Abstract
Besides typical fluid and melt inclusions specific for miarolitic pegmatites, unusual fluid inclusions filled with complex solid or liquid hydrocarbons were described. Raman spectroscopy was used as the principal method for first characterizing these inclusions. We briefly discuss the meaning of the hydrocarbons in inclusions in pegmatite quartz and think that Oparin’s thoughts on the origin of life became new impulses.
Keywords
Volyn pegmatites, Raman spectroscopy, Organic compounds in fluid inclusions
Introduction
An illustrative description and a short genetic interpretation of the mostly isometric to lens-shaped miarolitic Volyn pegmatites within the Korosten Pluton in Ukraine is in Pavlishin and Dovgyi [1]. Generally, the pegmatite bodies are often gigantic: dimensions of 20 x 20 x 15 m are usual. Melt inclusion in quartz and topaz trace the magmatic stage of the Volyn chamber pegmatites [2] as a pseudo-binary solvus curve with the solvus crest at 732°C and 27.4% water down to temperatures of about 550°C. Besides melt inclusions, representing the magmatic state, the quartz from the giant miarolitic pegmatites often contains fluid inclusion with homogenization temperatures around 375 and 400°C, meaning the hydrothermal-metasomatic stage in the mineralization [1,3]. According to Pavlishin and Dovgyi [1], the postmagmatic stage is characterized by an intense dissolution of quartz at about 300-400°C. At this stage, or slightly lower temperatures, the inclusions with relatively pure organic liquids were probably trapped. The hard bitumoid kerite [C491H386O87(S)N] formed under the same conditions [4]. Kerite was found in the center of a pegmatite body and comprises heavy black aggregates of up to 3 kg of abiogenic origin (according to Ginsburg et al., [5], cited in Gorlenko et al. Last time, an intense and controversial discussion started on the 1.5-billion-year-old Volyn ‘biota’ by Franz et al. [5] and Head et al. [6].
Methods
We used for all microscopic and Raman spectrometric studies a petrographic polarization microscope with a rotating stage coupled with the RamMics R532 Raman spectrometer working in the spectral range of 0-4000 cm-1 using a 50 mW single mode 532nm laser. Details are in Thomas et al. 2022a [7] and 2022b [2]. For the Raman spectroscopic routine measurements, we used the Olympus long-distance LMPLN100x as a 100x objective. For the identification of the organic compounds, we applied the Spectral Database for Organic Compounds [8].
Sample
The studied sample, a polished quartz plate about 1.5 mm thick, is from the root of a giant quartz crystal (~1000 kg) from Volyn pegmatite body № 402. The original sample is in the Mineralogical Museum of the Taras Ševčenko National University Kyiv, and the studied plate is from D.K. Voznyak (Kyiv, Ukraine). A description of the sample cross-section is in Voznyak (2007). The inclusions in question are between the crystal zones IV and V. According to Voznyak [3], the fluid inclusions beside the new type of inclusions homogenize at 390-395°C in the vapor phase. Also, other quartz samples of the same pegmatite from different, unclear positions contain inclusions with organic liquids.
Results
The presence of graphite and diamonds in the pegmatite quartz shows mantle components participate in pegmatite formation. By the spheric form, they are intruded fast by supercritical fluids. Table 1 shows Raman spectroscopic data on diamonds and graphite. According to Zaitsev [9], the spectral position of the diamond Raman line can vary considerably. Values between 1331 and 1346 cm-1 are possible depending on crystal perfection.
Table 1: Raman data of spherical diamond and graphite inclusions in pegmatite quartz from Volyn (11 different aggregates).
