Abstract
Modifications of pro-inflammatory processes and neurotransmitter changes underpin the cognitive and neuro-behavioural consequences of traumatic brain injury (TBI). Amantadine has the potential to promote dopaminergic activity via multiple mechanisms involving facilitation of synaptic dopamine (DA) release, blockade of presynaptic DA re-uptake and increased DA synthesis via stimulation of Dopa Decarboxylase. Amantadine is also a non-competitive antagonist of glutamatergic (NMDA) receptors. Evidence from randomized controlled trials (RCTs) together with systematic reviews suggest that treatment with amantadine [100-300mg/d] is effective for improvements in level of consciousness and cognitive function in both acute and chronic care phases for up to 6 months post-TBI resulting, for example, in functional recovery in patients with TBI-related MCS or VS/UWS over 4 weeks of treatment. The majority of good-quality RCT’s also provide evidence for efficacy of amantadine in the treatment of the major neuro-behavioural sequelae of TBI such as agitation, irritability and aggression. These findings have resulted in updates of clinical practice guidelines for disorders of consciousness including those of the American Academy of Neurology which recommends that amantadine (100-200mg bid) be prescribed for adults with traumatic VS/UWS or MCS [4-16 weeks post-injury] to hasten recovery and reduce disability early in recovery [Level B].
Keywords
Amantadine, Disorders of consciousness, Neuro-behavioural outcomes, Practice guidelines, Traumatic brain injury
Background
Traumatic brain injury (TBI) has wide-ranging consequences for survivors’ quality of life. Disabilities include decreased level of consciousness (LoC) as well as cognitive, neuropsychiatric (anxiety, depression) and neurobehavioral sequelae the latter often taking the form of irritability, hyperexcitability, disinhibition, poor impulse control, agitation and aggression. Amantadine has the potential to increase the concentrations of dopamine (DA) in the brain and the agent is one of the most commonly prescribed medications for the management and treatment of patients with disorders of consciousness undergoing neurorehabilitation following TBI. The current review was initiated in order to (A) clarify current opinion relating to the mechanism of action of amantadine as an agent for the treatment of TBI and its associated CNS disorders and (B) to critically review the evidence in support of the efficacy of amantadine for the treatment of TBI and its associated cognitive and neuro-behavioural complications. Findings from individual published randomized controlled trials (RCTs) as well as related systematic reviews and meta-analyses are compiled and compared and some implications of the findings for the updating of practice guidelines are reviewed.
Mechanisms of action of amantadine in TBI
TBI and its attending alterations of central functional and chemical imbalances lead to region-selective modifications of pro-inflammatory processes and neurotransmitter changes that underpin the cognitive and neuro-behavioural consequences of the injury. The acute phase of recovery from severe TBI is characterized by a brief period of hyperexcitability followed by a longer period of hypo-excitability resulting from the depletion of multiple neurotransmitters one of which is dopamine (DA). [1] Amantadine has the capacity to promote dopaminergic activity via multiple mechanisms including the facilitation of the synaptic release of DA together with the blockade of DA re-uptake. Furthermore, amantadine has the capacity to stimulate the enzyme L-Dopa decarboxylase (DDC) resulting in increased DA synthesis, a process that is functionally-related to the antagonism of NMDA receptors. Stimulation of DDC activity secondary to NMDA receptor antagonism has been demonstrated in humans by the technique of Positron Emission Tomography (PET). [2] Moreover, PET studies in TBI patients lend credence to the notion that amantadine has the potential to improve CNS function via actions on the dopaminergic system that include significant improvements in prefrontal energy metabolism and function indicated by increased F18-deoxyglucose-PET with concomitant increases in dopamine-D2 receptor availability [3].
Evidence-based review of the efficacy of amantadine for the treatment of TBI and its associated loss of consciousness and cognitive dysfunction
Evidence from systematic reviews and meta-analyses
Amantadine continues to find widespread use in TBI as a means of increasing the speed and efficacy of cognitive recovery and rehabilitation. Results of systematic reviews of clinical trials have helped to fuel the debate on the comparative efficacy and safety of amantadine. Such reports include the following:
A report published in 2009 described the results of a review of the impact of pharmacological agents on cognitive outcomes in early stages post-TBI based upon reports published between January 1980 and May 2008 following searches of PubMed and PsycINFO databases using appropriate keywords and inclusion criteria. Amantadine treatment produced marked benefits by assessment of Glasgow Coma Scale (GCS); drug dosage and choice of outcome measures appeared to influence the probability of treatment benefit [4].
