Abstract
Introduction: Pterocarpus osun is a deciduous leguminous plant that has been used overtime in ethno medicine in the treatment of different types of ailments including inflammatory diseases.
Aim: The aim of this study is to identify the anti-inflammatory compounds present in Pterocarpus osun heartwood organic extract and to determine the acute and sub-chronic toxicity effects of the plant extract.
Method: The phytochemical constituents of the plant were determined using gas chromatograph-flame ionization detector. The acute and sub-chronic toxicity study was conducted using modified Lorke’s method. The anti-inflammatory activities of the plant extract were determined using fresh egg-albumin induced rat paw oedema method. Gas chromatography-mass spectrometry (GC-MS) was used to determine the non-polar compounds present in the plant organic extract.
Result: The GC-FID revealed that the organic extract contains more of flavonoid. Acute toxicity evaluation showed that the organic extract LD50 is >5000 mg/kg. The sub-chronic toxicity study revealed that the long term use of the extract is toxic to the kidney and liver tissue. GC-MS analysis of Pterocarpus osun heartwood eluted eight non polar compounds. The compounds responsible for the anti-inflammatory activity the plant extract could be 9,12-Octadecadienoic acid (Z,Z)- Linoelaidic acid (38.41%), and Homopterocarpin (5.03%).
Conclusion: The 9,12-Octadecadienoic acid (Z,Z)- Linoelaidic acid (38.41%), and Homopterocarpin (5.03%) present in Pterocarpus osun heartwood extract may be responsible for the plant’s anti-inflammatory activity. Also, single dose of the plant is safe while the long-term use is toxic to the kidney and liver.
Keywords
Anti-inflammatory, LD50, Sub-chronic toxicity, Gas chromatography-flame ionization detector (GC-FID) and gas chromatography-mass spectrometry (GC-MS)
Introduction
Among the costs associated with inflammatory disorders include the high prevalence of comorbid ailments, lost workdays, poor quality of life, financial load, and shorter lifespan [1]. Inflammation is a major risk factor for the emergence of chronic illnesses such as cancer, diabetes, cardiovascular disease, alzheimer’s, dementia, chronic obstructive pulmonary disease, endometriosis, inflammatory bowel disease, autoimmune diseases, etc. Conventional anti-inflammatory medications are used to treat inflammatory illnesses, although their usage is discouraged due to their unfavourable side effects like gastro intestinal irritation. There medicinal plants that are used to treat inflammatory diseases in traditional medicine without side effects. Pterocarpus osun is among the medicinal plants that are used to treat inflammation in traditional medicine. It is a leguminous tree that belongs to the Fabaceae family and genus Pterocarpus. The ethnopharmacological qualities of the genus Pterocarpus have been diversified, and nearly all plant components, including the bark, root, leaves, and sap, have been used in the traditional treatment of numerous ailments [2]. Pterocarpus osun is found in lowland evergreen and semi-deciduous forests in southern Nigeria, African savannahs, Cameroon, Asia, and Equatorial Guinea [3]. It is also known by the names Bloodwood (English), Akwara (Igbo), Osun dudu, Gbingbin (Yoruba), Ukme (Edo), Madubiya (Hausa), Isele (Delta), Ukpa (Efik), Vene (French), Palissandre (Senegal), Kino (Gambia), Bani or Banuhi (Burkina Faso), and others [3]. The stem is a component of traditional medicines used in ethnomedicine to treat sickle-cell disease, amenorrhea, anaemia, cough, ulcer, diarrhoea, and asthma. Collagen fibres are coloured using a dye made from the stem bark extract [4]. Heartwood, stem bark, leaves, and root powder are applied topically to the skin to cure rashes, skin infections, acne, rheumatism, mental abnormalities, chicken pox, and dislocated joints [5]. The dry leaf is one of the ingredients in traditional black soap called Dudu Osun soap. In the Eastern part of Nigeria, children with chicken pox are treated using P. osun’s crude extract [6]. Johnson-Ajinwo et al. [7] had showed that Pterocarpus osun has anti-inflammatory activity. This study is expected to throw more light on the anti-inflammatory effect of Pterocarpus osun heartwood extract using fractionated guided activity to determine where the anti-inflammatory activity lies. This study will also provide information on the acute toxicity and sub-chronic toxicity of Pterocarpus osun heartwood extract. This study aim is to determine the anti-inflammatory compounds present in the organic plant extract.
Methods and Materials
Collection of Plant Materials
The plant material was collected in March, 2021 from a bioreservior in Delta state, Nigeria. It was identified by a botanist, Dr. Suleiman Mikailu of the Department of Pharmacognosy and Phytotherapy, University of Port Harcourt. It was then cleaned, dried and pulverized into powdered form by use of an electric blender. The plant powder was stored in airtight container.
Extraction of the Plant Materials
Using the American National Cancer Institute (NCI) method of extraction [8]. Three thousand grams (3000 g) of the pulverized plant materials was macerated in 1:1 mixture of dichloromethane and methanol for 24 hours. After which, the extract was filtered out from the residue. Using rotary evaporator, the organic solvent was evaporated from the organic extract which was further dried in a desiccator at 40°C to remove any trace of solvent. The extract was weighed, stored in airtight container and preserved in refrigerator for further analysis. The percentage yield of the extract was calculated using the formula below.
The method of extraction used was maceration method.
Weight of Pterocarpus osun heartwood powder used=3000 g
Weight of Pterocarpus osun heartwood organic extract obtained=375.14 g

Materials and Apparatus
Grinding machine, weighing balance, measuring cylinders, wide mouth bottles, spatula, foils, rotary evaporator, water bath, fresh egg albumin, digital Vernier caliper, hand towels, needle and syringes, nose mask, hand gloves, Wistar rats (male and female), desiccator, airtight container, separating funnel etc.