|
Phase |
Line position (cm-1) | FWHM
(cm-1) |
Line position (cm-1) |
FWHM (cm-1) |
| Diamond |
1336.4 ± 6.4 |
83 ± 5.0 | ||
|
Graphite G |
1580.6 ± 5.9 |
65.0 ± 7.0 |
||
| Graphite D2 |
1609.7 ± 1.0 |
39.0 ± 10.0 |
FWHM-full width at half maximum
Because we studied only diamonds about 100µm deep under the sample surface, we used a laser power of 50 mW on the sample and a counting time of 200 seconds for the Raman spectra of diamond and graphite. The main concern of the present contribution is the description and composition of the inclusion type filled with hydrocarbon compounds. Most inclusions contain a small black ball-like carbon aggregate and small colorless to pale yellow strip-shaped crystals. Rare are tiny vapor bubbles. They form primarily at the Raman measurements. Their movement inside such inclusion demonstrates their liquid consistency. However, there are also inclusions with solid organic compounds with low melting temperatures, around 30°C, trapped as liquid droplets in the quartz. The following compounds found Thomas and Voznyak (2023) [10] in the inclusions: isoprene [C5H8], dimethyl cyclohexane [C8H16], 2,3-xylenol [C8H10O], and diisobutyl phthalate [C16H22O4]. Figure 1 shows the new fluid inclusions type filled with organic liquids, sometimes tiny crystals of organic composition, and mainly with a small black aggregate of complex hydrocarbons.
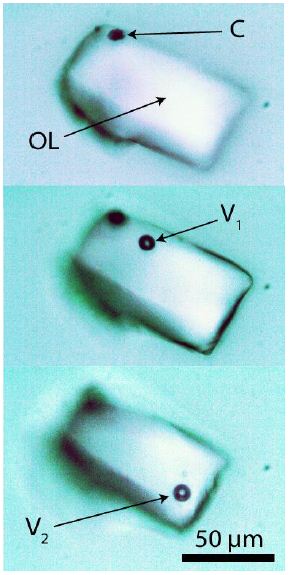
Figure 1: Typical fluid inclusion in pegmatite quartz from Volyn. The fluid is a liquid organic compound or a mixture (OL). C-hydrocarbon, V1, and V2 are the vapor bubbles at positions 1 and 2. The vapor bubble forms at the Raman measurement and disappears after the measurement. The change in the bubble position demonstrates the liquid state of the inclusion content.
The fluid inclusions, composed of organic material in the pegmatite quartz, are mostly isometric and have diameters between 20 and 100 µm. Figure 2 shows typical inclusions with organic material in quartz.
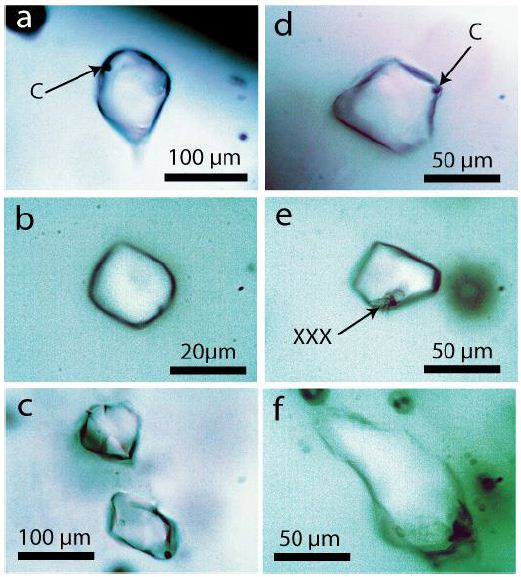
Figure 2: Typical organic fluid inclusions in Volyn pegmatite quartz. C with arrow shows small spherical hydrocarbon material. XXX-pale yellow colored crystals of organic material, similar to the liquid phase.
Clear indications are missing that the new kind with organic compounds-filled inclusions are impurities. However, up to now, the trapping conditions are not clear. The relatively isolated position far from the sample surfaces, microcracks, and the local distribution of these inclusions clearly show that they are not contaminations-they formed during the pegmatite crystallization. Generally, the small black ball-like aggregates inside the fluid inclusions are complex hydrocarbon material, not graphite or simple carbonaceous material, with the characteristic D1, D2, D3, and G bands in the Raman first-order region [11].