A study from The University of Toronto reviewed evidence of efficacy of pharmacological interventions for TBI based upon available published literature. Multiple studies found that amantadine (100-300mg/d) was effective in both the acute and chronic care phase post-TBI particularly for cognitivedifficulties and for improvement in level of consciousness as measured by GCS [5].
A focussed report described the results of a systematic review of the efficacy of medications for cognitive disorders post-TBI. Articles were searched via the Medline database from 1990 to 2012 along PRISMA guidelines. 89 references were analysed for a total of 1306 cases of TBI, 295 of which were treated with amantadine (50-400mg/d) leading to improvements in the level of vigilance, orientation, attention, processing speed and motor learning. Results of the review resulted in recommendations for good practise under the auspices of The French High Authority for Health [HAS] in collaboration with The French Society for Physical and Rehabilitation Medicine [SOFMER] [6].
In a review aimed at determining the efficacy of amantadine for improvement of cognitive function post-TBI, PubMed and CINAH databases were searched for articles published in the 1994-2004 period included a Cochrane review, a meta-analysis and several RCTs. Key points and recommendations included the effective use of amantadine leading to increased arousal and cognition compared to placebo leading to the conclusion that amantadine therapy(100mg/d) may be beneficial from 3 days to 6 months post-TBI [7].
A comprehensive review of the literature relating to the diagnosis, natural history, prognosis and treatment of disorders of consciousness (DoC) lasting more than 28 days was conducted with a view to updating American Academy of Neurology (AAN) practice guidelines [8]. The natural history of recovery from prolonged vegetative state/unresponsive wakefulness syndrome (VS/UWS) was found to be better in traumatic compared to non-traumatic cases and prognosis followed a similar pattern. Amantadine hastened functional recovery in patients with minimally conscious state (MCS) or VS/UWS secondary to severe TBI over 4 weeks of treatment. These findings led to an update of the practice guidelines for the treatment of patients with prolonged DoCs. It is recommended that clinicians prescribe amantadine (100-200mg bid) for adults with traumatic VS/UWS or MCS (4-16 weeks post-injury) to hasten functional recovery and reduce disability early in recovery (level B evidence) [9].
Evidence from the individual RCTs
An international RCT was undertaken in 184 patients who were in a VS or MCS 4 to 16 weeks following severe TBI. Patients received amantadine or placebo for 4 weeks followed by a 2-week washout period post treatment. The rate of functional recovery was assessed using the Disability Rating Scale (DRS). During the 4-week treatment period, recovery was significantly faster in the amantadine group compared to placebo. Post-hoc analysis of the distribution of DRS scores by outcome category revealed that more patients in the amantadine group had favourable outcomes on DRS compared to placebo with fewer remaining in a VS and a greater percentage manifesting recovery of key behavioural indices on the Coma Recovery Scale-Revised (CRS-R) at the end of the 4-week treatment period. It was concluded that amantadine is effective in accelerating the pace of recovery during acute rehabilitation in patients with prolonged post-TBI DoC [10], (Figures 1 A, B).
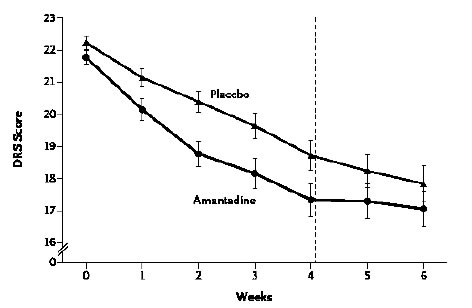
Figure 1a. Rate of functional recovery (DRS score) as a function of duration of treatment with amantadine compared to placebo in patients with severe TBI. DRS scores were improved significantly more rapidly following amantadine during the 4-week treatment period compared to placebo. On weeks 5 and 6 (washout interval), recovery rate in the amantadine group were significantly slower. Error bars indicate mean values ± SE.
