Abstract
Objectives: The objective of this explorative in vitro study was to evaluate the ability of a novel self-assembling peptide matrix gel with calcium phosphate in effectively occluding dentine tubules compared to selected desensitizing toothpastes.
Methods: Mid-coronal dentine discs with a thickness of 1 mm were sectioned from caries-free human molars. The discs were etched with 6% citric acid for 2 minutes, halved and subjected to a 2-minute brushing with a novel gel (SAPM) and three selected desensitizing toothpastes ([SRP], [SRR] and [CSP]). The ability of the desensitizing gel and toothpastes to occlude the dentine tubules was assessed and compared before and after brushing using Scanning Electron Microscopy (SEM) on both etched and fractured dentine surfaces. The SEM observations were supplemented by hydraulic conductance measurements using a modified Pashley model before and after tooth brushing (n=5).
Results: The results demonstrated that there was a reduction in both the number and the diameter of the open dentine tubules, which was evident for all the treated dentine discs. The particles that occluded the open dentine tubules, however had different morphologies and distribution. The self-assembling peptide matrix gel (SAPM) demonstrated greater reduction in the number of open tubules compared to the other desensitizing toothpastes. Reductions in the hydraulic conductance measurements were observed for all tested materials (mean [SD, %]: SAPM 55.1 [12.5], SRP 64.9 [18.5], SRR 39.1 [17.1] and CSP 27.6 [6.8]). No statistically differences were observed between the SAPM and SRP, SRR toothpastes (paired t-Test; ≤0.05) although a significant difference was noted between the SAPM and the CSP toothpaste. There was an overall trend for reduction for the SAPM compared to the SRR toothpaste.
Conclusion: The results would suggest that a novel self-assembling peptide matrix gel (SAPM) was effective in blocking the dentine tubules and may therefore have the potential to be an effective desensitizing product for the treatment of Dentine Hypersensitivity.
Keywords
Self-assembling Peptide Matrix, Desensitizing Toothpastes, Tubular Occlusion, Hydraulic Conductance
Introduction
According to Hill & Gillam [1] Dentine Hypersensitivity (DH) is a clinical problem that may have impact on the Quality of Life of individuals who experience discomfort when eating and drinking hot and cold food during their day to day activities. The condition is postulated in the Hydrodynamic Theory [2] to be a result of minute fluid shifts within the dentine tubules following an external stimulus (e.g., cold, heat etc.). Currently toothpastes, gels and mouthwashes are designed to reduce or relieve pain arising from DH based on either their 1) tubular occluding components (e.g., silica, calcium carbonate, hydroxy- or nano-hydroxy apatite(s), oxalates or bioactive glass) or 2) nerve desensitization properties (e.g., potassium ions). Most of today’s commercial products have been reformulated from well-established technologies [3–5]. One of the few new developments representing a biomimetic approach to remineralization and thus being an alternative to the traditional desensitizing products for treating DH, is a self-assembling peptide matrix (SAPM) gel. The biomimetic self-assembling peptide P11–4 (SAP P11–4) has been shown to be effective as a non-invasive treatment for early stage dental caries [6–12]. In the treatment of early caries SAP P11–4 has been shown to diffuse into the subsurface micro-pores of enamel and form a 3D scaffold/matrix/hydrogel thereby mimicking the enamel matrix original function during tooth development, to support apatite crystallization, thus reversing tooth decay [6, 8]. According to previous reports SAP P11–4 (Ace-Gln-Gln-Arg-Phe-Glu-Trp-Glu-Phe-Glu-Gln-Gln-NH2) self-assembles into a matrix or hydrogel [13] under defined conditions. This matrix can support biomimetic mineralization and enamel regeneration [9]. The glutamic acid residues on the surface have been shown to act as nucleation sites for apatite formation and result in remineralisation of the lesion body [14].
The first product based on SAPM for treatment of Dentine Hypersensitivity (DH) has been marketed (Curodont D’Senz, Credentis ag, Switzerland). It has been recently shown to be effective in reducing DH in a randomised clinical study by Schlee et al. [15], demonstrating faster desensitization than a toothpaste including 5% Arginine and Calcium Carbonate. This product uses the same self-assembling peptide as included in the product for remineralization of carious lesions but was formulated as a gel with the self-assembling peptide in its assembled (i.e. matrix, SAPM) state. The SAPM is able to form a film on the dentine surface, without undergoing any chemical or physical transformation, solely by binding to the available Calcium ions on the tooth surface [14].
The in vitro measurement of fluid flow (hydraulic conductance [Lp]) in the dentine disk model has been used to assess the ability of desensitizing agents in treating Dentine Hypersensitivity (DH) [16]. According to Bränström [2] the mechanism underpinning the hydrodynamic theory is associated with rapid minute shifts in dentine fluid flow within the open dentine tubules, which acts as a capillary bore when a stimulus (e.g., cold) is applied to an exposed dentine surface. Fluid flow is dependent on the fourth power of the radius and therefore, any reduction in the diameter of the dentine tubule lumen should in theory reduce the fluid flow within the tubule, which in turn will decrease DH [16]. These investigators used a dentine section of approximately 1.0 mm in thickness to measure the hydraulic conductance of each desensitizing product applied on a dentine disc according to Greenhill & Pashley [16]. The aim of this explorative in vitro study was to investigate the ability of a commercially available SAPM containing gel in occluding dentinal tubules and thus reducing hydraulic conductance (fluid flow) through dentine.
Objectives
The main objective of this explorative in vitro study was to access the ability of a novel SAPM gel in reducing fluid flow (hydraulic conductance [Lp]) by tubular occlusion and to compare the effectiveness of the SAPM to three desensitizing technologies incorporated into toothpastes with established tubular occluding properties e.g., Colgate Sensitive ProRelief [CSP], Sensodyne Repair and Protect [SRP], and Sensodyne Rapid Relief [SRR].
Methods: In the present experiments a mid coronal dentine disc was used in a modified Pashley cell (Figure 1) [10, 17]. The fluid flow through the dentine discs was measured for the SAPM Gel and three selected desensitizing toothpastes (Table 1). The non-treated dentine discs were used as a control to the measurements obtained from the treated dentine discs.
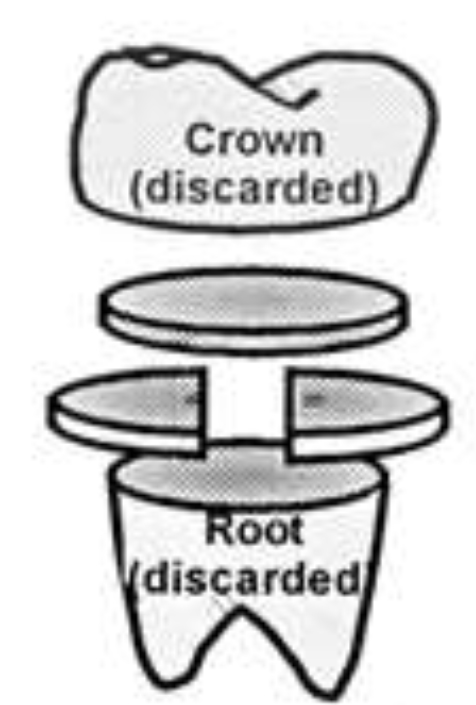
Figure 1: Dentine Disc Preparation (Based on Mordan et al., [17]).
