Abstract
Reduced immune reactivity RIR has been documented in some phases of sars-cov-2 infected and vaccinee. RIR attributed to; anergy, tolerance, exhaustion and hypo-activity. The present opinion was an at glance review of anergy in COVID-19 infected and COVID vaccinee. Anergy has gross organ system presentation and cellular basis. Lymphocyte anergy and lymphocyte exhaustion are associated with changes in genetic landscape, epigenetic control and metabolic reprograming. Anergic B cells are found of multiple phenotypes and some of which associated with COVID-19 vaccine boost anergy as that found in Indian health care workers. Both CD4+ and CD8+ T cells were associated with immune protection among COVID infected and vaccinee. CD4+ anergy and CD8+ exhaustion [Though some debate] were reported in covid vaccinee.
Keywords
Anergy, COVID-19, Exhaustion, Hypo-function, Infected, Vaccine
Introduction
The immune system ensemble in human being living in continuum with his own ecologic niche may express three immune-biological phases as; normogy, allergy and anergy [1-5]. This came in accordance with normal, abnormal and subnormal functioning immune system. Three descriptive concepts are known in evaluation of this subnormal functioning immune system. Though they are being not well demarcated. Exhausted, tolerant and anergic immune cells are actually allied but not identical and may be overlapping both in sense and use [2-6]. The present opinion stands as an attempt to visualize the immunobiology of lymphocyte anergy and to pinpoint the role of anergy in COVID-19 infection and COVID-19 vaccination.
Reduced Immune Reactivity
Normogy is a state of normal functioning human immune system. Allergy is a condition of hyper-functioning immune system towards certain exo- or endogenous materials, the allergens. While anergy is a state of reduced or hypo-functioning immune system in presence of certain antigens within the continuum of the immune cells niche. Anergy is one of the processes that induce tolerance and modify immune cells to in order to prevent self –destruction [1-6]. Allied to anergy are; clonal deletion, immune regulation and immune cell exhaustion, Figures 1 and 2. These allied reduced immune reactivity concepts can be delineated on the bases of three related, none-identical but overlapping senses which are identity, cause and function, the convergence strategy. Exhaustion can be defined on the bases of the aforementioned concepts, as that cells when exposed to chronic high, antigen exposure, are activated and proliferated before becoming dysfunctional. The first is that cell is evident in absence of effector response. Second is that cell that are produced in presence of a given cause like exposure to an antigen and the third is that cell presenting same molecular marker of death the programmed cell death protein-1 (PD-1) [2]. In this opinion reduced immune cell function was defined and anergic cells were visualized, their potential role played in COVID-19 was mapped.
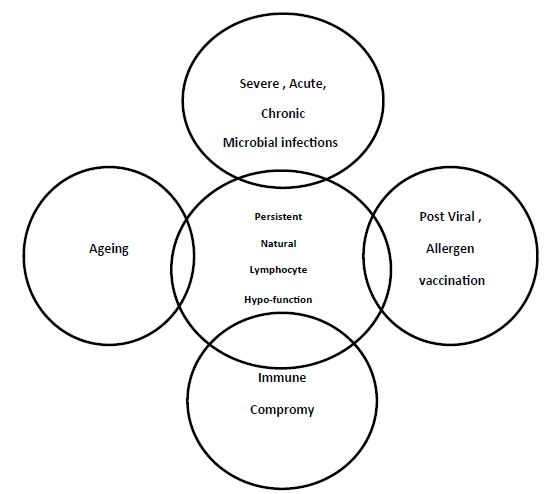
Figure 1: Effectors of lymphocyte hypo-function
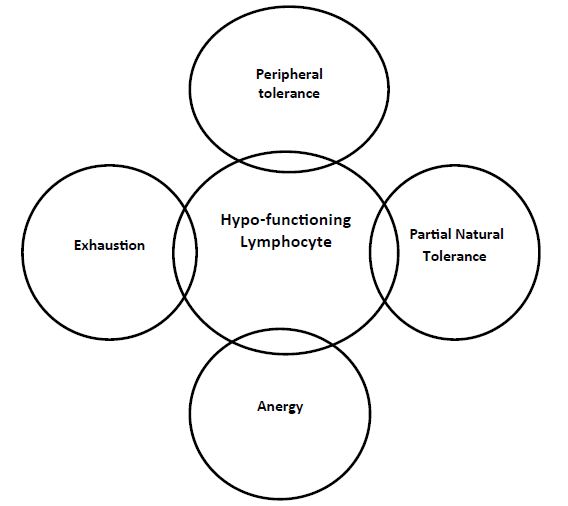
Figure 2: The Allied forms of lymphocyte Hypo-function
Cellular Basis of Anergy
Anergy in practice have two identifying facets. First, the reduced or absent gross clinical presentation and second the reduced or absent cellular functions. Herein, the study concerned with the cellular facets. The anergy is mainly tackled in lymphocytes [7-12] though there was report on anergy in basophils [13].