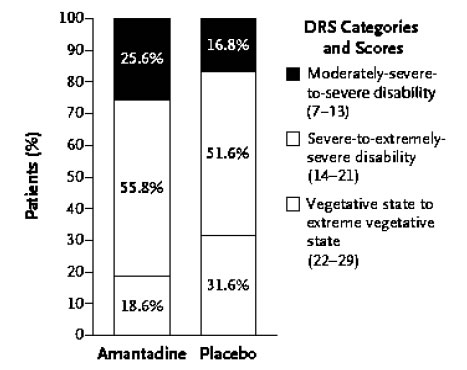
Figure 1b. Effects of amantadine treatment compared to placebo on the distribution of scores on DRS as a function of the category of functional disability (DRS score). After 4 weeks of treatment, the proportion of patients in a vegetative-to-extreme vegetative state was significantly lower in the amantadine group by post-hoc analysis.
In order to evaluate the efficacy of amantadine sulphate for improvement of outcome, 90 patients with moderate to severe TBI were randomly assigned to one of two groups (n=45each). Group A received standard ICU protocol; Group B received additionally amantadine sulphate infusions (200mg/12h for 14 days). LoC was assessed by GCS 1, 2 and 4weeks post-injury with patient outcome assessed after 4 weeks by Glasgow Outcome Scale (GOS) Patients in Group A (amantadine) showed better improvement in GCS compared to group B (p<0.005) together with better outcome at the end of week 4 by GOS [11].
To evaluate the effects of amantadine on cognition in individuals with a history of TBI, a multi-site, parallel-group RCT of amantadine (100mg/d, twice daily for 60d) was made in 119 individuals with chronic TBI (> 6months post-injury). Cognitive function was measured on treatment days 0, 28 and 60 using a battery of psychological tests. Composite indices were generated for General Cognitive, Learning Memory and Attention/Processing Speed Indices. Repeated measures ANOVA revealed statistically-significant between-group differences favouring placebo for General Cognitive (p<0.002) and Learning Memory (p<0.001) Indices at day 28. Consequently, in contrast to the general consensus of opinion expressed in the studies described above, the use of amantadine for enhancement of cognitive function in chronic TBI was not supported by the findings of this trial [12].
Evidence-based review of the efficacy of amantadine for the treatment of TBI and associated neurobehavioral disorders
Systematic Reviews
A Canadian study reviewed evidence of efficacy of pharmacological interventions for TBI based upon published literature. Multiple studies found that amantadine (100-300mg/d) was effective in both the acute and chronic care phase post-TBI for the treatment of neuro-behavioural sequelae (agitation, anxiety) [5].
A Systematic review of RCTs aimed at determining the efficacy of dopaminergic agents on apathy, psychomotor retardation and behavioural management post-brain injury made use of searches of Medline, EMBASE, PsychInfo and Cochrane Clinical Trials databases. Six trials and 150 patients met inclusion criteria. Results suggested benefit for treatment ofagitation and aggression, but trial quality was compromised by faulty design, small numbers and heterogeneous outcome measures. One good quality trial demonstrated efficacy of amantadine for behavioural management [13].
A focussed report described the results of a systematic review of medications for behavioural disorders after TBI. Articles were searched via the Medline database from 1990 to 2012 along PRISMA guidelines. Eighty-nine references were analysed for a total of 1306 cases of TBI, 295 of which were treated with amantadine (50-400mg/d) leading to improvements in the level of vigilance, orientation, attention, processing speed and motor learning but insufficient evidence for the treatment of agitation, aggressiveness or anxiety. A note added in proof subsequently withdrew this latter statement. Results of this systematic review resulting in recommendations for good practise under the auspices of The French High Authority for Health [HAS] in collaboration with The French Society for Physical and Rehabilitation Medicine [SOFMER] [6].
The aim of a subsequent systematic review to critically evaluate evidence on the efficacy of pharmacological interventions for the treatment of aggression (primary outcome) following TBI in adults making use of databases from Medline, PubMed, CINSHL, EMBASE, PsychInfo and Central with use of the Cochrane Risk of Bias Tool. Ten studies were included, 5 of which were RCTs 2 of which reported evidence of efficacy of amantadine for the treatment of irritability with a further two positives for treatment of aggression [14].