The origin of the organic material is related directly to the pegmatite crystallization, which follows from Figure 3, a melt inclusion in pegmatite quartz with a drop of organic material separated now from the volatile phase (V + L). Figures 2a and 2d show a faint meniscus in the liquid phases. In Thomas and Vosnyak (2023) [10], the meniscus is seen very well for inclusion 2a and shows three different organic liquid phases: dimethyl cyclohexane, 2,3-xylenol, and isoprene. That means at least the composition of the inclusions is not homogenous-from inclusion to inclusion, we see with Raman an inevitable variability by mixing different compounds. Mixing also happens by the laser light heating used for the Raman measurements. A surprise was the finding of DL-2-aminobutyric acid in fluid inclusions. α-Aminobutyric acid (AABA) is a non-proteinogenic amino acid with the structural formula H2N-C(CH3)2-COOH. It is rare and found in nature only in meteorites [12]. Table 2 lists the measured Raman data (532 nm laser) against the reference SDBS No. 1076 [8] using the 488 nm laser.
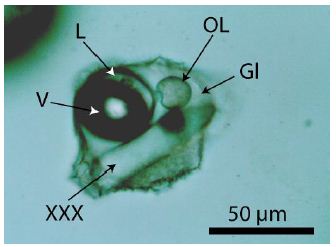
Figure 3: Melt inclusion in pegmatite quartz from Volyn. Gl-silicate glass, L-water-rich fluid phase. This phase also contains a tiny droplet of organic material (OL). OL-drop of a liquid organic compound. V-vapor phase, XXX-crystal.
Table 2: Comparison of the Raman data between natural inclusion in pegmatite quartz from Volyn and the SDBS database reference No. 1076: DL-2-amino-n-butyric acid.
|
DL-2-amino-n-butyric acid Volyn inclusions |
DL-2-amino-n-butyric acid C4H9NO2 SDBS No. 1076 | ||
| Band position in cm-1 | Relative Intensity | Band position in cm-1 |
Relative Intensity |
|
2964 |
48 | 2979 | 87 |
| 2944 | 67 | 2943 |
96 |
|
2872 |
98 | 2881 | 55 |
| 1454 | 55 | 1455 |
39 |
|
1436 |
59 | 1426 | 31 |
| 1384 | 10 | 1382 |
20 |
|
1346 |
11 | 1337 | 23 |
| 1319 | 16 | 1319 |
32 |
|
1274 |
9 | 1274 | 11 |
| 1124 | 7 | 1120 |
10 |
|
1070 |
21 | 1069 | 29 |
| 1046 | 14 | 1046 |
28 |
|
987 |
4 | 985 | 15 |
| 919 | 16 | 926 |
17 |
|
863 |
11 | 871 | 28 |
| 839 | 26 | 847 |
21 |
|
808 |
27 | 811 | 29 |
| 770 | 7 | 769 |
15 |
|
648 |
6 | 643 | 32 |
| 420 | 5 | 424 |
22 |
|
n.d. |
– | 226 | 24 |
| n.d. | – | 186 |
49 |
|
n.d. |
– | 166 |
48 |
n.d.: not detected: by the strong background of the host (quartz), an unambiguous Raman measurement was impossible.
Figure 4 shows the structure formula of DL-2-amino-n-butyric acid [C4H9NO2] according to the database [8]. The linear formula is [C2H5CH(NH2)CO2H]. The melting point of DL-2-amino-n-butyric acid, a white solid, is 295°C. That means that the trapping of this substance must be higher than this temperature.
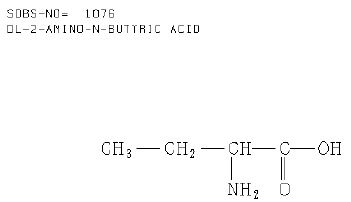
Figure 4: Structure formula of DL-2-amino-n-butyric acid according to the database (Author Collective, 1987)
The differences (relative intensity) in both spectra (Table 2) result mainly from the laser wavelengths used-532 vs. 488nm (database) and by mixing with small amounts of other organic components in the natural sample.
The unambiguous determination of the inclusion content alone with Raman spectroscopy is complicated by varying composition. The complex Raman band between 2800 and 3020 cm-1 is typical for all inclusions. A strong Raman band sometimes appears at 3079 cm-1 (e.g., at diisobytyl phthalate and butyl cyclohexane).