Mechanisms For Induction of Anergy In Lymphocytes
The target deletion in immune repose genes are associated with hyper-proliferation of T or B lymphocytes, frank autoimmune disease. These immune suppression genes represents the negative regulators that suppress self – reacting ability by enforcing negative selection in thymus for T cells and in bone marrow for B cells. The expression of these genes interferes with generation and/or function of regulatory T cells, altering signaling through T or B cells antigen receptors or promote apoptosis of peripheral T or B cells these two immune events outcomes with either T or B cell anergy. Such lymphocyte anergy might represent default genetic program, globally imposed on either mentioned lymphocyte types by low-level intracellular calcium influx occurring in response to recognition of self- antigen [6,8,13,14].
Mechanism of B cell Anergy
Yarkoni and associates [15] and Waterman and Cambier [12] have been thoroughly reviewing the multiple possible mechanisms for induction of B cell anergy. Going through these reviews, a hypothesis was arise from a collective viewing to the presented facts that may unify the mentioned mechanisms and was phrased as in the following;
- B cell face chronic antigen stimulation
- Surface immunoglobulin undergoes re –arrangement.
- Blockade of the second transducing signal.
- Notable Intracellular collective biochemical changes
- Activation of anergy specific gene circuit expression
- Genetic reprograming as a default genetic program
- Metabolic reprograming.
Mechanisms for Induction of Anergy In T cells
The induction of T cell anergy was based on the work of Valdor and Macian [7,16] and depicted in the following steps;
- Engagement of TCR and CD28.
- Free intracellular Ca influx.
- Activation of transcription factors
- NFAT form complex with AP1 with AP-1 inactivation
- NFAT direct the expression of anergy inducing genes.
- Expression of anergy inducing genes.
Mechanisms of induction of Exhaustion in T cells
Rha and Shin [17] were briefing the features of exhausted T cells which may in some way explained the mechanisms of induction of exhaustion in T cells, and were depicted in the followings
- Loss of effector function
- Sustained expression of inhibitory receptors
- Alteration in genetic and epigenetic landscape.
- Metabolic reprograming
B Cell Anergy
Anergic B cells are characterized by; Low surface IgM, normal surface IgD levels with reduction in their functional capacity. Reduced life span in absence of T cell help, inhibition of second signal transduction so that B cell does not release calcium as a critical pathway in activation of B cells. Anergic B cell undergoes change in migration patterns and distinctive recirculation fashion through lymphoid system as compared to normal functioning B cells. Normal B cells becomes anergic upon chronic exposure to self- antigen and reactivated to normal state by the expression to CD40 ligand in presence of IL4 (Table 1) [18].
Table 1: Comparative view to the immunobiologic features of anergic and exhausted lymphocytes as compared to normal
|
Features |
Normal B cells | Anergic B cell [10] | Normal T cell | Anergic T cell [7,8,14] |
Exhausted T cell [17] |
| Origin and migration | Bone marrow, peripheral lymphoid tissues | Bone marrow, peripheral lymphoid tissue | Bone Marrow, Thymus | Bone Marrow, thymus | Bone marrow, thymus |
| Developmental stage on migration for maturation | Mature, bone marrow | Mature, bone marrow | Immature, thymus | Immature, thymus | Immature, Thymus |
| Surface Markers | CD19, CD20, CD22, CD5, CD35, BCR, B7 | IgD, CD10, CD24, CD36, CD9 | TCR, CD2, CD3, CD28, CD5, CD7, CD45, CD4 or CD8 | TCR and CD28 engagement | CD28, CD5, CD2, CD3, CD5, CD7, CD45, CD8 |
| Signaling | Two signals | Stimulatory | Two signals | Stimulatory | Two signals |
| Anergy/phenotypic changes | Down-regulation of IgM, antigen irresponsive | Inhibition of cytokine production, Irresponsive to antigen | |||
| Exhaustion phenotypic changes | PD-1, TIM-3, LAG-3, CTLA-4, TIGIT, Markers & Expression of exhaust associated genes | ||||
| Reactivation | BAFF, TLR, INF gamma, CD40L with IL4 | Fas and Bcl-2 proteins | In-vivo patient recovery |
Auto-reactive B cells not controlled by receptor editing or clonal deletion may become anergic. Mature human B cell subsets that are naturally auto-reactive and controlled by the tolerizing mechanisms are those of functional anergy [9].
