir F (2003) Mechanism of aspartame-induced antinociception in indian J Pharmacol 35: 37-41.
- Salvemini D, Wang ZQ, Wyatt PS, Bourdon DM, et al. (1996) Nitric oxide: a key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br J Pharmacol 118: 829-838.
- Kimura Y, Okuda H, Okuda T, Hatano T, et al. (1985) Studies on the activities of tannins and related compounds from medicinal plants and drugs. Inhibitory effects of caffeoylquinic acids on histamine release from rat peritoneal mast cells. Chem Pharm Bull 33: 690-696. [crossref]
- Kimata M, Shichijo M, Miura T, Serizawa I, et (2000) Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells. Clin Exp Allergy 30: 501-508. [crossref]
- Jayaprakasam B, Vanisree M, Zhang Y, Dewitt DL, Nair (2006) Impact of alkyl esters of caffeic and ferulic acids on tumor cell proliferation, cyclooxygenase enzyme, and lipid peroxidation. J Agric Food Chem 54: 5375-5381.
- Kim SH, Jun CD, Suk K, Choi BJ, et (2006) Gallic acid inhibits histamine release and pro-inflammatory cytokine production in mast cells. Toxicol Sci 91:123-131. [crossref]
- Legba B, Dougnon V, Deguenon E, Agbankpe J, et al. (2019) Toxicological Characterization of Six Plants of the Beninese Pharmacopoeia Used in the Treatment of Salmonellosis. J Toxicol.
- Suffness M, Pezzuto JM (1990) Assays related to cancer drug discovery. In: Hostettmann K (ed) Methods in Plant Biochemistry: Assays for Academic Press London 6: 71-133.