Table 1. The desensitizing toothpastes investigated in the study.
|
Toothpaste Investigated |
Company |
Principal Ingredients |
|
Self-Assembling Peptide Matrix Gel (Curodont D’Senz)(SAPM) |
Credentis AG |
P11–4
|
|
Colgate Sensitive ProRelief (CSP) |
Colgate Palmolive |
Pro-Argin(5% Arginine) Calcium Carbonate |
|
Sensodyne Repair and Protect (SRP) |
GSK Consumer Healthcare |
Calcium phosphosilicate (Novamin) Bioactive Glass |
|
Sensodyne Rapid Relief (SRR) |
GSK Consumer Healthcare |
Strontium Acetate* |
|
*Current formulations include Stannous Fluoride instead of Strontium Acetate |
||
Dentine Disc preparation: Caries free extracted mandibular and maxillary molars were used for the study. Teeth were obtained from the tooth bank at the Royal London Dental Hospital under agreed Ethics Committee approval (QMREC 2011/99). Teeth were stored in a 70/30 ethanol/water solution within the Department of Dental Physical Sciences under the Human Tissue Act (2004) regulations prior to the evaluation of the selected products.
The criteria for the teeth selection were as follows:
- Molar teeth.
- Large molar teeth, with a dentine area of at least 6 mm in diameter.
- Carious free.
The selected teeth were cleaned and stored in a 3% sodium hypochlorite solution (prepared from 14% sodium hypochlorite solution after dilution) for 24 hours to allow for disinfection. After the disinfection procedure, the teeth were stored in 70/30 ethanol/water prior to use. Each tooth was fixed in a sample holder using impression material compound (Kerr, Model: 813–00425) and placed perpendicular to the annular diamond coated blade of a precision slicer (Microslice II, Malvern Instruments, UK). The crown of the tooth was discarded, and the cutting continued below the dentine-enamel junction to produce discs consisting of mid coronal dentine (Figure 1). The diamond blade was adjusted to result in discs with a thickness of 1 mm. The prepared discs were then stored in 70/30 ethanol/water.
Polishing, Cleaning and Etching of Dentine Discs
Prior to the assembling of the dentine discs into the Pashley permeability holder (cell), they were polished, cleaned and etched. The polishing step was completed by wet polishing both sides of the dentine discs against a series of silicon carbide grinding paper (P800 – P4000 equivalent to 5 µm) using a polishing machine (Kent 4 Automatic Lapping & Polishing Unit, Kemet International Ltd., UK). The thickness of the polished dentine disc was measured using a digital Vernier calliper (AK962EV, Sealey). The polished dentine discs were cleaned (2x5min) in an Ultrasonic bath (Kerry Ultrasonic bath), the water was changed after the first five minutes.
Dentine Tubule Occlusion Evaluation by Scanning Electron microscopy (SEM)
The dentine discs were etched (6% citric acid; 2 min) and rinsed thoroughly with de-ionised water. Each test surface was marked and then fractured into two halves using dental pliers, one half was used as a control, and the other half was subjected to two minutes brushing with 0.1 ml toothpaste by an electrical toothbrush with a sensitive brush head (Oral-B Trizone 5000, UK). Following brushing, the discs were rinsed by water and dried in air. All four test products described in Table 1 were evaluated and new brush heads were used for each of the test toothpastes.The control-half dentine disc and the test-half treated were again halved into quarter discs. A quarter from each half was prepared for SEM analysis. The specimens were gold coated and viewed under a field emission scanning electron microscope (FEI Inspect F, Oxford Instruments, Oxfordshire, UK) in the secondary scanning imaging mode at a voltage of 10 KV and a working distant of 10 mm. The deposit on the surface of the dentine and the distribution of the particles around and within the dentine tubules was investigated. SEM images were taken at the same region (close to the centre of the dentine disc) of the control and treated dentine disc with same magnification(s) (2000x, x 5000x, 10,000x and 20,000x) to evaluate the effectiveness of the ability of each toothpaste in occluding the dentine tubules. A cross section of a dentine disc was prepared by cutting one dentine disc in half using dental pliers [17]. The section designated the ‘test’ half was treated by applying the SAPM according to the Manufacturer’s instructions and viewed under the SEM.
Permeability Measurement (Hydraulic Conductance [Lp])
Following cleaning, the etched dentine disc was then placed in the Pashley specimen holder and prepared for the measurement of dentine permeability (hydraulic conductance). An air bubble (0.1 ml gas) was introduced into the system via a disposable plastic syringe, which then passed through the tubing and traversed the micro-capillary set up. The distance (in cm) that the air bubble travelled in the micro-capillary was recorded in one min intervals. Overall a continuous 10 min measurement was performed. The distance that the air bubble travelled was plotted against time, where a linear correlation was expected. The slope of the linear relationship was the fluid flow rate for the acid etched dentine disc. Following the application of the test products (see above) the toothpaste residual was rinsed with de-ionised water for five seconds and the fluid flow measurements were repeated in the same manner as described previously. The percentage reduction of the fluid flow was calculated using the following equation and presented in percentage (%).

The selected desensitizing toothpastes investigated in the present study are listed in Table 1. 20 dentine discs were used for the permeability aspect of the study. Five discs were used in each of the four toothpaste groups.
Results
Figure 2–7 show the SEM images of the dentine discs 1) control (un-brushed sections) and 2) after brushing with the tested toothpastes at 3 different magnifications (2000x, 5000x and 20,000x) and a 3) cross-sectional view of the dentinal tubuli (Figure 2-3 only for SAPM). A magnification of 2000x gives an overall impression of the dentine tubules occlusion, where high magnification shows how the particles were distributed in and around the dentine tubules. The dentine disc treated with SAPM showed almost complete dentine tubule coverage by placing a hydrogel film over the dentine surface. The dentine disc surface was rather smooth with some big clusters presumably Dicalcium Phosphate particles from the formulation (Figure 2–4).
SEM images of dentine tubules treated SAPM before (left) and after treatment (right) can also be observed in Figure 4.
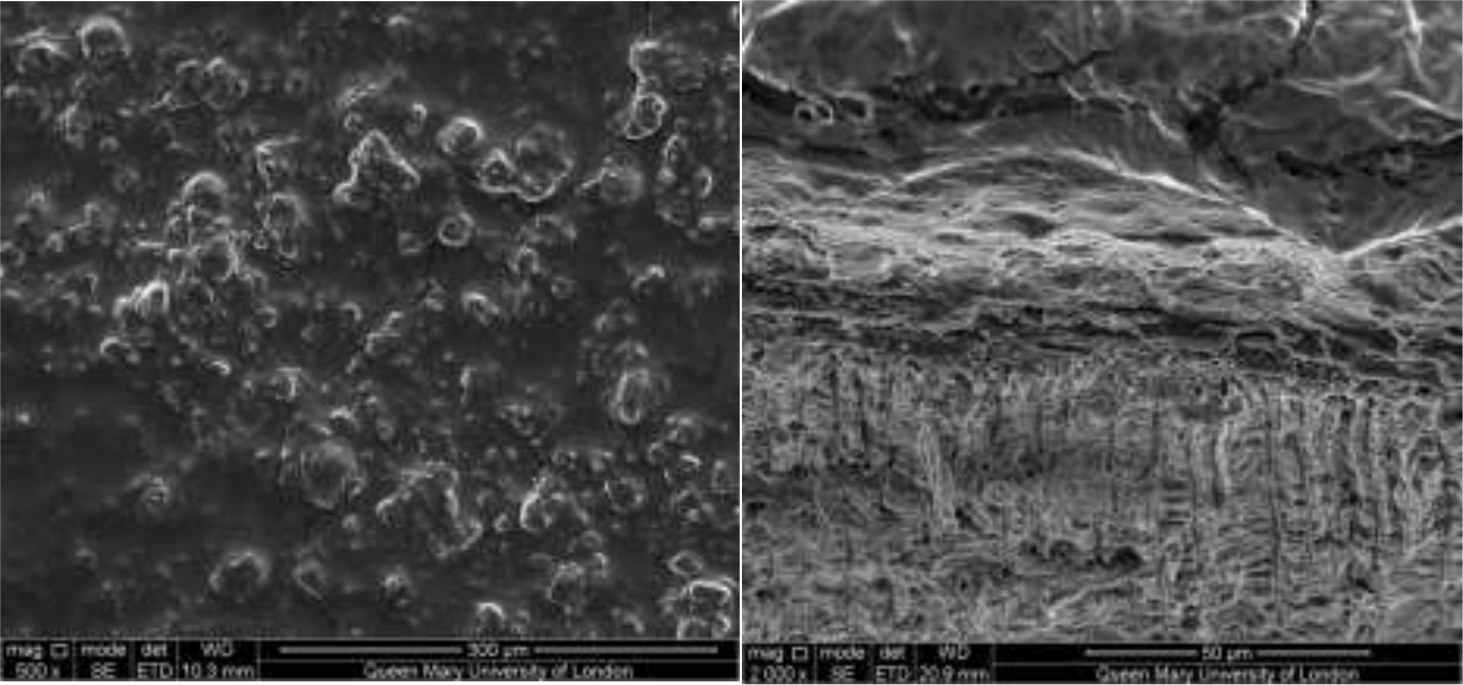
Figures 2–3: Showing the hydrogel film of the SAPM gel covering the dentine surface: Figure 2 shows the top down view whilst Figure 3 shows the cross-section. The presence of large clusters was also observed which may be result of the deposition of dicalcium phosphate particles from the formulation.