Another exceptional compound (tetrabutylgermane) is present as a liquid phase in the fluid inclusion (Figure 2e). Tetrabutylgermane is [C16H36Ge] or as a linear formula [CH3(CH2)3]4Ge. This compound is a colorless to light yellow liquid.
Table 3 lists the Raman data of the measured and given tetrabutylgermane. Differences result from the applied Raman stimulation light (532 vs. 488 nm) and possible impurities.
Table 3: Comparison of the Raman data between natural inclusion in pegmatite quartz from Volyn and the SDBS database reference No. 18433 (see Author collective, 1987): tetrabutylgermane.
|
Tetrabutylgermane in Volyn inclusions |
Tetrabutylgermane C16H36Ge SDBS No. 18433 | ||
| Band position in cm-1 | Relative Intensity | Band position in cm-1 |
Relative Intensity |
|
2961 |
48 | 2964 | 34 |
| 2930 | 90 | 2938 |
67 |
|
2895 |
99 | 2895 | 95 |
| 2875 | 99 | 2877 |
72 |
|
2856 |
99 | 2869 | 60 |
| 1450 | 49 | 1449 |
29 |
|
1437 |
55 | 1425 | 13 |
| 1305 | 12 | 1311 |
12 |
|
1286 |
12 | 1295 | 12 |
| 1060 | 14 | 1067 |
19 |
|
888 |
10 | 889 | 29 |
| 845 | 13 | 853 |
16 |
|
628 |
5 | 626 | 27 |
| 236 | 41 | 231 |
33 |
The occurrence of tetrabutylgermane, a metalorganic compound, is a surprise. However, Ge can enriched during pegmatite-forming processes in considerable amounts. The Raman bands’ slight deviation can also be caused by substituting Ge by Si contribution. The Raman Spectrum is presented in Figure 5 [8].
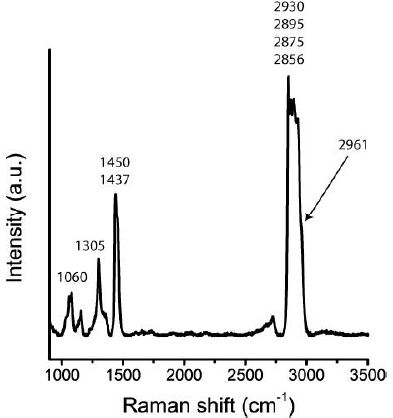
Figure 5: Part of the tetrabutylgermane Raman spectrum in a fluid inclusion in quartz of the Volyn pegmatite (Figure 2e).
Besides the compounds called here, there are a lot of others, often simpler in composition, for example, p-nitrophenol [C6H5NO3] or butyl cyclohexane [C10H20].
Discussion
The age of the Volyn pegmatites is, according to Popov [13], about 1760 ± 3 Ma. During the crystallization of the chamber pegmatites, starting at about 750°C [2], organic material is formed, enriched, and suspended in the late liquid phase. For the origin, we think of a catalytic formation from methane, CO2, or carbon coming from the mantle region, indicated by the finding of nanodiamonds and graphite in pegmatite quartz [10]. Freund [14] states that highly active carbon is present near all minerals and fluids coming from mantle depths and can form in water abiotic hydrocarbons. Carbonic material is often current during experimental work at high pressure and temperature due to contamination using carbon-bearing solutions such as acetone or CO2 [15]. So strong Raman bands between 2800 and 3000 cm-1 are, according to our Raman work, characteristic of hydrocarbons in synthetic stishovite, coesite, and buddingtonite [NH4AlSi3O8]. Carbon is often present in nature, particularly in supercritical fluids [16]. The transition from the supercritical into the critical and under-critical fluid is related to unusual processes, such as heterogeneous catalyzing [17], which can be responsible for the direct formation of the liquid organic material, including metalorganic compounds. The appearance of the ball-like carbon in almost all inclusions with organic material is unusual because, generally, carbon is graphite or a graphite-like material in fluid and melt inclusions. Unlike graphite, that black material is also unstable under the Raman laser light. Maybe this stuff forms the kerite aggregates found in the chamber center of Volyn pegmatites [1,4].
