Abstract
Bovine Salmonellosis is the zoonotic disease caused by pathogenic Salmonella Species. The feco-oral route is the most important mode of transmission of Salmonellosis in animals. It is an important worldwide public health challenge causing substantial morbidity and has a significant economic loss. Human salmonellosis is mainly foodborne which is transmitted through consumption of contaminated food of animal origin which includes meat, milk, poultry meat and eggs. Some studies conducted in Ethiopia on prevalence of Salmonella provided that there were different levels of prevalence of disease in different parts of the country. Epidemiological pattern, prevalence and incidences of disease differ greatly between geographical areas. This is affected by pathogens themselves, industrialization, urbanization and change of lifestyles, knowledge, belief and practices of food handlers and consumers, demographic changes, international travel and migration, international trade in food, animal feed and poverty and lack of safe food preparation facilities. Having animals and raw products, it is not possible to be free from zoonotic agents like Salmonella; however the occurrences can be minimized by applying high standard of hygiene in all steps of food production. Some topics highlighted in this paper are the epidemiology, mode of transmission, treatment and control, public health importance, conclusion and recommendations.
Keywords
Antimicrobial Resistance, Food borne, Salmonella species, Zoonosis
Abbreviations
ARG: Antibiotic Resistance Gene; EU: European Union; FDA: Food and Drug Administration; HGT: Horizontal Gene Transfer; MRSA: Methicillin Resistance Staphylococcus aureus; NTS: Nontyphoid Salmonellosis; US: United State
Introduction
The genus Salmonella was named after Daniel E. Salmon first reported the isolation of Salmonella from a pig in 1885 and named the organism Bacterium choleraesuis. The bacterium is currently known as Salmonella enterica serovar Choleraesuis. Salmonella causes typhoid fever and gastroenteritis, and it is one of the major foodborne pathogens of significant public health concern in both developed and developing countries. Meat, poultry, eggs, nuts, fruits and vegetables, and humans are the major source of infection [1]. Salmonellae are common in cattle. They are often concern due to disease of cattle and the potential to infect human that come in contact with cattle or consume dairy product or bovine meat product. Meat processing and packaging at the whole sale or retail level contribute to higher levels of contamination in minced beef product compared to beef carcass. Bovine salmonelosis usually manifest clinically as a syndrome of septicemia, acute or chronic enteritis and abortion. There are few serotypes that are associated with cattle and of this Salmonella enterica serotype Dublin (S. dublin) and Salmonella enterica subspecies enterica serotype Typhimurium (S. typhimurium) is the most common. The presence of S. typhimurium in cattle and the cross contamination of beef carcass tissue is one of the most common cause of Salmonella infection in developed countries [2]. Bovine Salmonellosis causes gastro enteritis and typhoid fever and is one of the major foodborne pathogens of significant public health concern. Salmonellosis is a disease caused by many serotypes of Salmonella and characterized clinically by one or more of the three major syndromes; septicemia, acute and chronic enteritis. Salmonellae to be familiarized in the digestive system of humans and animals. Hence, the presence of Salmonellae in water, food, and environment is elucidated by fecal contamination [3]. There are more than 2500 serovars of Salmonella worldwide. In humans, Salmonella enterica typhi (S. typhi) and Salmonella enterica paratyphi (S. paratyphi) cause typhoid fever and paratyphoid fever, respectively. Animals and poultry are commonly infected with S. enteritidis and S. typhimurium that can be transmitted to human. Animal products including; poultry meat, eggs and milk, water, domestic and wild animals, rodents and pets have been implicated as important sources for human salmonellosis outbreaks [4]. Nontyphoidal Salmonella are most important zoonotic bacterial food-borne pathogens of humans. Salmonellae are widely distributed in nature, and they are the major pathogenic bacteria in humans as well as in animals. They are most frequently isolated bacterial agents of food-borne disease outbreaks, and they account around 93.8 million food-borne illnesses and 155,000 deaths per year worldwide [5]. Non-typhoid salmonellosis (NTS) sources are red meat, meat products, dairy products, vegetable origin, pet animals can harbor and shed Salmonella Serovars [6]. Bovine Salmonellosis in farm