In healthy human IgMlo phenotypes are characterized by the absence of activation markersCD69,CD86and CD95,reduced expression of co-stimulatory molecules CD19 and CD21 with an evident inhibition levels of CD22. Therein the major fractions of mature B cell have a reversible anergic phenotypes determined impart by a down-regulation of sIgM that appears to determine a higher threshold for their activation through BCR [whether IgM or IgD] [10].
IgM but not IgD is down-regulated on auto-reactive B cells. IgD is less sensitive than IgM to endogenous antigens in-vivo. So the decision for the B cell developmental fate are shifted accordingly. The role of B cell surface IgD is in maintaining the quiescence of their auto-reactivity and restoring their differentiation into antibody secreting cells [11].
Clonal anergy was proposed as a way for inactivation of B cells stimulated early in their developmental phases when only auto-antigen would be present Anergic B cells are naïve-like B cells identified by the down-regulation of the complement -2 cell surface receptor (CD21) [10]. Though, the human anergic B cells are quite divergent and of multiple phenotypic forms [19].
There are about 2.5%-30% of human peripheral CD27- B cells are auto-reactive and anergic based on un-responsiveness to antigen-receptor BCR stimulation and auto-reactivity of cloned and expressed BCR. Human anergy is maintained by elevated expression of PTEN [a phosphatidylinositol 3 4 5 P-3- phosphatase],Reduction in expression of micro-RNA coding PTEN was associated with up-regulation PTEN and anergy in B cells (Table 1) [20].
Functions of Anergic B Cells
Rosenspire and Chen [6] deduced four main functions of anergic B lymphocytes in human welfare as:
- In healthy individuals it is found inactive and self -reactive.
- In autoimmune conditions, activated B cells found binding to self- antigenic epitopes.
- In state of infection with microbe bearing self- mimicking epitope, anergic cell found binding to cross-reacting epitope of the microbe.
- In fulminant Infection conditions, TLR signaling synergize with the anergic B cells allowing them to transition from the anergic cell pool, this in turn will lead to expression of active B cells which recognize host mimicking epitope on the pathogen as well as antigens on the host.
T Cell Anergy
Anergy in T cells is a state of growth arrest designed to reduce their proliferation during T cell immune responses. Failure of normal naïve T cell to establish the second signal transduction due to any cause will lead to T cell anergy state. The T cell anergy inducers are; peptide-MHc occupation, cross-link with concavalin A in absence of APC, binding with anti-CD3 mAb. Anergic T cells showed reduced IL2 to 25 to 50 folds lower than normal T cells and inhibition of CD40 ligand (Table 1) [21].
T cell anergy is a tolerance mechanism in which the lymphocyte is intrinsically functionally inactive following an antigen encounter but remain alive for an extended period of time in an hypo-responsive state. Two model explanations have been held for CD4 and CD8 T cells. First was the clonal anergy which principally growth arrest arise from an incomplete T cell activation. The second was the in-vivo anergy which represent more generalized inhibition of proliferation and effector function due to naïve T cell stimulated by deficient co-stimulation. Both of clonal anergy and in-vivo anergy are found in T reg. subsets [22].
T cell anergy is induced in previously activated T cells or cell clones by re-stimulation through T cell receptor TCR in absence of co-stimulating signal. Such anergic cells were found to be of reduced calcium flux, expression of protein enriched specifically by anergic program to induce T cell anergy. The induction ability of anergy in T cells depends on the intracellular assembly of E3 ubiquitin ligase activity [23].