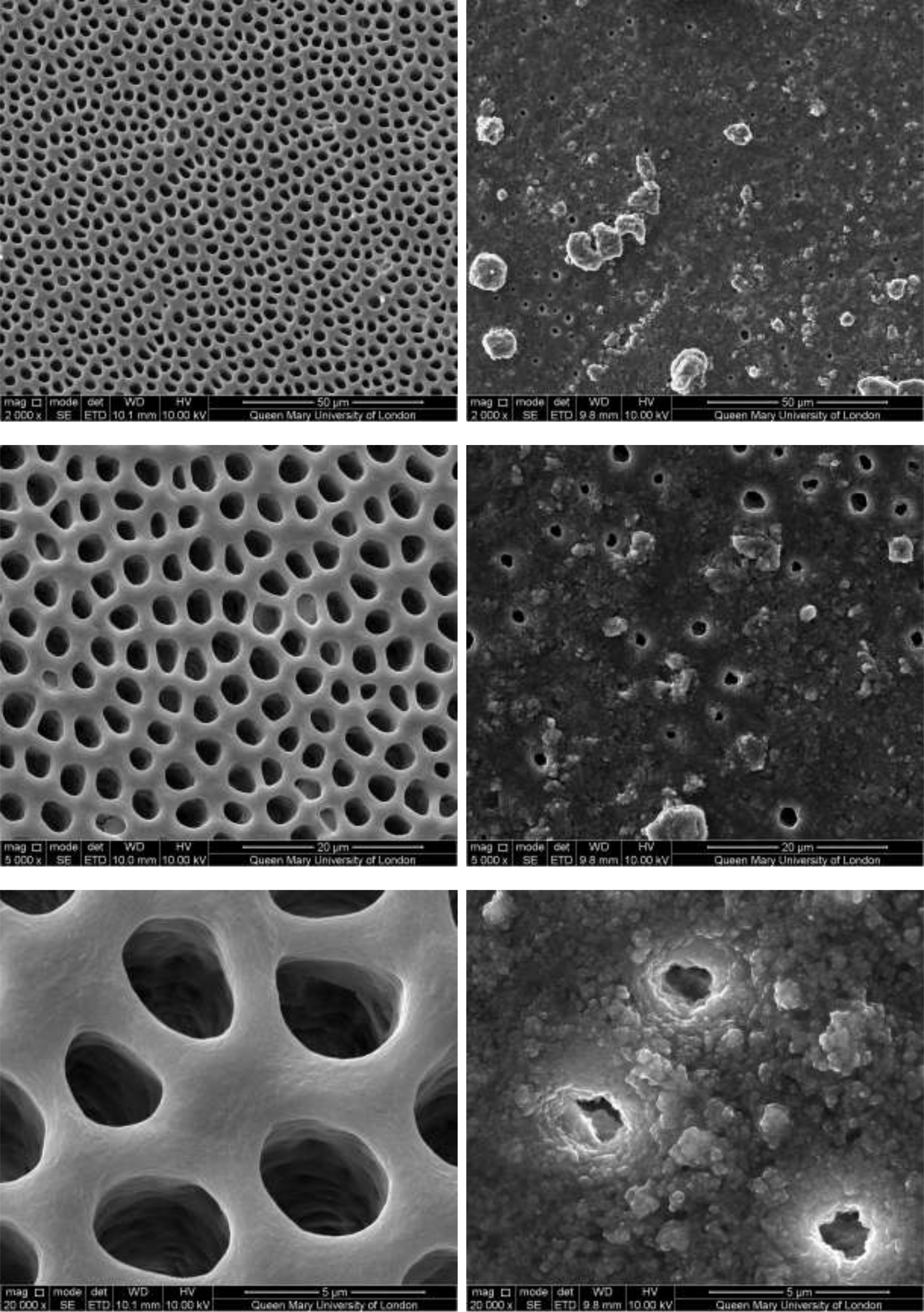
Figure 4: SEM images of dentine tubules treated with self-assembling peptide matrix (SAPM) before treatment (left) and after treatment (right).
CSP treated dentine discs showed levels of dentine tubule occlusion and a different morphology of the elongated particle, which has a tendency of forming clusters was observed (Figure 5). The dentine discs treated with SRP (Figure 6) provided levels of occlusion, however, a large area of open dentine tubules was observed. A small amount of fine particles (submicron level) was also observed on the dentine surface. Large clusters of particles also occluded some of the tubules. In contrast, the specimens treated with SRR formulation (Figure 7) provided better coverage compared with the SRP formulation (Figure 6). It was clear that there was precipitation of particles within the dentinal tubules as well as on the dentine surface.