livestock and its association with human infection has attracted a great deal of attention, particularly in recent years. The appearance of a chloramphenicol resistant strain of Salmonella typhimurium phage type D T204 in calves in Great Britain highlighted the potential public health risks and since then chloramphenicol resistant strains of the same organism, thought to have in some cases been derived from calves, have been isolated from sick humans. More recently, chloramphenicol resistance has been demonstrated in other phage types of S. typhimurium and Salmonella dublin isolated from calves and other animals [7]. Bovine Salmonellosis is one of the most common foodborne diseases worldwide, accounting around 93.8 million foodborne illnesses and 155,000 deaths per year worldwide [8]. Reports in the United States account for more than one million people sickened by Salmonella each year from 2000 to 2008 give an estimated average cost in health care of this foodborne illness of $55.5 to $93.2 billion, in the United States. Reports from the EU in 2015 showed 94,625 confirmed cases of salmonellosis in humans and 126 deaths [9]. The prevalence of bovine salmonellosis in Ethiopia was 8.4% [3]. Bovine Salmonellosis is a major economically important public health issue. Globally, an estimation indicates 33 million cases, and 0.5 million deaths associated with typhoid fever while NTS cause 93 million illnesses with 0.155 million deaths each year [10]. Economic loss is due to investigation, treatment and prevention of illness [11] and also related to restriction of animal products from international trade (market). Therefore, the objective of this paper is to review the public health importance of bovine salmonellosis.
Bovine Salmonellosis
In cattle salmonellosis is primarily associated with two serotypes, the host-adapted S. dublin and the ubiquitous S. typhimurium, although other types are sometimes involved [12]. The incidence of the serotypes varies, but generally S. typhimurium is more common in adults and S. dublin in calves. The disease in adult cattle is usually sporadic, although S. dublin has become established in some areas of the country and on some farms, and acute and sub-acute forms of the disease are recognised [13]. Characteristically severe form of the disease produced by S. dublin in adult cattle, onset is usually sudden. Cattle suffer a high temperature, become dull and stop eating. Although their faeces are initially firm, severe diarrhoea often with blood soon develops. The high temperature usually persists form several days after which animals become cold and death may occur in up to 75% of untreated animals. With S. dublin this may result in pregnant cattle aborting, although abortion may also occur in the absence of any other signs. In some cattle the disease progresses more slowly and they become emaciated and dehydrated [14]. A similar disease is produced by other serotypes including S. typhimurium, although abortion is not as common. Survivors of S. dublin infection often remain as ‘carriers’, possibly for life, while the carrier state is rarer with other serotypes. The disease in calves usually occurs between two to six weeks of age, although animals may become infected soon after birth, or with S. dublin, may be born infected [15]. Characteristically, calves become dull, refuse to drink and develop a fever. Diarrhoea follows which in young calves involves the excretion of faeces with the colour and consistency of putty. It may be stained with blood and contain mucus. Eventually the faeces become dark brown and watery with an offensive odour, or may be very bloody. In older calves the faces is usually dark brown and watery. The disease is, however, very variable. Some calves become systemically infected and, especially those two to three days old, may collapse suddenly and die, even if treated. In other animals the disease is so mild as to pass unnoticed. Alternatively the diarrhoea is prolonged and they may eventually die of dehydration and loss of salts. Complications such as pneumonia, meningitis, arthritis and gangrene may occur. Mortality from acute salmonellosis in calves may be as high as 60% without treatment and all animals may become infected [16]. Bovine Salmonella is widespread and can be found on a large number of dairy farms and in many species of animals, including mammals, birds, insects, reptiles and humans. It is often an opportunistic bacterium, meaning it infects an animal when its immune system is suppressed, when other competing gut bacteria are absent (common after antibiotic therapy), or when the animal is very young. It also infects healthy animals when they are exposed to high doses. There are many Bovine Salmonella species that are able to