Drugs, Chemicals and Reagents
Dichloromethane (Sigma-Aldrich brand), methanol (Sigma-Aldrich brand), water, n-hexane (Sigma-Aldrich brand), dimethylsulfoxide, (JHD company, Guangdong. Guanghua Sci-Tech. Co. Ltd. China), ethyl acetate (Sigma-Aldrich brand), n-butanol (Sigma-Aldrich brand), hydrochloric acid, magnesium metal, sodium hydroxide, ferric chloride, dragendorff’s reagent, wagner’s reagent, olive oil, hydrochloride acid, chloroform, acetic anhydride, sulphuric acid, fehling’s solution a + b, molisch reagent, million’s reagent, glacial acetic acid, picric acid solution, distilled water, diclofenac injection 20 mg/kg (Novartis brand) etc.
Fractionation of Organic Extract
To produce the n-hexane fraction, ethyl acetate fraction and n-butanol fraction of the organic extract, organic extract (50 g) was sequentially partitioned with organic solvents based on increasing order of polarity. Methanol, n-hexane, ethyl acetate and n-butanol are the chemicals utilized in the fractionation process.
Qualitative Phytochemical Screening
The organic extract was phytochemically screened using the method described by Trease and Evans [9].
Gas Chromatography-Flame ionization Detector (GC-FID) Analysis
Methanol (15 ml) was used to dissolve 100 mg of organic extract of Pterocarpus osun heartwood. The GC-FID device was test run to make sure it is in good operating condition and is free of impurities. Injecting 1 ul of the sample solution into the gas chromatograph caused it to evaporate quickly into the gaseous phase and separate into its components. As the separated components exit the GC column, they are combined with hydrogen and an appropriate oxidant (oxygen) in the flame-ionization chamber. This mixture is then burned with a hydrogen flame, causing any chemical components of the organic extract to become ionized and giving them a positive charge. Above the flame was a negatively charged collecting plate. As the positive ions emerge, they are accelerated towards the negatively charged collector plate where, upon making contact, they induce an electric current. This current was measured, with the amount of current being related to the number of carbon atoms burned and quantity of each component present in the organic extract. The GC has 15 m x 250 0.15 RESTEK MXT-1 column. Helium 5.0 p.a.s was used as the carrier gas, flowing at a rate of 40 ml/min, with a splitless injection of 1 ul of sample at a linear velocity of 30 cm/s. The 280°C was the temperature of the injector. At first, the oven was operating at 200°C, it was then increased to 330°C at a rate of 3°C per minute, and this temperature was maintained for 5 min. An operating temperature of 320°C was used by the detector.
Acute Toxicological Evaluation
A total of twelve wistar rats of both sexes with an average weight of 155 g were used for the study. The rats were obtained from animal house of the department of Experimental Pharmacology and Toxicology, Faculty of Pharmaceutical Sciences, University of Port Harcourt, Rivers state. The rats were allowed to get acclimatised for one week. The animals were allotted into three groups of four animals each and had continuous access to feed and water throughout the study period. Pterocarpus osun heartwood organic extract was administered in doses of 1000, 2000, and 5000 mg/kg to Groups 1-3, respectively via the oral route. The rats were placed under observation for 48 hours to check if mortality would occur. No death was recorded. Following that, the rats were further observed for 14 days for signs of mortality. No fatalities were noted. Lorke’s [10] method was modified and used to evaluate the acute toxicity for the determination of LD50.
Evaluation of Anti-inflammatory Activities
Fifty healthy Wistar rats of both sex with the average weight of 125 g were used for this study. The rats were obtained from animal house of Pharmacology and Toxicology department, Faculty of Pharmaceutical Sciences, University of Port Harcourt, Rivers state. They were allowed to get acclimatised with the environment for two weeks. They had free access to feed and water continuously throughout the period of the study. The rats were grouped into 8 test groups and 2 control groups with 5 rats per group and weighed respectively. The extract was given to the rats intraperitonealy.
Group 1 received distilled water (1 ml/kg), group 2 received diclofenac (20 mg/kg), group 3 and 4 received 200 mg/kg and 400 mg/kg of Pterocarpus osun heartwood organic extract respectively. Group 5 and 6 received 200 mg/kg and 400 mg/kg of n-hexane fraction respectively. Group 7 and 8 received 200 mg/kg and 400 mg/kg of ethyl acetate fraction respectively. Group 9 and 10 received 200 mg/kg and 400 mg/kg of n-butanol fraction respectively.
After allotting the rats into groups, the initial paw size was measured before the induction of inflammation. Acute Inflammation (edema) was induced in the right paws of the animals by administering 0.1 ml egg albumin to rats plantar. After 30 minutes of inducing edema, the paw thickness was measured and treatments were given to the rats intraperitoneally according to their body weights after which their paw thickness were determined at time intervals (T) of 1 hour, 2 hours, 3 hours and 4 hours using a digital vernier caliper. A modification of the Barung et al. [11] method used by Johnson-Ajinwo et al. 2021, was employed to evaluate the anti-inflammatory activity of Pterocarpus osun heartwood in this study. The percentage inhibition was determined and recorded.
The percentage reductions of inflammation were calculated using the formula below:
Percentage Reduction %=
![]()
Where; Tc=Mean paw size in control group (distilled water group)
Tc initial paw size=Mean initial paw size in control group (distilled water group)
Tt=Mean paw size in the treatment group
Tt initial paw size=Mean initial paw size in the treatment group
Sub-chronic Toxicological Evaluation
Wistar rats of both sexes with average weight of 150 g were assigned randomly to four groups (n=6/group). Group i received distilled water (1 ml/kg) while Groups ii – iv received 250, 500 and 1000 mg/kg of the Pterocarpus osun heartwood organic extract respectively. Using stainless steel feeding needle, the rats were dosed by oral gavage for 28 days. The rats had free access to feed and water continuously throughout the period of the study. Their feed and water intake pattern were monitored through the period of the study. The rats were sacrificed on day 29. Shorinwa et al. 2020 method of sub chronic toxicity evaluation was used [12].
Histopathological Evaluation
The liver and kidneys tissues were excised and then fixed in Bouin’s solution for histological analysis. The tissue samples of liver and kidney were dehydrated in an ascending series of alcohol, cleared in xylene, and embedded in paraffin wax melting at 60°C. Serial sections (5 μm thick) obtained by sectioning the embedded tissue with a microtome were mounted on slides coated with 3-aminopropyltriethylsilane and dried at 37°C for 24 hours. Sections on slides were deparaffinised with xylene and hydrated with a descending alcohol series. They were then stained with Mayer’s hematoxylin and eosin stain, dried, and mounted on a light microscope (x400) for histopathological examination.
Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
Blank runs were carried out on the GC-MS device thrice by injecting 1 ul of methanol into the injection port of the instrument during each program run. 0.1 g of organic Pterocarpus osun heartwood extract was dissolved in 15 ml of methanol and filtered before analysis on the GC-MS. Three times, the microsyringe was rinsed with the sample solution before commencement of the program run. 1 ul of the sample solution was injected into the GC inlet where it was vaporized and swept onto a chromatographic column by the carrier gas (usually helium). The sample flows through the column and the compounds present in Pterocarpus osun heartwood organic extract were separated based on their interaction with the coating of the column (stationary phase) and the carrier gas (mobile phase). The final part of the column is connected to the ion source where compounds eluted from the column were converted to ions. Finally, the detector displayed the number of compounds present in Pterocarpus osun heartwood and their molecular weights. The GC−MS system used for this study consist of an Agilent 7890A GC system with split injection (250°C; 1:10), coupled to an Agilent MS model 5975C mass selective detector (MSD) with a triple axis detector. The system was fitted with a 30 m HP5-MS column [(5% phenyl)-methylpolysiloxane, with an internal diameter of 0.25 mm and a film thickness (df) of 0.25 μm, (Agilent Technologies, Santa Clara, CA). Mass spectra was acquired using MSD ChemStation. Spectroscopic detection by GC-MS required an electron ionization system using high-energy electrons (70 eV). Helium gas (99.995% pure) with a flow rate of 1 ml/min was used as the carrier gas. Relative quantity of the chemical compounds present in the organic extracts of Pterocarpus osun was expressed as percentage based on peak area produced in the chromatogram. Bioactive compounds were identified based on GC retention time on HP5-MS column and matching of the spectra with the NIST 11 MS Library, (National Institute of Standards and Technology Library).
Statistical Analysis
The means and standard error of mean (SEM), was determined for all animal-based experiments carried out using Graph pad PRISM version 8. The statistical difference between the treatment groups and the control groups, were determined using Two-way analysis of variance (ANOVA); with Greisser-Greenhouse used as post hoc test. P<0.05 was considered statistically significant.
Results and Discussion
The results of this study were presented in Tables and Figures.
Percentage Yield of Extraction
Pterocarpus osun (3000 g) yield 12.50% extract as shown in Table 1. This was a good yield.
Table 1: Percentage yield of Pterocarpus osun using Dichloromethane + Methanol (organic extract)
|
Solvent |
Weight of Pterocarpus osun heartwood powder |
Weight of Pterocarpus osun heartwood organic extract |
Percentage yield (%) |
| Dichloromethane+Methanol (organic extract) | 3000 g | 375.14 g | 12.50 |
Qualitative Phytochemical Analysis
The qualitative phytochemical analysis of Pterocarpus osun heartwood organic extract revealed that secondary metabolites: carbohydrates, steroids, anthraquinones, saponins, triterpenoids, cardiac glycosides, phenols, flavonoids, proteins, cyanogenic glycosides and tannins are present in the organic extract as shown in Table 2.
Table 2: Qualitative phytochemical profile of Pterocarpus osun heartwood organic extract
|
S/N |
Test |
Observation |
| 1 | Steroids |
+ |
| 2 | Anthraquinones |
+ |
| 3 | Saponins | + |
| 4 | Triterpenoids | + |
| 5 | Cardiac glycosides | + |
| 6 | Phenols | + |
| 7 | Flavonoids | + |
| 8 | Proteins | + |
| 9 | Tannins | + |
| 10 | Carbohydrate | + |
| 11 | Cyanogenic glycoside | + |
Key: Present; +
This indicates that this plant is a rich source of phytochemical agents.
Gas chromatography – Flame Ionization Detector Analysis
The GC-FID revealed that plant extract contained high level of cyanogenic glycosides compound (15.91 μg/ml) followed by steroids (15.31 μg/ml). Another compounds that were present in appreciable quantity include anthocyanin (11.62 μg/ml), Rutin (11.26 μg/ml), Naringin (10.54 μg/ml), Kaempferol (9.01 μg/ml), Flavone (8.90 μg/ml), epicathechin (7.84 μg/ml), flavonones (7.55 μg/ml) proanthocyanin (7.19 μg/ml), oxalate (7.18 μg/ml), as well as sapogernin (7.15 μg/ml). Other constituents such as Resveratol (4.42 μg/ml), Lunamarine (4.36 μg/ml), Naringenin (1.68 μg/ml), Tannin (1.09 μg/ml), Flavan-3-ol (4.42 μg/ml), sparteine (2.63 μg/ml), and cardiac glycosides (1.36 μg/ml) were present in trace quantity. Pterocarpus osun heartwood organic extract contained high quantities of polyphenol; flavonoids, stilbene and tannin compared to other phytochemical constituents as showed in Table 3. Alkaloids, saponins, steroids, oxalate, glycosides and phytate are also present in the organic plant extract.
Table 3: GC-FID profile of Pterocarpus osun heartwood extract
|
Type of Phytochemical |
Phytochemical compounds |
Concentration (µg/ml) |
| Stillbenes (0.17 µg/ml) | Resveratol | 0.17 |
| Flavonoids (90.56 µg/ml) | Naringenin | 1.68 |
| Flavan-3-ol | 4.42 | |
| Rutin | 11.27 | |
| Anthocyanin | 11.62 | |
| Naringin | 10.54 | |
| Flavonones | 7.55 | |
| Naringin | 10.54 | |
| Kaemferol | 9.01 | |
| Flavone | 8.90 | |
| Epicatechin | 7.84 | |
| Proanthocyanin | 7.19 | |
| Tannin (1.09 µg/ml) | Tannin | 1.09 |
| Glycosides (17.27 µg/ml) | Cardiac glycosides | 1.36 |
| Cyanogenic glycosides | 15.91 | |
| Alkaloids (6.99 µg/ml) | Lunamarine | 4.36 |
| Sparteine | 2.63 | |
| Saponins (13.39 µg/ml) | Sapogenix | 6.24 |
| Sapogernin | 7.15 | |
| Steriods (15.31 µg/ml) | Steroids | 15.31 |
| Anti-nutrient (14.36 µg/ml) | Phytate | 7.18 |
| Oxalate | 7.18 |
Key: microgram/millilitre; μg/ml
Acute Toxicity Evaluation
Modified Lorke’s method (1983) was used in the acute toxicity evaluation [10]. Acute toxicity evaluation showed that Pterocarpus osun heartwood organic extract recorded zero mortality in the rats used in the study, as shown in Table 4. Also there were no changes in the behavior and physiology of the rats throughout the period of the study. This means that the single dose of Pterocarpus osun heartwood is safe and the LD50 exceeded 5000 mg/kg.