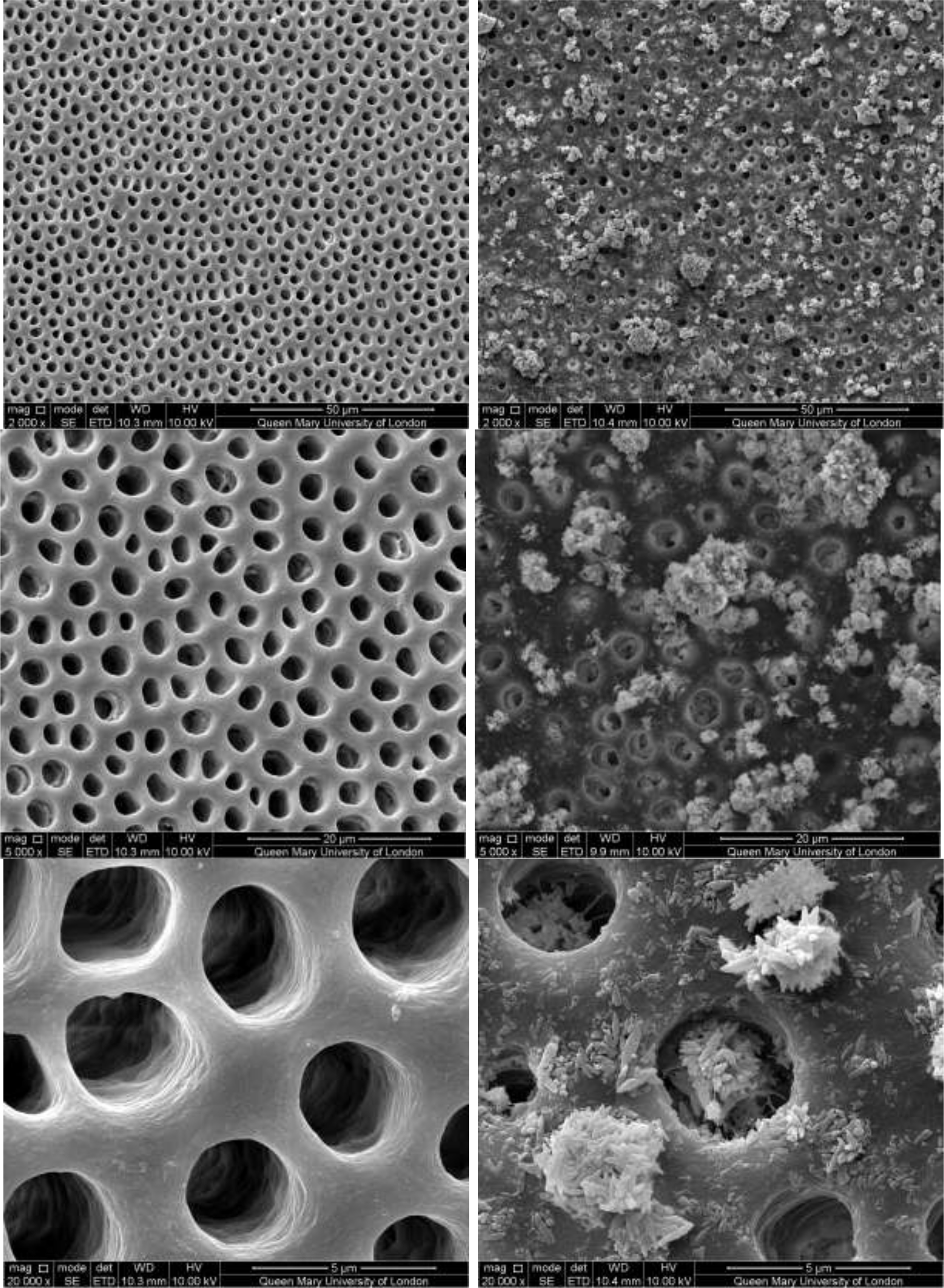
Figure 5: SEM images of dentine tubules treated with Colgate Sensitive ProRelief Toothpaste, before treatment (left) and after treatment (right).
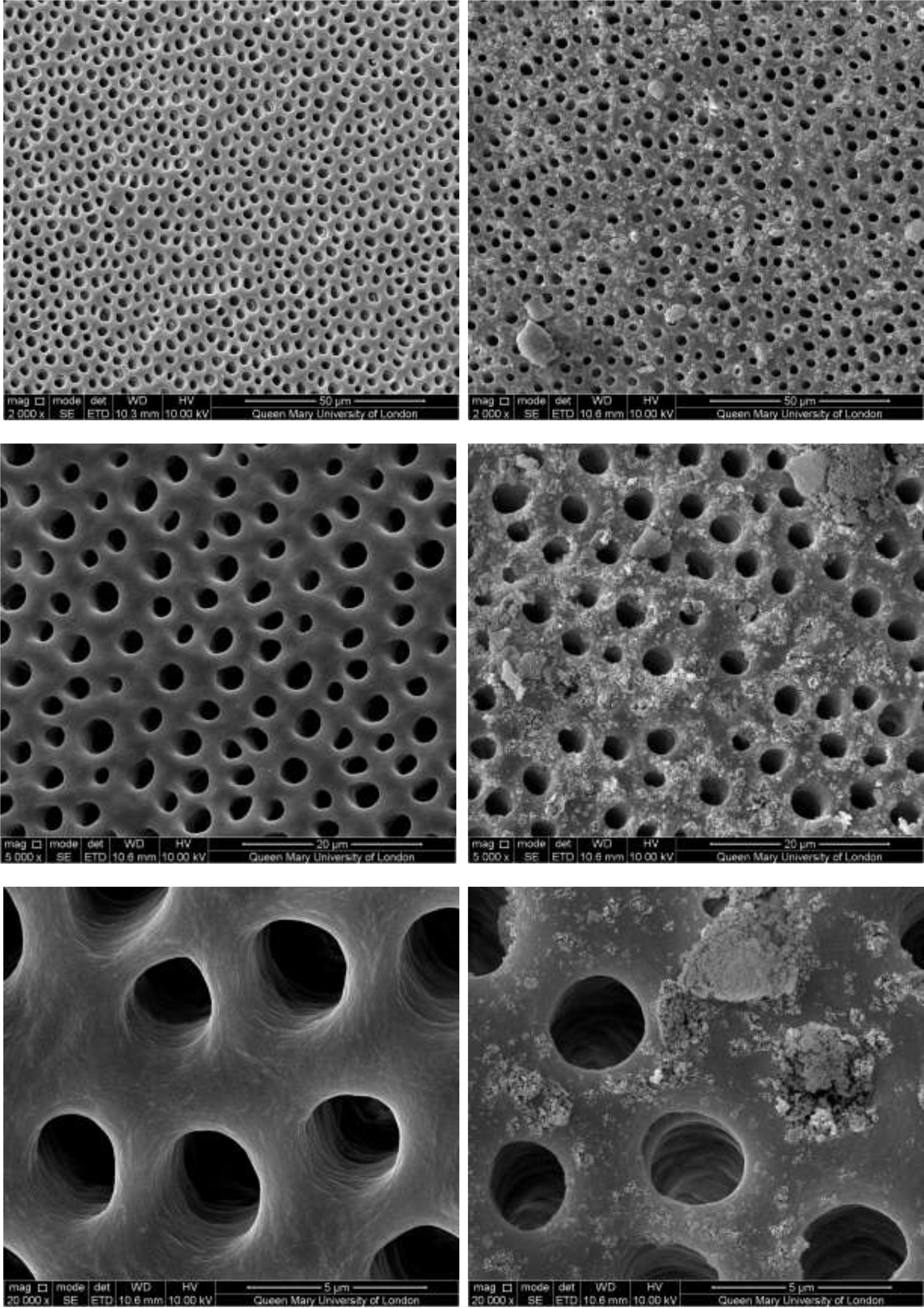
Figure 6: SEM images of dentine tubules treated with Sensodyne Repair and Protect Toothpaste, before treatment (left) and after treatment (right).