Among a number of mechanisms that coevolved to control the adaptive immunity is the anergy. Which is the functional inactivation of T lymphocyte that responds to antigens in absence of inflammation. There found to be an intracellular protein in quiescent T cell that function to integrate signals for antigens co-stimulation and growth factor receptors. These factors ensure that all cells that fail to engage them from all the three pathways are shunted into alternative transcriptional program designed to dissuade them from participating in the subsequent immune response. Anergy is a combine result of factors that negatively regulate with a program of active transcriptional silencing that reinforced through epigenetic mechanisms [24].
One mechanism that is in place to control the activation of mature T cells that bears self- reactive antigen receptor is anergy, a long term state of hypo-responsiveness that establish T cell in response to sub-optimal antigen re-stimulation. T cell receive signals not only from antigen recognition and co-stimulation but also from other sources including cytokine receptor, inhibitory receptor or metabolic sensors. Under conditions that induce anergy, T cells activate program of gene expression that lead to the production of protein that block T cell receptor signaling and inhibit cytokine gene expression [8].
T cell anergy is already present in non-ventilated COVID-19 patients and strongly associated with virus persistence and reversible with clinical recovery [25].
Functions of T cell Anergy
T cell anergy may have the biological potentials to function in several immune conditions [7,14,25-28];
- Silence the auto-reactive T cells, so that in absence of such slience provoke marked autoimmune condition [7,14].
- Silence the allergenic reactions post-allergen vaccination [7,28]
- The developmental path of T cell anergy phases may serve potential target of pharmaceutical interventions [7].
- Serve as a pathognomic marker for viral persistence in severe viral infections including COVID-19 [25].
- Share part with T reg, functions in regulation of an aberrant immune responses [7].
Exhausted T Cells
It is a T cell that express hypo-functioning as well as reversible functional mode. Current information have develop a number of characteristics for this T cell functional form. Though its phenotype referred to as CD8+ T cells. Exhausted T cells have shown to be with; loss of effector function, alteration in the transcriptional and epigenetic landscape and metabolic reprograming. The presence of these cells in COVID-19 vaccine is contra-versial [17].
Anergy in Normal Human Being
Like the state of normal human being in whom there are percentages of auto-reactive cells and autoantibodies that represent the baseline levels of normal physiological autoimmune reactions [29-33]. There are normal levels of anergic immune cells like anergic B, anergic T and anergic myeloid cells [9].
Anergy in Sars-Cov-2 Human Infections
Both B and T cell immunity are involved in sars-cov-2 human infections. B cell produce Sars-cov-2 specific antibodies and T cells produce a spectrum of cytokines that take a role in the pathogenesis and immune-pathogenesis of sars-cov-2 infections. CD4 T cells helps B cells in production of antibodies. CD8+ T cells kills virus infected cells. T cells interferon gamma controls viral infection. Lymphopenia is evident in sars-cov-2 infection affecting CD4+,CD8+ and B cells. T cell responses in severe COVID infection forms may be over-activated, impaired or inappropriate. Post infection immunity yield memory B, memory CD4 and memory CD8 phenotypes [1]. Severe fatal sars-cov-2 infections in man showed a spectrum of immune responses that involve three phases; i-normal or hypo-immune, ii-hyper-activation and iii-Anergy [5]. COVID-19 infectious epidemic pneumonia is associated with; i-hyper-inflammation and ii- clear lymphocyte hypo-function that are mainly noted in hospitalized severe infection needing ventilation and associated with viral persistence [25]. Thus severe cod-19 infection is presenting both lymphopenia and lymphocyte hypo-function or anergy.
Anergy versus Antigens
Immune tolerance is either central genetic or peripheral, acquired. It can be of low or high dose tolerance depending on the toleragen dose, exposure frequency and continual coexistence facing the immune cells. On the absence of the antigen cells becomes reactive normal. Anergy need continual exposure of lymphocytes to the antigen which impacts the phenotypic, the migration behavior and the function. Regulatory T cell may function both in tolerance and anergy [26,27]. Vaccine for allergy in post-vaccination period may influence anergy in immune cells [28].
Anergy and Sars-Cov-2 Antigens
Sars cov-2 virus have an array of antigens; like spike, neucleo-capsed membranous as well as nonstructural protein [Grifoni et al. [29], Le Bert et al. [30]. This virus appeared to have super-antigen, super-antigen like and/or super-antigen trigger host cytokine storm, lymphocyte differentiation, lymphocyte apoptosis, anergy and autoimmunity [31].
The order of sars-cov-2 antigen exposure determine the nature of the immune response. Since it impacts the distribution between spike specific and non-spike specific responses. Thus the exposure history shapes phenotype and specificity of memory T cells [32].