Table 4: Acute toxicity of Pterocarpus osun heartwood organic extract (n = 4)
|
Duration (days) |
Dose (mg/kg ) |
No of rats per group |
No of death |
| 14 | 1000 | 4 | 0 |
| 2000 | 4 | 0 | |
| 5000 | 4 | 0 |
Evaluation of Anti-inflammation
This study revealed that the organic extract and fractions reduced inflammation induced by fresh egg albumin as shown in Table 5 and Figure 1 below.
Table 5: Mean paw size(mm) ± standard error of mean
|
TREATMENT |
Dose (mg/kg) |
Mean paw size (mm) ± standard error of mean |
|||||
|
Initial Paw size (mm) |
30 minutes |
I hr |
2 hrs |
3 hrs |
4 hrs |
||
| Negative control (distilled water) | 1ml | 2.74 ± 0.07 | 6.78 ± 0.15 | 6.73 ± 0.03 | 6.68 ± 0.03 | 6.63 ± 0.07 | 6.47 ± 0.05 |
| Diclofenac | 20 | 2.74 ± 0.14 | 5.34 ± 0.03 | 4.11 ± 0.12 | 3.72 ± 0.02 | 3.47 ± 0.01 | 3.30 ± 0.44 |
| Organic extract | 200 | 2.40 ± 0.09 | 5.58 ± 0.33 | 5.06 ± 0.33 | 3.77 ± 0.07 | 3.14 ± 0.03
** |
3.14 ± 0.03
* |
| Organic extract | 400 | 2.69 ± 0.07 | 5.52 ± 0.04 | 4.01 ± 0.06
|
3.70 ± 0.03 | 3.31 ± 0.03
|
2.93 ± 0.03
|
| N-hexane | 200 | 2.68 ± 0.21 | 7.37 ± 0.33 | 4.86 ± 0.14
** |
4.39 ± 0.16
** |
3.76 ± 0.14
** |
3.24 ± 0.12 |
| N-hexane | 400 | 2.66 ± 0.07 | 6.13 ± 0.10 | 4.32 ± 0.05
* |
3.71 ± 0.06 | 3.50 ± 0.08 |
3.36 ± 0.02 * |
| Ethyl acetate | 200 | 2.76 ± 0.12 | 7.32 ± 0.13 | 5.06 ± 0.02
* ** |
4.29 ± 0.24
* ** |
3.75 ± 0.06
* ** |
3.11 ± 0.05 |
| Ethyl acetate | 400 | 2.85 ± 0.09 | 6.06 ± 0.07 | 4.89 ± 0.31 | 3.56 ± 0.10 | 3.37 ± 0.08 | 2.96 ± 0.20 |
| N-butanol | 200 | 2.73 ± 0.06
|
7.49 ± 0.10 | 4.93 ± 0.14
** |
4.50 ± 0.13
* ** |
4.27 ± 0.05
* ** |
3.13 ± 0.30 |
| N-butanol | 400 | 2.45 ± 0.06 | 5.69 ± 0.14 | 4.90 ± 0.06
** |
3.78 ± 0.18 | 3.36 ± 0.11 | 2.75 ± 0.30 |
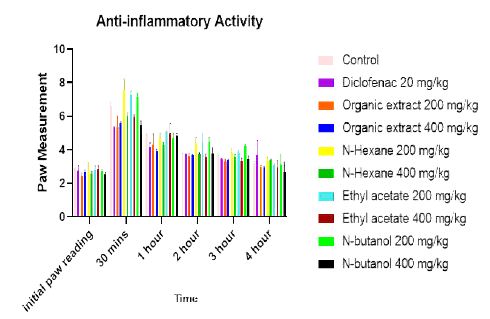
Figure 1: Mean paw size against time
The mean paw size decreased with increase in time as observed in Figure 1. This means that Pterocarpus osun had time-dependent effect.
Also, 400 mg/kg dose demonstrated better anti-inflammatory effect compared to 200 mg/kg. This implies that Pterocarpus osun had dose-dependent effect as observed in Table 6. The ethyl-acetate fraction 400 mg/kg had the highest percentage inhibition followed by organic extract 400 mg/kg and other fractions as presented in Table 6. By implication, ethyl-acetate fraction and organic extract were better than diclofenac. Also 400 mg/kg of organic extract had the enhanced anti-inflammation effect at 1 hour while the 400 mg/kg ethyl acetate achieved optimum anti-inflammatory effects at 2 hours, 3 hours and 4 hours compared to Diclofenac 20 mg/kg. This showed that Pterocarpus osun had fast onset of action compared to Diclofenac 20 mg/kg as shown in Table 6.
Table 6: Percentage reduction of inflammation (%)
|
Treatment |
DOSE (mg/kg) |
Percentage reduction of inflammation (%). |
|||
|
1hr |
2 hrs |
3 hrs |
4 hrs |
||
| Control | 1ml | 0.7 | 1.5 | 2.2 | 4.6 |
| Diclofenac | 20 | 65.67 | 75.13 | 81.23 | 84.98 |
| Organic extract | 200 | 33.33 | 65.23 | 69.15 | 80.16 |
| Organic extract | 400 | 66.92 | 74.37 | 84.06 | 93.57 |
| N-hexane | 200 | 45.36 | 56.60 | 72.23 | 81.77 |
| N-hexane | 400 | 58.40 | 73.35 | 78.41 | 84.45 |
| Ethyl acetate | 200 | 46.62 | 61.17 | 74.55 | 90.62 |
| Ethyl acetate | 400 | 44.61 | 81.97 | 86.63 | 97.05 |
| n-butanol | 200 | 44.61 | 55.08 | 60.41 | 89.28 |
| n-butanol | 400 | 38.59 | 66.24 | 76.61 | 91.96 |
The 400 mg/kg dose had a better anti-inflammatory effect compared to 200 mg/kg for all the extracts and fractions. This means that Pterocarpus osun had dose-dependent effect as observed in Figure 2.