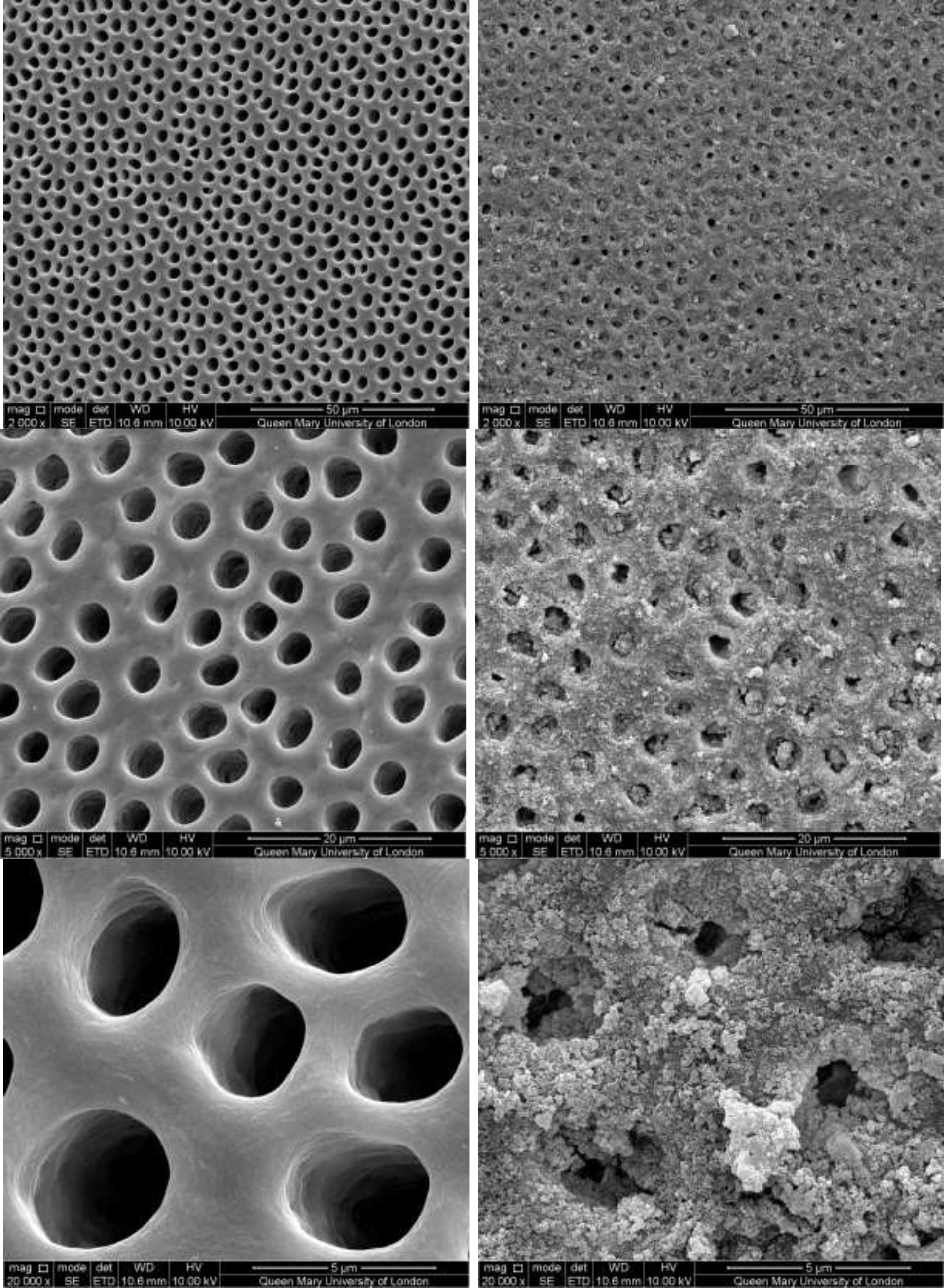
Figure 7: SEM images of dentine tubules treated with Sensodyne Rapid Relief Toothpaste, before treatment (left) and after treatment (right).
Figure 8 demonstrated the variation of the fluid flow reduction (FFR) of the five discs used following the application of SAPM Gel (39.4%-65.7%). The mean FFR for the SAPM Gel was 55.1% which compared favourably to the other desensitizing products (Table 2). The percentage of fluid flow reduction by the SAPM Gel was statistically