infect cattle; some species are also able to infect man (referred to as zoonoses or zoonotic infections), and other farm animals such as dogs and cats. Salmonellosis is more severe in the very young and old in all animal species. Disease can be serious in those people with concurrent diseases and immuno-suppressant conditions. Infection can be acquired from contact with faeces, contaminated clothing, aborted material, and un-pasteurised milk. Salmonella species can cause a wide range of clinical signs in cattle including diarrhoea and possible dysentery, joint infections, chronic pneumonia, abortion and sudden death from septicaemia. An outbreak of salmonellosis can have serious economic consequences on a farm as well as public health implications [17]. Non-typhoidal Salmonella typically causes acute gastroenteritis resulting in diarrhoea, vomiting and abdominal pain, and occasionally more serious conditions such as septicaemia, meningitis and chronic arthritis, which require treatment with effective antibiotics. In addition to these human health impacts, Salmonella can also cause production losses in livestock systems. Animals typically contract Salmonella when they consume contaminated feed or water. All livestock species can be affected by salmonellosis with young, debilitated and parturient animals most susceptible to clinical disease. While research shows that a relatively high proportion of feed and water are contaminated with Salmonella, normal adult livestock can typically tolerate small numbers of the bacteria and avoid infection [18].
Public Health Importance of Bovine Salmonellosis
Bovine Salmonellosis is an important global public health problem causing substantial morbidity and thus also has a significant economic impact. Although most infections cause mild to moderate self-limited disease, serious infections leading to deaths do occur. In spite of the improvement in hygiene, food processing, education of food handlers and information to the consumers, foodborne diseases still dominate as the most important public health problem in most countries. Public health issues and the capability for foodborne zoonotic spread have made bovine Salmonellosis the focus of various international, national, and regional surveillance platforms [19]. Bovine Salmonellosis is a major and economically important public health issue. Globally, an estimation indicates 33 million cases, and 0.5 million deaths associated with typhoid fever, while NTS cause 93 million illnesses with 0.155 million deaths each year. Bovine Salmonellosis incidence is defined as the identification of Salmonella from animals or group of animal’s product or surrounding which can be specifically related to identifiable animals or from animals feed. On the human side, a registered medical practitioner in the US required under the Public Health (Control of Disease) act to notify the local authority, if the patient is suffering from or suspected of having foodborne disease. Studies provide increasing evidence of adverse human health consequences due to the occurrence of resistant microorganisms. Use of antimicrobial agents in human and animal affects the intestinal tract placing those concerned at increased risk of certain infection. This is defined as the proportion of Salmonella that would not have occurred if the Salmonella were not resistant. In addition antimicrobial agent used in animal can result in increased transmission of resistant microorganisms between animal and therefore would results in case of transmission of such microorganisms to human through food. Increased frequency of treatment failure and increase severity of infection may be manifested by prolonged duration of illness. Salmonella dublin is largely but not entirely specific to cattle with average 10 human case reported in each year in Ireland. Apart from its pathogenicity two other characteristics of S. dublin make it particularly important for Ireland from a public health viewpoints. First, it is very prevalent on Irish farms and secondly in evolutionary terms, it is only one step away from S. enteritidis, a common Salmonella serotype in poultry and the main case of clinical salmonellosis in humans [20]. In genetic terms, difference between the serovars S. dublin and S. enteritidis are no greater than those found within each serotypes. This indicates that, S. dublin and S. enteritidis share a common ancestor. One branch evolved in to a poultry adapted serotype capable of causing disease in human, the other in to host specific cattle pathogen. If S. dublin has been confirmed in breeding herd there is a significant risk of persistent infection in carrier cows for as long as animal which were present at the time of the outbreak remain in the herd [21].