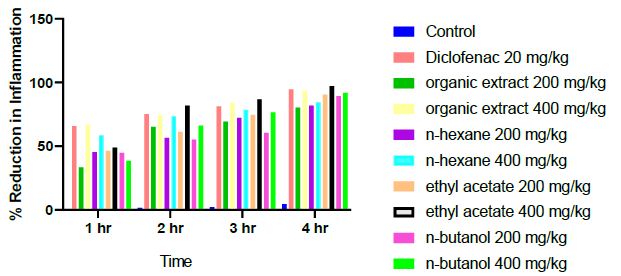
Figure 2: Percentage reduction of inflammation of the different treatment at time intervals
Ethyl-acetate 400 mg/kg had the highest percentage reduction of inflammation while the organic extract, n-hexane and n-butanol at 200 mg/kg doses had low percentage reductions as presented in Figure 3. This implies that ethyl acetate 400 mg/kg demonstrated optimum activity compared to the organic extract/fractions and diclofenac.
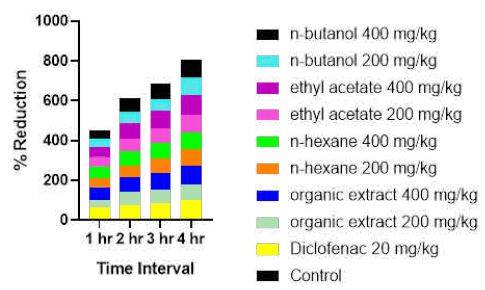
Figure 3: Percentage reduction of inflammation of the different treatment at time intervals
Hourly comparison of the activities of the extract/fractions was carried out and the results displayed in Figure 3. The 400 mg/kg of organic extract displayed early onset of anti-inflammatory activity with optimum effect at 1 hour while the 400 mg/kg ethyl acetate demonstrated superior anti-inflammatory effects from 2 hour to 4 hour compared to Diclofenac 20 mg/kg. This showed that Pterocarpus osun had fast onset of action compared to Diclofenac 20 mg/kg (Figure 3).
Sub-chronic Toxicological Evaluation
Shorinwa et al. method of sub chronic toxicity evaluation was used [12]. No mortality was recorded throughout the period of the study. There was no change in the rat behavior and physiology. No change in food and water intake as compared to control group.
Histopathology of the Kidney
The kidney tissue of the rats in control group showed normal morphology. The kidney section at 250 mg/kg of Pterocarpus osun heartwood organic extract showed mild inflammatory cell infiltration, 500 mg/kg showed interstitial edema and mild inflammatory cell infiltration and 1000 mg/kg showed glomerular degeneration as observed in Figure 4. This implies that long term use of Pterocarpus osun heartwood is toxic to the kidney.
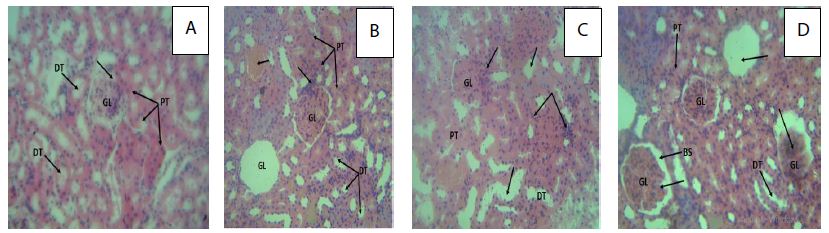
Figure 4: Photomicrograph (H&E X400) of the rat’s kidney in control and treated groups.
Key; A: A cross section of the rat kidney in control group with arrows showing normal showing glomerulus (GL), proximal tubules (PT) and distal tubules (DT); (arrows) enclosed bowman’s space (arrow). B: A cross section of the rat kidney treated with 250 mg/kg of the organic extract of Pterocarpus osun with arrows showing glomerulus (GL), proximal tubules (PT) and distal tubules (DT); complete loss of the glomeruli: mild inflammatory cell infiltration, with blood deposit in the interstitium. C: A cross section of the rat kidney treated with 500 mg/kg of the organic extract of Pterocarpus osun with arrows showing interstitial oedema of the renal tubules; mild inflammatory cell infiltration of the renal tubules and glomerulus. D: A cross section of the rat kidney treated with 1000 mg/kg organic extract of Pterocarpus osun with arrows showing glomerulus (GL), bowman’s space (BS), Proximal (PT) and distal tubules (DT) with moderately diffused renal tubular inflammatory cell infiltration, glomerular degeneration (complete loss and glomeruli shrinkage).
Histopathology of the Liver
The liver tissue of the rats in control group showed normal morphology.
The liver section at 250 mg/kg of Pterocarpus osun heartwood organic extract showed mild inflammatory cell infiltration, 500 mg/kg showed moderate distortion of the periportal area associated with hepatocyte degeneration and 1000 mg/kg showed moderate cytoplasmic degeneration as presented in Figure 5. This implies that long term use of Pterocarpus osun heartwood may be toxic to the liver.
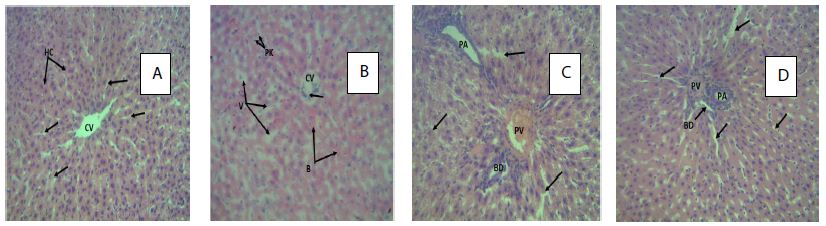
Figure 5: Photomicrograph (H&E X400) of the rat’s liver in control and treated groups.