higher than for CSP, however no significantly differences were observed between the SAPM Gel and both SRP and SRR (paired t-test).
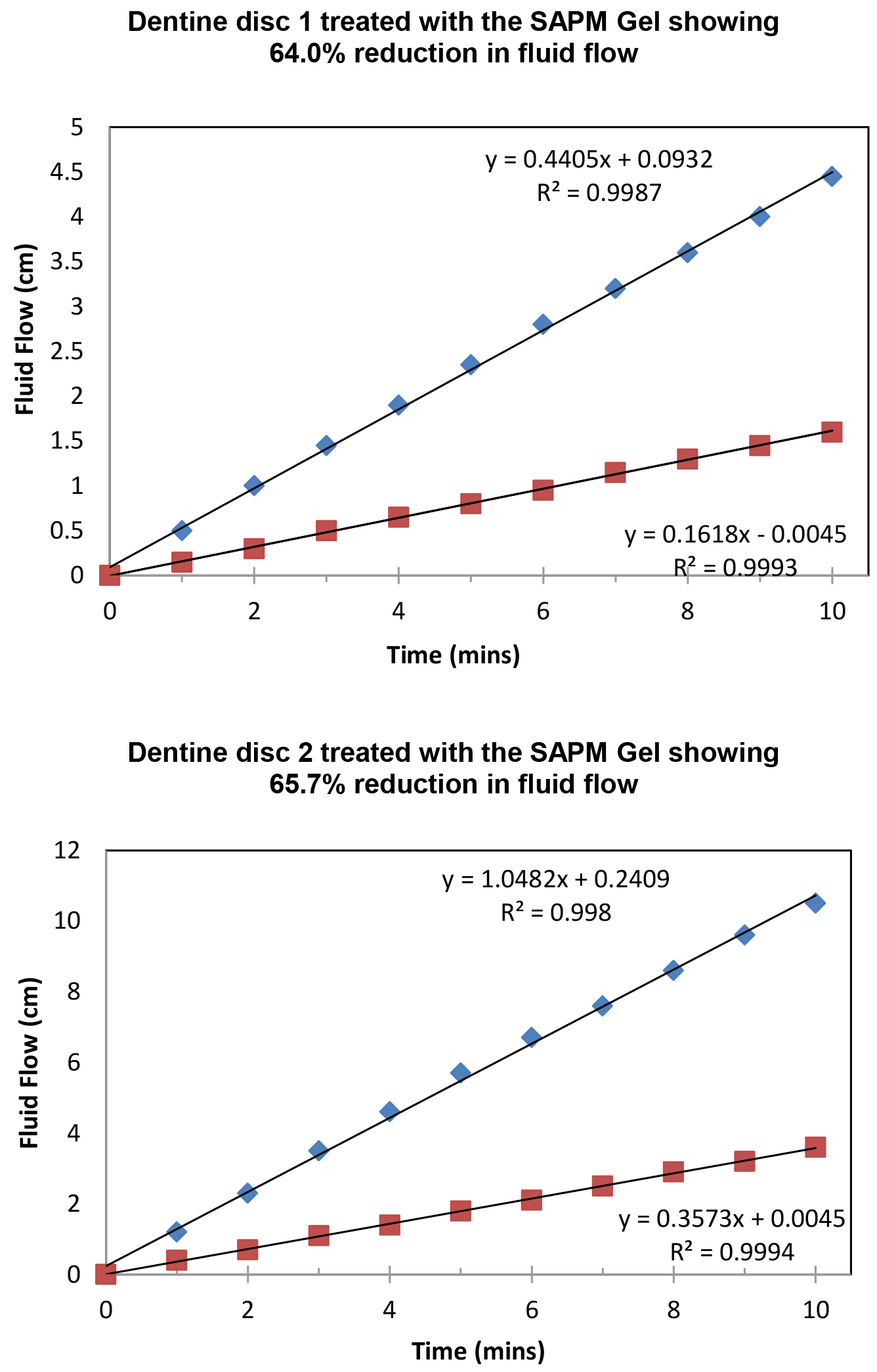
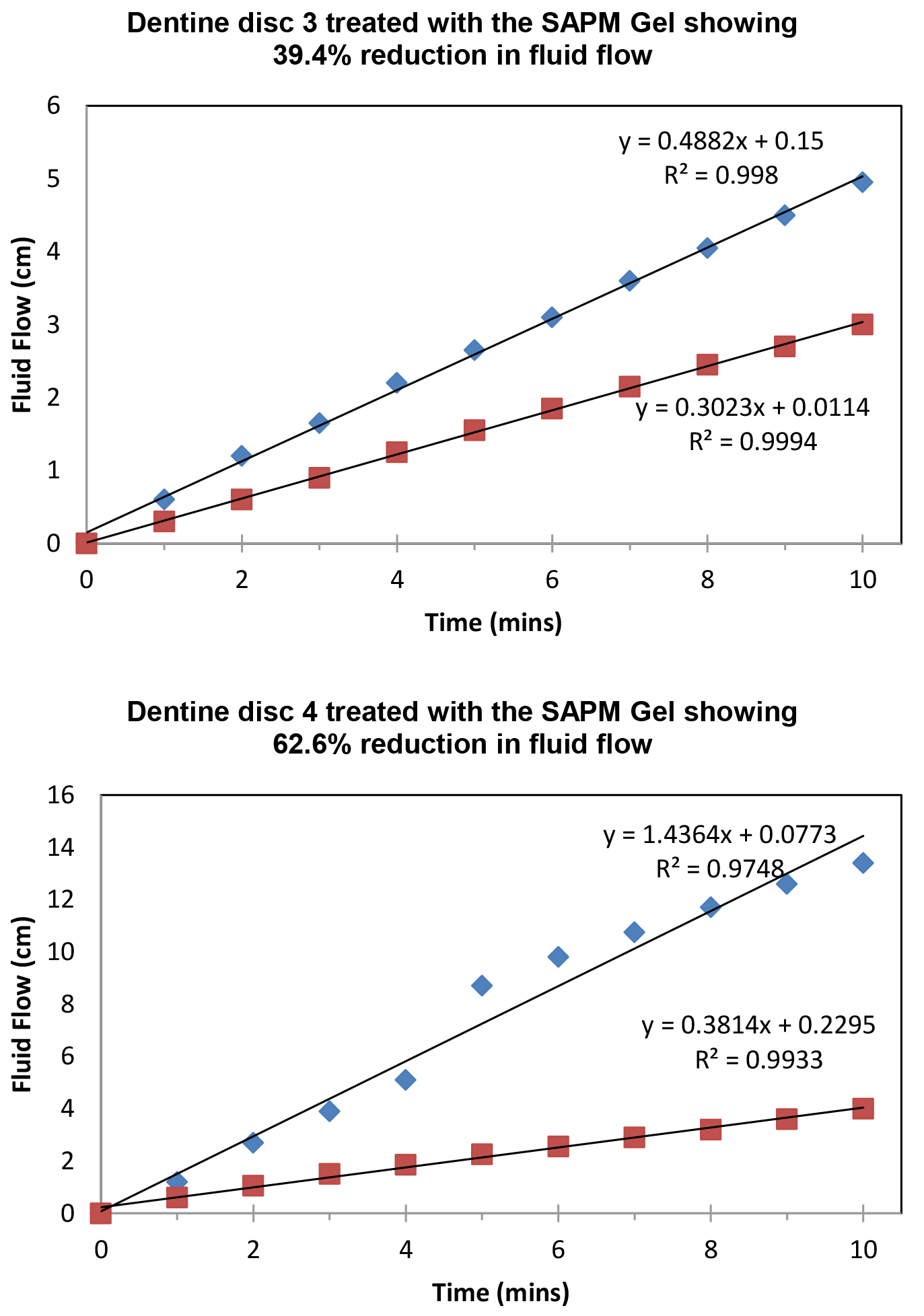
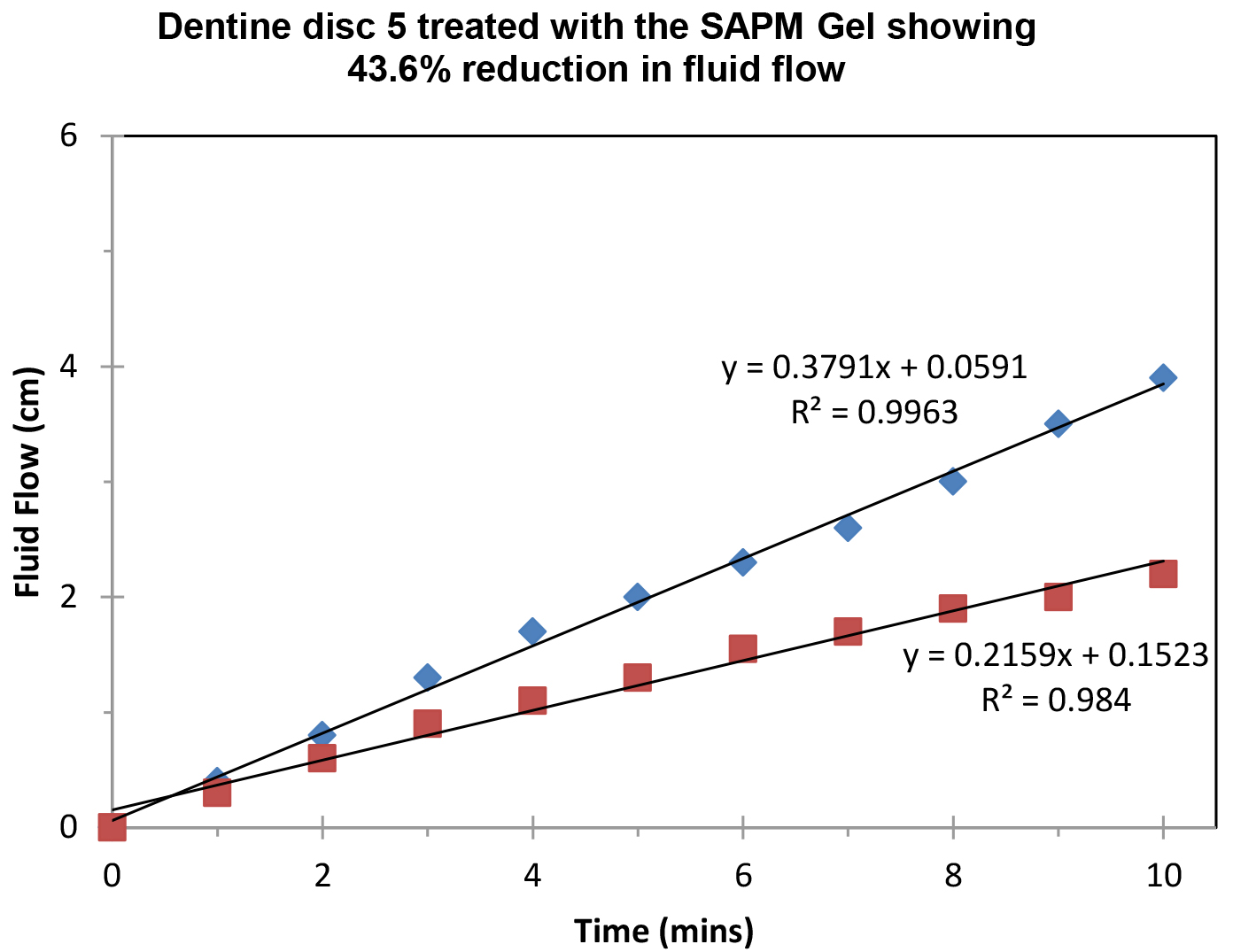
Figure 8: Plot showing the fluid low changes of the dentine discs after 2 min brushing with the SAPM Gels (discs 1-5), where blue diamond represents etched control, and red square for after treatment (Fluid Flow Reduction [FFR] range: 39.4%-65.7%).