Individual RCTs
An international RCT was undertaken in 184 patients who were in a VS or MCS 4 to 16 weeks after severe TBI.Patients received amantadine or placebo for 4 weeks followed by a 2-week washout period post treatment. The rate of functional recovery was assessed using the Disability Rating Scale (DRS). Clinically-relevant behavioural benchmarks were assessed by CRS-R. During the 4-week treatment period, recovery was significantly faster in the amantadine group compared to placebo in terms of key behavioural benchmarks including consistent command following, intelligible verbalization, reliable yes/no communication and other related tasks [10].
To evaluate a priori the hypothesis that amantadine reduces irritability and aggression in individuals more than 6 months post-TBI, 76 subjects were enrolled in a parallel group RCT of amantadine (100mg twice daily, n=38) versus placebo (n=38). Symptoms of irritability and aggression were assessed using NPI-I and NPI-A respectively as well as NPI-Distress domains. Amantadine resulted in 3-point improvements on NPI-I compared to placebo (p<0.0016) [15].
To further test the hypothesis that amantadine reduces irritability in TBI of greater than 6 months duration, 168 patients were enrolled in a multi-site RCT of amantadine versus placebo. Participants received amantadine hydrochloride (100mg bid) versus placebo for 28 and 60 days. Symptoms of irritability were measured before and after treatment using the Neuropsychiatric Inventory Irritability (NPI-1) domain as well as the NPI-Distress. In the amantadine group, significant improvements were observed compared to placebo on NP-1 (p<0.04) and NP-1 Distress (p<0.04). Results were not significantly different following correction for multiple comparisons. CGI scale demonstrated greater improvements for amantadine compared to placebo (p<0.04). It was concluded that amantadine 100mg every morning and noon to reduce irritability was not positive from the observer perspective although there were indications of benefit at day 60 from the perspective of patients with TBI and their clinicians that may warrant further study [16].
A subsequent report from the same group of investigators described un # 3.2.3 (above) described findings related to the potential benefits of amantadine 100mg twice daily on anger and aggression in 168 patients with chronic TBI. Measurements of anger and aggression were made using State-Trait Anger Inventory Expression-2 (STAXI-2) and NPI-A Most Problematic and Distress scores. Amantadine 100mg bid appeared to be beneficial in decreasing aggression from the patient with TBI standpoint but had no impact on anger [17].
Implications for the updating of national practice guidelines for disorders of consciousness
The results of two high quality systematic reviews summarized under sections 3.1 and 3.3 of the current review provide the basis for the updating of clinical practice guidelines relating to disorders of consciousness. The first one from France resulted in recommendations for good practice (RGP) under the auspices of The French High Authority for Health (HAS) in collaboration of the SOFMER Scientific Society of Physical and Rehabilitation Medicine. The second one from the United States appeared in the form of a report of the Guideline Development, Dissemination and Implementation Subcommittee of The American Academy of Neurology (AAN), the American Congress of Rehabilitation Medicine (ACRM) and the National Institute on Disability, Independent Living and Rehabilitation Research (NIDLRR).
The specific recommendations based on the findings of these reviews are as follows:
United States (American Academy of Neurology) 2018 [8]
A. Patients with traumatic VS/UWS or MCS who are from 4-16 weeks post-injury should be prescribed amantadine 100-200mg bid to hasten functional recovery and reduce degree of disability in the early stages of recovery providing there are no medical contraindications or other case-specific risks for use [level B].
B. Amantadine (100-200mg bid) when administered over a period of 4 weeks in patients aged 16-65 yr with traumatic DoC between 4-16 weeks post-injury probably hastens functional recovery in the early stages. Faster recovery reduces the burden of disability, lessens health care costs and minimizes psychosocial stressors in both patients and caregivers.
C. No identified therapeutic studies have enrolled paediatric populations so far. The only therapeutic intervention shown to have efficacy in adults (16-65 yr) is amantadine. A retrospective case-controlled study of amantadine use in patients with TBI reported that 9% of children taking this treatment had side effects but methodological concerns limit therapeutic conclusions in this study.