Key; A: A cross section of the rat liver in control group with the arrows showing normal structure of the centrilobar area of the central venules (CV): visible hepatocytes (HC), and sinusoids (arrows). B: A cross section of the rat liver treated with 250 mg/kg of the organic extract of Pterocarpus osun heartwood. The arrows showing vacoulation of the hepatic lobules (V) with pyknosis of cell nucleus (PK): mild diffused blood deposit in the sinusoids (B) associated with inflammatory hepatocyte infiltration of the central vein (CV). C: A cross section of the rat liver treated with 500 mg/kg of organic extract of Pterocarpus osun heartwood. The arrows showing distortion and constriction of the Portal area (portal vein (PV), portal artery (PA) and bile duct (BD)). Diffused cytoplasmic and hepatocyte degeneration (arrows) D: A cross section of the rat liver treated with 1000 mg/kg of the organic extract of Pterocarpus osun heartwood. The arrows showing moderate constriction of the portal area (portal vein (PV), portal artery (PA) causing collapsing of the bile duct (BD)): with cytoplasmic degeneration associated with sinusoids dilation (arrows).
Gas Chromatography-Mass Spectrometry (GC-MS) Analysis Results
This study showed that Pterocarpus osun heartwood continued eight (8) compounds: 1,1-Dodecanediol, diaconate (9.99%), 2-Acetamido-2-deoxy-d-mannolactone (10.0%), cis-9-Hexadecenal (0.07%), E,E-10,12-Hexadecadien-1-ol acetate (0.07%), 9-Methyl-z,z-10,12-hexadecadien-1-ol acetate (0.24%), Homopterocarpin (5.03%), 9,12-Octadecadienoic acid (Z,Z) (38.41%) and Z-1,9-Hexadecadiene (36.13%).
The major phytochemical compound present is: 9,12-Octadecadienoic acid (Z,Z) (38.41%) followed by Z-1,9-Hexadecadiene (36.13%), 2-Acetamido-2-deoxy-d-mannolactone (10.0%), 1,1-Dodecanediol,diaconate (9.99%) and Homopterocarpin (5.03%) as shown in Table 7 and Figure 6.
Table 7: GC-MS Profile of Pterocarpus osun heartwood organic extract
|
S/N |
Non-polar compounds |
Molecular weight (g) |
Molecular formula |
Retention time (minutes) |
Area percentage (%) |
Biological activities |
| 1 | 1,1-Dodecanediol, diacetate | 275 | C15H30O2 | 19.361 | 9.99 | Unknown activity |
| 2 | 2-Acetamido-2-deoxy-d-mannolactone | 217 | C8H11NO6 | 19.897 | 10.06 | Unknown activity |
| 3 | cis-9-Hexadecenal | 238 | C16H30O | 29.354 | 0.07 | Antifungal activity,antimicrobia[13] |
| 4 | E,E-10,12-Hexadecadien-1-ol acetate | 280 | C18H32O2 | 30.042 | 0.07 | Unknown activity |
| 5 | 9-Methyl-Z,Z-10,12-hexadecadien-1-ol acetate | 294 | C19H34O2 | 30.474 | 0.24 | Unknown activity |
| 6 | Homopterocarpin | 284 | C16H17O4 | 31.996 | 5.03 | Anti-inflammatory, anticancer, anti-oxidant, antimicrobial and anti-ulcer activities. [14,15] |
| 7 | 9,12-Octadecadienoic acid (Z,Z)- Linoelaidic acid | 280.5 | C18H32O | 33.276 | 38.41 | Anti-inflammatory analgesic, antioxidant activity, Anti-cancer, and anti-microbial activities[16,17]. |
| 8 | Z-1,9-Hexadecadiene | 222.4 | C16H30 | 36.790 | 36.13 | Unknown activity |
Key: P value <0.05 is significance at a given time. (n= 5)
* means negative control significance.
**means positive control significance.
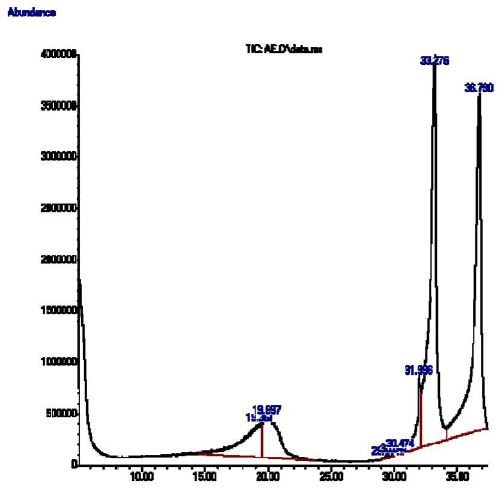
Figure 6: GC-MS profile of Pterocarpus osun
Discussion
The use of medicinal plants extract to treat acute and chronic inflammation is on increase. This is due to the unwanted side effects that are associated with conventional anti-inflammatory drugs like steroidal and non-steroidal anti-inflammatory drugs.
The 3000 g of heartwood of P. osun extracted with organic solvents (1:1 ratio of methanol and dichloromethane) yielded 12.50% extract (Table 1). This was a good yield and is in consonance with another study that reported that the use of equal volumes of polar and non-polar solvent for extraction enhances the yield of the extract [13-18].
The phytochemical evaluation of the plant Pterocarpus osun heartwood extracts revealed the presence of various Phytoactive compounds such as carbohydrates, steroids, anthraquinones, saponins, triterpenoids, cardiac glycosides, cyanogenic glycosides, phenols, flavonoids, proteins and tannins (Table 2). The presence of a wide range of phytoactive compounds in Pterocarpus osun heartwood indicated that the plant would be useful as a medicinal plant.