Life Extreme and Vaccines
Life extremes are childhood and senescence. Ageing as life extreme impacts human immune system mainly as brook of immune tolerance, appearance of autoimmune reactions and reduction of other immune functions as; Decreased phagocytic activity, disturbed processing and presentation of antigens, decrease in functionality of innate and adaptive immune system, impaired responses to microbial vaccines in general and to sars-cov-2 vaccine. Low NK function, poor priming of T cells. B cell produces non-protective antibodies. These impacts may make aged subject vulnerable to respiratory infection and pneumonia [4].
Anergy versus COVID-19 Vaccines
In a population of 205 health care workers that were elected in Jan 2021. They were enrolled in a covisheild vaccine [same as ChAdOxl] vaccine. Vaccinated subjects were subjected to blood collection at the day14 and 28 after the first shot and three months after the second shot. Non-responder rate was 35:205 17% at the day 14 and 5;205 2% at the day 28. At the second dose all vaccinated were responders with 17 fold increase in anti-spike protein antibodies. Non-responder rates were higher in male and senior citizens. A 1.5 folds decrease in ASSA titers in the previously exposed. Thus, vaccination in non-exposed express prime-boost effects and in previously exposed express boost anergy effects [33,34]. Continual exposure to COVID vaccine induced anergy.
Vaccination after infection leads to expansion of spike specific T cell and differentiation of CCR-CD 4RA+ effector. In contrast Individuals after break through infection mount vigorous non-spike specific responses and diversify the T cell memory repertoire. Current vaccination protocols continue to expand and differentiate spike specific memory [32].
Conclusion
Reduction of immune reactivity of lymphocytes may be attributed to hypo-function, tolerance, exhaustion or anergy. Genetic versus epigenetic change, loss of function, identity, cause and function may be a helpful parameters for evaluation of these cell entities. Anergy influence change in phenotype, migration behavior and function of lymphocyte. Anergic lymphocytes were noted in; normal, chronic infected and vaccinated. Chronic viral infections and viral vaccination forms an insult for initiation of anergy. Post vaccination by viral vaccines and allegen vaccines can be terminate by anergic lymphocytes. Since continual antigen exposure to lymphocytes is one of the known ways for induction of anergic state in lymphocytes. One of the possible potentials of anergic cell is to silence auto-reactive cells and mediators. COVID-19 vaccination in exposed health care workers lead to boost anergy.
References
- Puddemann A, Aranson (2020) what is the role of T cells in covid-19 infection. why immunity is about more than antibodies. Oxford Covid-19 evidence team, Centre for evidence based medicine, Nuffield Department of Primary Care Health Sciences, University of Oxford. Oct.19.2020.
- Kaminski H, Lemone M, Paradeu T (2021) Immunological exhaustion; How to make a disparate concept operational? Plos Pathogen 17: e1009892. [crossref]
- Goldman M, Hermans C (2021) Thrombotic thrombocytopenia associated with covid-19 infection or vaccination; Possible to platelet factor 4 autoimmunity. Plos Medcine 18: 1003648. [crossref]
- Andryelkov BG, Besednova N (2021) Older adults: Panoramic view on covid-19 vaccination. Pub Hlth 8: 388-415. [crossref]
- Zhou X, Ye Q (2021) Cellular Immune responses to covid-19 and potential modulators. Front Immunol 12: 646333. [crossref]
- Rosenspire AJ, Chen K (2015) B cells precarious on call warriors at the nexus of autoimmunity and false flagged pathogens. Front Immunol 6: 580. [crossref]
- Valdor R, Macian F (2010) Mechanisms of Self inactivation in anergic T cells. Immunologia 29: 20-33.
- Valdor R, Macian F (2013) Induction and stability of anergic phenotype in T cells. Sem Immunol 25: 313-320. [crossref]
- Duty JA, Szodoray P, Zheng NY, Koelsch KA, Zhang Q, Swiatkowski M, et al. (2008) Functional anergy in subpopulation of naïve B cells from healthy human that express auto-reactive immunoglobulin receptor. J Exp Med 206: 139-151. [crossref]
- Quach TD (2010) human anergic B cells: IgMlo. PhD thesis. School of Medicine and Dentistry, University of Rochester, New York.