France (French Society of Physical and Rehabilitation Medicine SOFMER) 2016 [6]
A. There is insufficient evidence of the efficacy of amantadine in the treatment of agitation, aggressiveness and anxiety after TBI. Improvement of apathy, the decision-making process or motivational disorders was reported in case studies with amantadine (300mg/d). Amantadine has no marketing authorization (MA) to treat apathy. The prescription of this drug should be evaluated for each individual case according to the criteria associated with treatments prescribed outside the MA on top of the precautions of use [Expert consensus (EC)].
B. In a note added after 2012, the end of the time-line of their systematic review, the authors added the following note: Two articles published in 2014 and 2015 contradict recommendation (A) above. The work of Hammond et al., 2014 [15] tends to demonstrate with a high level of evidence [grade A] the efficacy of amantadine (200mg/d) for treatment of irritability and aggressiveness associated with chronic TBI. Thus, the frequency and severity of these symptoms are decreased. It was a single centre study and extension to a multicentre level [16] did not validate the result. A strong placebo effect (observation bias) was underlined in both studies. No different adverse events were reported compared to placebo.
C. Amantadine was well tolerated.
Health Canada indications of use: Recommendations on the pharmacological management of TBI-related impairments [18]
A. Consider amantadine to improve attention in individuals with TBI who are out of post-traumatic amnesia and who have not responded to other medications. Recommendation # J 3.3, level: B
B. May be considered to enhance arousal and consciousness and accelerate the pace of functional recovery in individuals in negative or minimally-responsive state following TBI.Recommendation Priority # J 3.4, level: A
C. The use of amantadine 100mg can be considered for individuals with TBI when impaired arousal and attention are suspected as a factor in agitation. Recommendation Priority # R 10.5 (New), level: B.
Brasil 2018 (Tratamento farmacológico do traumatismo cranioencefálico: recomendações)[19]
Making use of the methodological strategies advocated by the Appraisal of Guidelines for Research & Evaluation [AGREE II] and evidence strengths from A to D it was determined that:
A. Amantadine was safe and effective in reducing frequency and severity of irritability (p<0.0085) and aggression (p<0.046) post-TBI.
B. In patients in a persistent VS of MCS 4-16 weeks post-TBI, amantadine accelerated the rate of functional recovery during the 1st 4 weeks of treatment compared to placebo (0<007).
C. Consequently, the overall conclusion was that amantadine was recommended to improve functionality between 4 and 16 weeks post-TBI with a degree of recommendation and strength of evidence: level A .
Conclusion
The present review serves to identify multiple studies in both acute and chronic care phases of TBI in which significant benefit of amantadine (100-300mg/d) are recorded and it has been suggested that the agent is particularly useful for cases of diffuse, frontal or right-sided brain injury. Improvements in arousal and level of consciousness as determined by GCS were accompanied by improvements in the level of vigilance, orientation, attention and cognition that were beneficial from 3 days to 6 months post-TBI in many cases. Moreover, amantadine treatment was found to hasten functional recovery from prolonged VS/UWS particularly in traumatic cases. In contrast to the general consensus in most studies, one study failed to find benefit of amantadine for the enhancement of cognitive function in chronic TBI. Surprisingly, as of March 2020, there have been no meta-analyses conducted on the results of RCTs cited in the present review and elsewhere relating specifically to the efficacy of amantadine for the treatment of TBI or its associated disorders of consciousness.
In contrast to the consensus of opinion on the efficacy of amantadine for the treatment of levels of consciousness and cognitive function post-TBI, studies of the effects of the agent for the treatment of neuro-behavioural complications such as irritability, agitation and aggression gave inconsistent results. This was apparent from the results of both the RCTs themselves and in systematic reviews assessing these trials. Possible sources of variance raised in discussions of the findings of these trials include design issues and heterogeneity of outcome measures as well as statistical procedures. Further studies are clearly warranted in order to resolve these issues.
References
- Giacino JT, Whyte J, Bagiella E,Kathleen Kalmar, Nancy Childs, et al. (2012) Placebo-controlled trial of amantadine for severe traumatic brain injury. N Engl J Med 366: 819-826.