The GC-FID analysis revealed the presence of Polyphenols, alkaloids, saponins, steroids, glycosides, cyanogenic glycosides, phytate and oxalate in Pterocarpus osun heartwood Organic extract (Table 3). This extract contained mainly flavonoids (90.56 µg/ml). Flavonoids are known to have anti-inflammatory, antioxidant and anticancer activities. This means that the synergistic activities of flavonoids and other phytochemical constituents present in the plant extract is responsible for its anti-inflammatory activity. The hydroxyl functional groups in flavonoids donate electrons through resonance to stabilize free radicals and mediate antioxidant protection which contribute to the anti-inflammatory activity of the plant extract. Phytochemicals known as anti-nutrients are also present in the plant organic extract. Anti-nutrients such as tannins, phytate and oxalate are known to inhibit nutrient absorption for example oxalate inhibit calcium absorption [19]. These constituents tend to react with calcium and form calcium oxalate crystals that can accumulate in the body and cause kidney stones. The GC-FID evaluation also revealed the presence of high content of cyanogenic glycosides. Hence, it should be used with caution to avoid cyanide poisoning.
The acute toxicity evaluation of Pterocarpus osun heartwood organic extract that was administered to Wistar rats orally at various doses of 1000 mg/kg, 2000 mg/kg and 5000 mg/kg showed that none of the doses recorded any fatality in the tested animals (Table 4). Therefore, it could be inferred that oral LD50 of the organic extract is greater than 5000 mg/kg body weight of the tested Wistar rats. Thus, the organic extract could be said to have high safety margins since substances possessing LD50 higher than 5000 mg/kg were considered practically non-toxic [20,21].
An evaluation of the anti-inflammatory activity showed that Pterocarpus osun heartwood organic extract has anti-inflammatory effect which is dose dependent. The maximal inhibition of inflammation was observed at 4 hrs by 400 mg/kg dose of extract. The organic extract and fractions all exhibited anti-inflammatory activity but the Ethyl acetate demonstrated optimum anti-inflammatory effect, (Table 6). Johnson-Ajinwo et al. [7] previously reported that the anti-inflammatory effect of organic extract (400 mg/kg) of P. osun heartwood was better than that of indomethacin in fresh egg-albumin induced rat paw oedema method (acute inflammation). This study revealed that the anti-inflammatory effect of 400 mg/kg ethyl acetate fraction and the organic extract of P. osun heartwood were better than that of diclofenac in egg albumin induced rat paw oedema method (Table 6). This showed that P. osun heartwood extract might display superior anti-inflammatory activities than NSAIDs in the treatment of acute inflammatory conditions. An added advantage of P. osun over NSAIDS in the treatment of inflammation is its use as an antiulcer antidote in ethnomedicine.
Usually chronic disease conditions require repeated daily dose and daily dosing can be accumulated in the body and gradually affect the body tissues and organs. Acute toxicity data usually does not provide information on the effect of the plant extract on the body organ and blood. Therefore, sub-acute toxicity study was carried out to determine the effect of Pterocarpus osun heartwood organic extract on liver and kidney. Histological evaluations of the rats’ kidneys and livers showed that long term use of low dose of Pterocarpus osun heartwood organic extract may cause toxic effect to liver and kidney. The toxicity effects are dose dependent. The dose with the highest toxicity effect is 1000 mg/kg followed by 500 mg/kg while the least was 250 mg/kg. This implies that the plant extract should be taken with caution since healthy kidney and liver are important for the survival of animals. In addition, it should not be given to patients with existing liver and kidney disease because it will worsen their disease condition.
The Gas chromatography and mass spectrometry (GC-MS) analysis of organic extracts of heartwood of P. osun elicited 8 individual compounds. The major phytochemical compound present in Pterocarpus osun heartwood extract is: 9,12-Octadecadienoic acid (Z,Z), Linoelaidic acid (38.41%) followed by Z-1,9-Hexadecadiene (36.13%), 2-Acetamido-2-deoxy-d-mannolactone (10.0%), 1,1 Dodecanediol,diaconate (9.99%) and Homopterocarpin (5.03%). The 9,12-Octadecadienoic acid (Z,Z), Linoelaidic acid (38.41%) and Homopterocarpin (5.03%) have been reported to have Anti-inflammatory, anticancer, anti-oxidant, hepatoprotective, antimicrobial and anti-ulcer activities [22-26]. This implies that 9,12-Octadecadienoic acid (Z,Z) and Homopterocarpin are the anti-inflammatory compounds present in the Pterocarpus osun heartwood organic extract. Free radicals are implicated in inflammation and these compounds may be responsible for the antioxidant effects of P. osun leaf extract that was reported by Ajiboye et al. [27].
The cis-9-Hexadecenal is an organic compound that belongs to aldehyde groups. It is an aliphatic long chain aldehyde. It has antimelanogenic, antimicrobial and anti-fungi activities [28-30]. It was also reported that the anti-microbial activities of aldehydes seem to depend on the presence of the alpha, beta-double bond and on the chain length from the enal group [23]. This may be responsible for the antimicrobial activities of Pterocarpus osun that was reported by Adewale et al. [5].
Conclusion
This study conducted revealed that the anti-inflammatory compounds present in Pterocarpus osun heartwood are 9,12-Octadecadienoic acid (Z,Z), Linoelaidic acid (38.41%) and Homopterocarpin (5.03%). Single dose of the plant extract is safe, but using it for an extended period of time is toxic to the kidneys and liver. Hence, the plant extract should be used with caution and should be avoided in patient with kidney and liver diseases. It is recommended that the haematological and biochemical effect of the plant extract should be studied.
Conflict of Interest
The study’s authors affirm that there were no financial or commercial ties that might be viewed as having a potential conflict of interest.
References
- CDRG (2023) Chronic inflammation is related to a wide variety of conditions, from arteriosclerosis to diabetes. International Committee of Medical Journal Editors.
- Tchamadeu MC, Dzeufiet PD, Nana P, Kouambou Nouga CC, Ngueguim Tsofack F, et al. (2011) Acute and sub-chronic oral toxicity studies of an aqueous stem bark extract of Pterocarpus soyauxii Taub (Papilionaceae) in rodents. J Ethnopharmacol 133: 329-35. [crossref]
- Shobayo B, Ojo D, Agboola D (2015) Antibacterial Activity of Pterocarpus osun L. on Multi-Drug Resistant (MDR) Escherichia coli from Wound Infections in Abeokuta, South-West Nigeria. Open Access Library Journal 2: 1-6.