Table 2. Fluid Flow Reduction for the tested toothpastes (n=5).
|
Toothpaste |
Mean FFR (%) |
SD (%) |
|
SAPM Gel |
55.1a |
12.5 |
|
CSP |
27.6b |
6.8 |
|
SRP |
64.9ac |
18.5 |
|
SRR |
39.1ab |
17.1 |
Discussion
According to Hill and Gillam [1] despite the vast array of commercial products designed either as professionally applied products or techniques (Dentist applied) or as over-the counter products (OTC) (Home use) there is no universally accepted product that can completely resolve DH. The desensitising technologies evaluated in the current in vitro study included a novel self-assembling peptide matrix (SAPM) gel and three selected desensitizing toothpastes with established tubular occluding properties namely 1) a technology consisting of arginine, a naturally occurring amino acid, and an insoluble calcium compound, in the form of calcium carbonate (CSP), 2) a 45S5 bioactive glass formulation (SRP) and 3) a strontium acetate formulation (SRR). The use of SAP P11–4 as a non-invasive regenerative treatment for early stage dental caries has been documented [3–12]. Recent reports on favourable remineralisation after SAPM application have been published [18–19]. A randomised clinical trial investigating an SAPM Gel (as investigated in this report) demonstrated fast relief of DH compared to CSP [15]. An in vitro study by João-Souza et al. [20] also compared the desensitizing effects of selected toothpastes including a diluted SAPM formulation under erosive conditions. The other three test products of the present study (CSP, SRP, and SRR) have also been documented as effective desensitizing toothpastes based on both in vitro and in vivo evidence [3–4, 21–27].
The in vitro evaluation of desensitizing products was undertaken using the established methodology described by Greenhill & Pashley [16] and Mordan et al. [17], although one of the limitations using dentine discs from different teeth is the variability between and within the discs particularly when investigating hydraulic conductance (see variability with the fluid low within the five discs). This is due in part to the variation of the regional flow which may be affected by the proximity of the dentine disk to the pulp [27–29]. Other limitations of using this particular model relate to 1) the effect of the storage medium and the etching agents such as a sodium hypochlorite solution and citric acid which may impact and modify the dentine surface as well as the collagen; 2) the unknown age and history of the teeth and 3) the thickness, location and orientation of the dentinal tubules which may have an impact on any of the results from studies of this nature [30–34]. It should be noted, however that one of the original objectives of using the dentine disc was to identify the mode of action of the desensitizing agents as well as their potential as a therapeutic formulated product to treat DH [17].
All the treated discs in the present study resulted in a reduction in the number and the size of open dentine tubules on the surface of the dentine disc. This would suggest that there was a degree of effective tubular occlusion within all groups. The particles that occluded the open dentine tubules, however had different morphologies and distribution across the disc surface. The SAPM gel demonstrated the highest tubuli occlusion compared to the other groups, based on the SEM analysis. The SEMs of the treated discs suggested that the tubule occlusion originated by the placement of a hydrogel film of SAPM on the tooth surface. Yet, it is important to supplement the evidence from the SEM data by relating it to the hydraulic conductance measurements, as this new type of surface occlusion might result in a different degree of fluid flow inhibition as seen by insoluble particles from the conventional desensitisation groups. It is also important to recognise that the occlusion by the SAPM gel, does not involve any chemical or physical reaction, whereas all the other products rely on a precipitation reaction within the dentinal tubuli. For example, according to several investigators, arginine in the CSP formulation has been postulated to form a calcium arginine complex with calcium carbonate on the tooth surface and within the dentine tubules [1, 4]. The chemistry of this process however is poorly understood and has not been characterized in any detail [1]. The 45S5 bioactive glass composition in the SRP formulation was originally designed as a bone substitute and not as an additive in toothpastes for treating DH. It has been postulated that the glass particles dissolve in the mouth releasing Ca2+ and PO43- ions forming a hydroxycarbonated apatite (HCA) on the tooth surface and as such may not be acid resistant for permanent tubuli occlusion [1]. The main mode of action of strontium acetate (and strontium chloride) (SRR) for treatment of DH, has been suggested to be by tubular occlusion although the actual effect attributed to strontium in clinical studies has yet to be defined [35, 36]. Current formulations of SRR have replaced Strontium Acetate with Stannous Fluoride.
Most of the toothpastes evaluated in the present in vitro study exhibited very good tubule occlusion and fluid flow inhibition following tooth brushing, the exception being SRP that was less effective in occluding the dentinal tubules (Figs 4–8, Table 2). One possible reason for this observation was that in the mouth this toothpaste reacts with saliva to form hydroxyapatite and as such may perform more effectively in the clinical environment rather than in the in vitro setting, as has been shown in clinical trials [23, 25].The reductions in the dentine hydraulic conductance measurements were observed for all tested materials (mean [SD, %]: SAPM 55.1 [12.5], SRP 64.9 [18.5], SRR 39.1 [17.1] and CSP 27.6 [6.8]). No statistically differences were observed between the SAPM and SRP, SRR toothpastes (paired t-Test; ≤0.05) – possibly due to the small sample size in this explorative study – although a significant difference was noted between the SAPM and the CSP toothpastes. There was an overall trend for reduction for the SAPM compared to the SRR toothpaste.
Although the SAPM gel was effective in the present study compared to the other tested products there is currently only one report of a randomised clinical trial comparing its clinical efficacy to that of the CSP product [15]. An additional in vitro study has evaluated a SAPM containing product in a daily erosion toothpaste (with a much lower concentration of SAPM) [20], where the investigators reported that none of the desensitizing products showed any significant effects on tubular occlusion under the erosive conditions used. There are, however, significant differences in the methodology used in this study [20] compared to the current study. For example, the specimens were submitted to a 5-day erosion-abrasion cycling model and the fluid flow reductions were not shown as a time dependent graph, but rather as a value (with uncommonly large error bars). Furthermore, the toothpaste used in the João-Souza et al. study [20] included SAP P11–4 at a significantly lower level (1/30) than the product investigated in the current study. It would therefore be of interest to compare these two SAPM containing products in a future study to determine their individual effects on tubular occlusion and identify the optimal concentration and application frequency for SAPM.
Conclusion
The results from the current explorative in vitro study would suggest that a novel self-assembling peptide matrix gel was effective in occluding the dentine tubules and may therefore have the potential to be an effective desensitizing product for the treatment of dentine hypersensitivity.
Acknowledgement
The research was funded by Credentis AG, Switzerland. The work of Hawshan Abdulrahman Mustafa was supported by the Kurdistan Regional Government- Human Capacity Development Program (KRG-HCDP).
References
- Hill RG & Gillam DG: Future strategies for the development of desensitising products. In: Dentine Hypersensitivity: Advances in Diagnosis, Management, and Treatment. Chapter 11, David G. Gillam (ed). DOI 10.1007/978-3-319-14577-8, © Springer International Publishing Switzerland. Pg No: 157–179.