- Deep P, Dagher A, Sadikot A, Gjedde A, Cumming P (1999)Stimulation of dopa decarboxylase activity in striatum of healthy human brain secondary to NMDA receptor antagonism with a low dose of amantadine. Synapse 34: 313-318. (Crossref)
- Kraus MF, Smith GS, Butters M, Donnell AJ, Dixon E et al. (2005) Effects of the dopaminergic agent and NMDA receptor antagonist amantadine on cognitive function, cerebral glucose metabolism and D2 receptor availability in chronic traumatic brain injury: a study using positron emission tomography (PET). Brain Inj 19: 471-479. (Crossref)
- Wheaton P, Mathias JL, Vink R (2009) Impact of early pharmacological treatment on cognitive and behavioral outcome after traumatic brain injury in adults: a meta-analysis. J ClinPsychopharmacol29: 468-477. (Crossref)
- Talsky A, Pacione LR, Shaw T, Wasserman L, Lenny AM, Verma A et al. (2011) Pharmacological interventions for traumatic brain injury. BC Medical Journal 53: 26-31.
- Plantier D, Luauté J. SOFMER group (2016)Drugs for behavior disorders after traumatic brain injury: Systematic review and expert consensus leading to French recommendations for good practice. Ann PhysRehabilMed 59: 42-57. (Crossref)
- Stelmaschuk S, Will MC, Meyers T (2015) .J Trauma Nurs 22: 194-203. (Crossref)
- Giacino JT, Katz DI, Schiff ND,Ashman EJ, Ashwal S et al. (2018) Practice guideline update recommendations summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology 91:450–460. (Crossref)
- Giacino JT, Katz DI, Schiff ND,Whyte J, Ashman EJ et al. (2018) Comprehensive systematic review update summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology. 91: 461-470. (Crossref)
- Giacino JT, Whyte J, Bagiella E,Kathleen Kalmar, Nancy Childs, et al. (2012) Placebo-controlled trial of amantadine for severe traumatic brain injury. N Engl J Med. 366: 819-826.
- Gafaar T, SaadElDin S, Salem H, οkasha F (2016) Amantadine sulfate effects on the outcome of patients with moderate and severe traumatic brain injury. Zagazig University Medical Journal 22: 1-8.
- Hammond FM, Sherer M, Malec JF,Zafonte RD, Dikmen S et al. (2018) Amantadine Did Not Positively Impact Cognition in Chronic Traumatic Brain Injury: A Multi-Site, Randomized, Controlled Trial.J Neurotrauma35: 2298-2305. (Crossref)
- Sami MB, Faruqui R (2015)The effectiveness of dopamine agonists for treatment of neuropsychiatric symptoms post brain injury and stroke. ActaNeuropsychiatr27: 317-326. (Crossref)
- Hicks AJ, Clay FJ, Hopwood M, James AC, Jayaram M et al. (2019)The Efficacy and Harms of Pharmacological Interventions for Aggression After Traumatic Brain Injury-Systematic Review. Front Neurol. (Crossref)
- Hammond FM, Bickett AK, Norton JH, Pershad R (2014) Effectiveness of amantadine hydrochloride in the reduction of chronic traumatic brain injury irritability and aggression. J Head Trauma Rehabil 29: 391-399. (Crossref)
- Hammond FM, Sherer M, Malec JF, Zafonte RD, Whitney M, et al. (2015) Amantadine Effect on Perceptions of Irritability after Traumatic Brain Injury: Results of the Amantadine Irritability Multisite Study. J Neurotrauma32: 1230-1238. (Crossref)
- Hammond FM, Malec JF, Zafonte RD,Sherer M, Bogner J, et al. (2017) Potential Impact of Amantadine on Aggression in Chronic Traumatic Brain Injury. J Head Trauma Rehabil. 32: 308-318. (Crossref)
- Ontario Neurotrauma Foundation. Clinical Practice Guideline for the rehabilitation of adults with moderatetosevereTBI2017.
- Anghinah R, Amorim RLO, Paiva WS, Schmidt MT, Ianof JN (2018)Traumatic brain injury pharmacological treatment: recommendations. ArqNeuropsiquiatr 76: 100-103.