- Avwioro OG, Aloamaka PC, Ojianya NU, Oduola T, Ekpo EO (2005) Extracts of Pterocarpus osun as a histological stain for collagen fibres. African Journal of Biotechnology 4: 460-462.
- Adewale EF, Oluremi IA, Olatunbosun A (2022) Nutrients, Phytochemical, Antioxidant and Antimicrobial Analysis of Pterocarpus osun Stem Bark and Leaf for Their Nutritional, Medicinal Capacity. Nigeria. Indo J Chem Res 10: 58-67.
- Ezeokonkwo MA, Okoro UC (2012) New dyes for petroleum products. J Emerg Trends Eng Appl Sci 3: 8-11.
- Johnson–Ajinwo OR, Udofia CE (2021) Chemical characterization, phytochemical evaluation and in vivo antinflammatory activities of Pterocarpus osun. IJMSIR 6: 239-249.
- Evans JR, Akee RK, Chanana S, McConachie GD, Thornburg CC, et al. (2023) National Cancer Institute (NCI) Program for Natural Product Discovery: Exploring NCI-60 Screening Data of Natural Product Samples with Artificial Neural Networks. ACS Omega 8: 9250-9256.
- Trease and Evans Pharmacognosy, 15th Ed, India: Elseiver pp. 191-393, 2002.
- Lorke D (1983) A new approach to practical acute toxicity testing. Arch Toxicol54: 275-87. [crossref]
- Barung EN, Dumanauw JM, Duri MF, Kalonio DE (2021) Egg white-induced inflammation models: A study of edema profile and histological change of rat’s paw. J Adv Pharm Technol Res 12: 109-112. [crossref]
- Shorinwa OA, Monsi B (2020) Toxicological implications of the fruit of Harungana madagascariensis on wistar rats. Clin Phytosci 6: 2.
- Galli C, Calder PC (2009) Effects of fat and fatty acid intake on inflammatory and immune responses: a critical review. Ann Nutr Metab 55: 123-39. [crossref]
- Kim B-R, Kim HM, Jin CH, Kang S-Y, Kim J-B, et al. (2020) Composition and Antioxidant Activities of Volatile Organic Compounds in Radiation-Bred Coreopsis Cultivars. Plants 9: 717. [crossref]
- Kapoor A, Jambheshwar G, Narasimhan B (2014) Antibacterial and antifungal evaluation of synthesized 9,12-octadecadienoic acid derivatives. Der Pharmacia Lettre 6: 246-251.
- Jiang T, Li K, Liu H, Yang L (2018) Extraction of biomedical compounds from the wood of Pterocarpus macarocarpus Kurz heartwood. Pak J Pharm Sci 31: 913-918.
- Oh JM, Jang H-J, Kang M-G, Mun S-K, Park D, et al. (2023) Medicarpin and Homopterocarpin Isolated from Canavalia lineata as Potent and Competitive Reversible Inhibitors of Human Monoamine Oxidase-B. Molecules 28: 258. [crossref]
- Ryckebosch E, Muylaert K, Foubert I (2012) Optimization of an Analytical Procedure for Extraction of Lipids from Microalgae. Journal of the American Oil Chemists’ Society 89: 189-198.
- Okoli EC, Umaru IJ, Otitoju O (2022) Phytochemical Screening, Antibacterial Effects and Antioxidant Activities of Ethanolic Stem Bark Extract of Pterocarpus erinaceus. Singapore Journal of Scientific Research 12: 170-179.
- Loomis TA, Hayes AW (1996) Loomis’s Essentials of Toxicology. (4th ed.), Academic Press, CA Google Scholar.
- The Organization of Economic Co-operation and Development (OECD) (1981) The OECD Guide-line for Testing of Chemical: 408 Subchronic Oral Toxicity-Rodent: 90-day Study, France.Google Scholar.
- Jiang T, Li K, Liu H, Yang L (2018) Extraction of biomedical compounds from the wood of Pterocarpus macarocarpus Kurz heartwood. Pak J Pharm Sci 31: 913-918. [crossref]
- Olaleye MT, Akinmoladun AC, Crown OO, Ahonsi KE, Adetuyi AO (2013) Homopterocarpin contributes to the restoration of gastric homeostasis by Pterocarpus erinaceus following indomethacin intoxication in rats. Asian Pac J Trop Med 6: 200-204.
- Kapoor R, Huang YS (2006) Gamma linolenic acid: an antiinflammatory omega-6 fatty acid. Curr Pharm Biotechnol 7: 531-4. [crossref]
- Galli C, Calder PC (2009) Effects of fat and fatty acid intake on inflammatory and immune responses: a critical review. Ann Nutr Metab 55: 123-39. [crossref]
- Kim B-R, Kim HM, Jin CH, Kang S-Y, Kim J-B, et al. (2020) Composition and Antioxidant Activities of Volatile Organic Compounds in Radiation-Bred Coreopsis Cultivars. Plants 9: 717.
- Ajiboye TO, Salau AK, Yakubu MT, Oladiji AT, Akanji MA, et al. (2010) Acetaminophen perturbed redox homeostasis in Wistar rat liver: protective role of aqueous Pterocarpus osun leaf extract. Drug Chem Toxicol 33: 77-87.
- Oh JM, Jang H-J, Kang M-G, Mun S-K, Park D, et al. (2023) Medicarpin and Homopterocarpin Isolated from Canavalia lineata as Potent and Competitive Reversible Inhibitors of Human Monoamine Oxidase-B. Molecules 28: 258. [crossref]
- Shults EE, Shakirov MM, Pokrovsky MA, Petrova TN, Pokrovsky AG, et al. (2016) Phenolic compounds from Glycyrrhiza pallidiflora Maxim and their cytotoxic activity. Nat Prod Res 31: 445-452. [crossref]
- Kapoor A, Jambheshwar G, Narasimhan B (2014) Antibacterial and antifungal evaluation of synthesized 9,12-octadecadienoic acid derivatives. Der Pharmacia Lettre 6: 246-251.