- Brännström M (1963) A hydrodynamic mechanism in the transmission of pain-produced stimuli through the dentine. In: Anderson D.J. (ed) Sensory mechanisms in dentine. Pergamon, Oxford, Pg No: 73–79,
- Cummins D (2009) Dentin hypersensitivity: from diagnosis to a breakthrough therapy for everyday sensitivity relief. J Clin Dent 20: 1–9. [Crossref]
- Cummins D (2010) Recent advances in dentin hypersensitivity: Clinically proven treatments for instant and lasting sensitivity relief. Am J Dent 23: 3A-13A. [Crossref]
- Gillam DG (2014) Chapter 5 Treatment Approaches for Dentin Hypersensitivity. In S. Taha, B.H. Clarkson (eds.), Clinician’s Guide to the Diagnosis and Management of Tooth Sensitivity, DOI 10.1007/978-3-642-45164-5_5, © Springer-Verlag Berlin Heidelberg, Pg No: 51–79.
- Kirkham J, Firth A, Vernals D, Boden N, Robinson C, Shore RC, et al. (2007) Self-assembling peptide scaffolds promote enamel remineralisation. J Dent Res 86: 426–430. [Crossref]
- Brunton PA, Davies RPW, Burke JL, Smith A, Aggeli A (2013) Treatment of early caries lesions using biomimetic self-assembling peptides – a clinical safety trial. Brit Dent J 215: 1–6. [Crossref]
- Kind L, Stevanovic S, Wuttig S, Wimberger S, Hofer J (2017) Biomimetic Remineralization of Carious Lesions by Self-Assembling Peptide. J Dent Res 96: 790–797. [Crossref]
- Alkilzy M, Tarabaih A, Santamaria RM, Splieth CH (2018) Self-assembling Peptide P11–4 and Fluoride for Regenerating Enamel. J Dent Res 97: 148–154. [Crossref]
- Chen X, Gillam DG, Lysek DA, Hill RG (2014) In Vitro Evaluation of Dentine Remineralisation by a Self-Assembling Peptide Using Scanning Electron Microscopy. 61th ORCA Congress July 2–5, Greifswald, Germany, Abstract no. 40.
- Schlee M, Schad T, Koch JH, Cattin PC, Rathe F (2018) Clinical performance of self-assembling peptide P11–4 in the treatment of initial proximal carious lesions: A practice-based case series. J Invest Clin Dent.
- Bröseler F, Tietmann C, Bommer C, Drechsel T, Heinzel-Gutenbrunner M, Jepsen S (2019) Randomised clinical trial investigating self-assembling peptide P11–4 in the treatment of early caries. Clin Oral Investig 2019 Apr 29. [Epub ahead of print].
- Aggeli A, Bell M, Boden N, Carrick LM, Strong AE (2003) Self-assembling peptide polyelectrolyte beta-sheet complexes form nematic hydrogels. Angew Chem Int Ed Engl 42: 5603–5606. [Crossref]
- Saha S, Yang XB Wijayathunga, N Harris, S Feichtinger, GA Davies, et al. (2019) A biomimetic self-assembling peptide promotes bone regeneration in vivo: A rat cranial defect study. Bone 127: 602–611.
- Schlee M, Rathe F, Bommer C, Bröseler F, et al. (2018) Self-assembling peptide matrix for treatment of dentin hypersensitivity: A randomized controlled clinical trial. J Periodontol 89: 653–660. [Crossref]
- Greenhill JD, Pashley DH (1981) The effects of desensitizing agents on the hydraulic conductance of human dentin in vitro. J Dent Res 60: 686–698. [Crossref]
- Mordan NJ, Barber PM, Gillam DG (1997) The dentine disc. A review of its applicability as a model for the in vitro testing of dentine hypersensitivity. J Oral Rehabil 24: 148–156. [Crossref]
- Soares R, de Ataide IDN, Fernandes M, Lambor R (2017) Assessment of Enamel Remineralisation with different Remineralising Agents. Journal of Clinical and Diagnostic Research 11: ZC136-ZC141.
- Jablonski-Momeni A, Korbmacher-Steiner H, Heinzel-Gutenbrunner M, Jablonski B, Jaquet W, et al. (2019) Randomised in situ clinical trial investigating self-assembling peptide matrix P11–4 in the prevention of artificial caries lesions. Sci Rep 9: 269. [Crossref]
- João-Souzaa SH, Scaramuccia T, Borges AB, Lussic A, Carvalhoc TS, Aranhaa ACC (2019) Influence of desensitizing and anti-erosive toothpastes on dentine permeability: An in vitro study. J Dent (in press) 89: 103176. [Crossref]
- Sharif M, Iram S, Brunton P (2013) Effectiveness of arginine containing toothpastes in treating dentine hypersensitivity: A systematic Review. J Dent 41: 483–492. [Crossref]
- Yan B, Yi J, Li Y, Chen Y, Shi Z (2013) Arginine-containing toothpastes for dentin hypersensitivity: systematic review and meta-analysis. Quintessence International 44: 709–723. [Crossref]
- Ramamoorthi S, Nivedhitha MS (2013) Effectiveness of Bioactive glass Containing Dentifrice on Dentin Hypersensitivity – A Systematic Review. JPR:BioMedRx: An International Journal 1: 803–809.
- Karim BFA, Gillam DG (2013) The Efficacy of Strontium and Potassium Toothpastes in Treating Dentine Hypersensitivity: A Systematic Review. Int J Dent.
- Talioti E, Hill R, Gillam DG (2014) The Efficacy of Selected Desensitizing OTC Products: A Systematic Review. ISRN Dent 2014: 865761. [Crossref]
- Gillam DG (2014) Treatment Approaches for Dentin Hypersensitivity. In Chapter 5 Clinician’s Guide to the Diagnosis and Management of Tooth Sensitivity S. Taha, B.H. Clarkson (eds.), DOI 10.1007/978-3-642-45164-5_5, © Springer-Verlag Berlin Heidelberg, Pg No: 51–79.
- Pashley D, Andringa HJ, Derkson GD, Derkson ME, Kalathoor SR (1987) Regional variability in the permeability of human dentine. Archs oral Biol 32: 519–523. [Crossref]
- Absi EG, Addy M, Adams D (1995) Dentine hypersensitivity: uptake of toothpastes onto dentine and effects of brushing, washing and dietary acid – SEM in vitro study. J Oral Rehab 22: 175–182. [Crossref]
- Ghazali FB (2003) Permeability of dentine. Malays J Med Sci 10: 27–36. [Crossref]
- Outhwaite WC, Livingston MJ, Pashley DH (1976) Effect of changes in surface area, thickness, temperature and post extraction time on dentine permeability. Archs oral Biol 21: 599–603. [Crossref]
- Secilmis A, Dilber E, Gokmen F, Ozturk N, Telatar T (2011) Effects of storage solutions on mineral contents of dentin. J Dent Sciences.
- Mjör IA, Nordahl I (1996) The density and branching of dentinal tubules in human teeth. Arch Oral Biol 41: 401–412. [Crossref]
- Komabayashi T, Nonomura G, Watanabe LG, Marshall GW Jr, Marshall SJ (2008) Dentin tubule numerical density variations below the CEJ. J Dent 36: 953–958. [Crossref]
- Phrukkanon S, Burrows MF, Tyas MJ (1999) The effect of dentine location and tubule orientation on the bond strengths between resin and dentine. J Dent 27: 265–274. [Crossref]
- Rösing CK, Fiorini T, Liberman DN, Cavagni J (2009) Dentine hypersensitivity: analysis of self-care products. Braz Oral Res 1: 56–63. [Crossref]
- Hu ML, Zheng G, Zhang YD, Yan X, Li XL, et al. (2018) Effect of desensitizing toothpastes on dentine hypersensitivity: A systematic review and meta-analysis. J Dent 75: 12–21. [Crossref]