- Noviski M, Mueller JL, Satterwaite A, Sinha LAG, BromBacher F, et al. (2018) IgM and IgD B cell receptors functionally respond to endogenous antigen and control B cell fate. eLife 7: 35074. [crossref]
- Waterman PM, Cambier JC (2010) The conundrum of inhibitory signaling by ITAM containing immune-receptors: Potential molecular mechanisms. FEBS Letters 584: 4878-4882. [crossref]
- Ma A, Patil G, Lund K et al. (2012) Antigen induced anergy in human Basophils is not antigen specific. J Allergy Clin Immunol 129: AB123.
- Macian F, Im SH, Gavacia-Cozar FJ, et al. (2004) T cell anergy. Cur Opin Immunol 16: 209-216.
- Yarkoni Y, Getahun A, Cambier JC (2010) Molecular underpinning of B cell anergy. Immunol Rev 237: 249-253. [crossref]
- Belanti J A(ed) (2012) Immunology IV: Clinical application in health and disease, Distruption of co-stimulatory signal and anergy. Care Press. MD. Immunopedia.Org.Za.
- Rha MS, Shin EC (2021) Activation or exhaustion of CD8+ T cells in patients with covid-19. Cellular Mol Immunol 18: 2325-2333. [crossref]
- Tartinton DM (1998) Anergy B cells, Delves PJ, Roitt IM(ed.). Encyclopedia of Immunology 2nd London. Academic Press 105-108.
- Andrews SF, Wilson PC (2010) The anergic B cells. Blood 115: 4976-4977.
- Smith MJ, Ford MR, Rihanek M, Coleman BM, Getahun A, et al. (2019) Elevated PTEN expression maintain anergy in human B cells and reavels unexpectedly high repertoire autoreactivity. JCI Insight 4: 123384. [crossref]
- Shwartz RH (1998) Anergy T cells, Delves and Roitt IM eds. Encyclopedia of Immunology, 2nd London, Academic Press 109-111.
- Shwartz RH (2003) T cell anergy. Ann Rev Immunol 21: 305-334. [crossref]
- Fathman CG, Lineberry NB (2007) Molecular mechanisms of CD4+ T cell anergy. Nat Rev Immunol 7: 599-609. [crossref]
- Wells AD (2009) New insight into molecular basis of T cell anergy, factors, avoidance, sensors and epigenetic imprinting. J Immunol 182: 7331-7341. [crossref]
- Renner K, Schwittay T, Chaabane S (2021) Severe T cell hypoactivity in ventilated covid-19 patients correlate with roploged virus persistence and poor outcomes. Nat Immunol 12: 3006. [crossref]
- Tolar P, Sohn HW, Pierce SK (2005) The initiation of antigen induced B cell receptor signaling viewed in living cells by fluorescence resonance energy transfer. Nat Immunol 6: 1168-1176. [crossref]
- Gauld SB, Benschop RJ, Merrell KT, Cambier JC (2005) Maintainence of B cell anergy requires constant antigen receptor occupancy and signaling. Nat Immunol 6: 1160-1167. [crossref]
- Cao Y, Yang M, Luo Z, Monapatra SS (1997) Vaccination with multi-epitope recombinant allergen induce deviation via T cell anergy. Immunol 90: 46-51. [crossref]
- Grifoni A, Weiskopf D, Ramirez SI (2020) Target T cell responses to sars-cov-2 coronavirus in human with covid-19 disease unexposed individual. Cell 181: 1489-1501. [crossref]
- Lebert N, Tan AT, Kabasegaran K, Tham CYL, Hafezi M, et al. (2020) Sars-cov-2 T cell immunity in cases of covid-19 and sars and un-infected controls. Nature 484: 457-462. [crossref]
- Hamdy A, Leonardi A (2022) Superantigens and sars-cov-2. Pathogens 11: 390. [crossref]
- Minervina AA, Pogorely MV, Kirk AM, et al. (2022) Sars-co-2 antigen exposure history shape phenotypes and specificity of memory CD8+ T cells. Nat Immunol 597: 268-273.
- Shnawa IMS (2022) Post-infection-post-vaccination autoimmune neural long covid. J Pharmaceutical Res Inter 34: 1-7.
- Kannian P et al (2022) Booster and anergic effects of covishield vaccine among health care workers in south India. medRxiv. a license to display pre-print in perpetuating. It is made available under a CC-By4.0, international licensed.