Freund [14] stated that the ancient ocean’s formation of appreciable amounts of biologically relevant essential molecules was ineffective. Alternatively, forming such molecules in a closed room like the Volyn pegmatites (as a natural laboratory) is possible. There are also significant older pegmatites with ages of 2900 Ma. Here forces the question: Is forming organic compounds also possible outside pegmatite bodies by the change of supercritical fluids coming from mantle regions into non-critical conditions in the ancient ocean? Oparin’s [18] thoughts obtain a new impulse here.
Acknowledgment
The present author thanks Dmytro K. Voznyak for providing the pegmatite samples
References
- Pavlishin VI, Dovgyi SA (2007) Mineralogy of the Volynian Chamber Pegmatites, Ukraine. Volodarsk- Mineralogical Almanac 12.
- Thomas R, Davidson P, Rericha A, Voznyak DK (2022b) Water-rich melt inclusions as “frozen” samples of the supercritical state in granites and pegmatites reveal extreme element enrichment resulting under non-equilibrium conditions. J. (Ukraine) 44: 3-15.
- Voznyak DK (2007) Microinclusions and reconstruction of conditions of endogenous mineral formation. Dumka, Kyiv, U.A. [in Ukrainian].
- Gorlenko VM, Zhmur SI, Duda VI, Suzina NE, Osipov GA, et. al. (2000) Fine structure of fossilized bacteria in Volyn kerite. Origins of Life and Evolution of the Biosphere 30: 567-577.
- Franz G, Lyckberg P, Khomenki V, Chournousenko V, Schulz HM, et al. (2022) Fossilization of Precambrian microfossils in the Volyn pegmatite, Ukraine. Biogeosciences 19: 1795-1811.
- Head MJ, Riding JB, O’Keefe JMK, Jeiter J, Gravendyck (2023) A reinterpretation of the 1.5 billion year old Voly’ biota’ of Ukraine, and discussion of the evolution of the eukaryotes. EGUsphere (Preprint repository).
- Thomas R, Davidson P, Rericha A, Recknagel U (2022a) Discovery of stishovite in the prismatine-bearing granulite from Waldheim, Germany: A possible role of supercritical fluids of ultrahigh-pressure origin. Geosciences 12: 1-13.
- Author Collective (1987) Spectral Database for Organic Compounds, SDBS, National Institute of Advanced Industrial Science and Technology (AIST), Japan.
- Zaitsev AM (2001) Optical Properties of Diamond. Data Handbook. Springer-Verlag Berlin, Heidelberg GmbH, 502.
- Thomas R, Voznyak DK (2023) Volyn Pegmatite: Unusual hydrocarbon-bearing fluid inclusions in pegmatite quartz. Aspects in Mining and Mineral Sciences 12: 1353-1360.
- Beyssac O, Coffee B, Chopin C, Rouzaud (2002) Raman spectra of carbonaceous material in metasediments: a new geothermometer. metamorphic Geol. 20: 859-871.
- Kvenvolden K, Lawless J, Pering K, Peterson E, Flores J, et al. (1970) Evidence for Extraterrestrial amino-acids and hydrocarbons in the Murchison meteorite. Nature 228: 923-926. [crossref]
- Popov DV (2023) Do pegmatites crystallise fast? A perspective from petrologically-constrained isotopic dating. Geosciences 13: 1-12.
- Freund F (1983) Atomarer Kohlenstoff in gesteinsbildenden Mineralen. Naturwissenschaftliche Rundschau. 36: 439-441.
- Mustart DA (1972) Phase relations in the peralkaline portion of the system Na2O-Al2O3-SiO2-H2 Dissertation, Stanford University, pg: 187 .
- Thomas R (2023) Growth of SiC whiskers in Beryl by a natural supercritical VLS process. Aspects in Mining & Mineral Science 11: 1292-1297.
- Thomas R, Rericha A (2023) The function of supercritical fluids for the solvus formation and enrichment of critical elements. (2023) Geology, Earth and Marine Sciences 5: 1-4.
- Oparin AI (1957) Die Enstehung des Lebens auf der Erde. VEB Deutscher Verlag der Wissenschaften Berlin 411.