Bovine Salmonellosis as a Food Born Disease
Bovine Salmonellosis is chiefly a foodborne infection and linked to the consumption of Salmonella-contaminated food products mostly from beef, poultry, pork and egg products. Humans, especially infected food handlers, and contaminated environments are also major reservoirs of Salmonella [22]. Human salmonellosis is generally foodborne and is contracted through consumption of contaminated food of animal origin such as meat, milk, poultry and eggs. Dairy products including cheese and ice cream were also implicated in the outbreak. However, fruits and vegetables such as lettuce, tomatoes, cilantro, alfalfa-sprouts and almonds have also been implicated in recent out-break [23]. Acute gastroenteritis is usually acquired from consumption of food which may be directly or indirectly contaminated with Salmonella [16]. Nontyphoidal Salmonella are most important zoonotic bacterial food-borne pathogens of humans. Salmonellae are widely distributed in nature and they are the major pathogenic bacteria in humans as well as in animals. they are most frequently isolated bacterial agents of food-borne disease outbreaks and they account around 93.8 million food-borne illnesses and 155,000 deaths per year worldwide. Salmonella has been found to be the major cause of food-borne diseases and a serious public health problem in the world, with an increasing concern for the emergence and spread of antimicrobial-resistant strains including in industrialized countries. Antibiotic-resistant Salmonella infections of both humans and animals are universal concerns, particularly in developing countries. Apart from the morbidity and mortality costs in humans and animals, restrictions to trade and discard contaminated food are important socioeconomic problems of the bacteria [5].
Antimicrobial Resistance
Antibiotics have consistently been viewed as one of the great revelations of the 20th century. The expansion in the use of antibiotics in emergency clinics, networks and the climate are increasing the antimicrobial resistance [24]. The misuse of microorganisms has resulted in the massive economical and financial losses, and enhanced the overall burden of diseases. Antimicrobial resistance of pathogenic microorganisms is a test related with high morbidity and mortality [25]. Antibiotics may be needed in high-risk groups, such as young children, the aged persons, and those with compromised immunity. With respect to the drugs, ampicillin, chloramphenicol, and trimethoprim sulfamethoxazole can be utilized for the treatment of Salmonellosis. However, resistance to these drugs has increased significantly in recent years. Fluoroquinolones have been recommended for the treatment of Salmonella infections for adults, while third generation cephalosporin are the drugs of choice to treat very young patients or when fluoroquinolone resistance is present (Tables 1 and 2) [26]. Multiple antimicrobial resistances (resistance to two or more antimicrobials). A total of seven different antimicrobial resistance patterns were observed.
Table 1: Antimicrobial Sensitivity Patterns for Salmonellae
|
Isolated from Infected Cattle* |
Total |
Resistant |
Sensitive |
| Aureomycin R (30 mcg) |
31 |
31 |
0 |
| Kanamycin (30 mcg) |
26 |
23 |
3 |
| Neomycin (30 mcg) |
26 |
23 |
3 |
| Sulfonamides (1 mcg) |
31 |
31 |
0 |
| Streptomycin (10 mcg) |
31 |
31 |
0 |
| Chloramphenicol (30 mcg) |
31 |
0 |
31 |
| Naladixic Acid (30 mcg) |
29 |
0 |
29 |
| Polymyxin B (300 U) |
31 |
0 |
31 |
| Gentamicin (10 mcg_) |
29 |
1 |
28 |
| Furacin (Furadantin) Macrodantin (300 mcg) |
31 |
0 |
31 |
Table 2: Antibiotic Susceptibility of Salmonella isolates in dairy farms
| Antimicrobials Antibiotic Susceptibility profile
No.sensitive (%) No. intermediate (%) No. resistant (%) Kanamycin 2 (7.1) 3 (10.7) 23 (82.1) Nalidixic acid 0 (0.00) 7 (25.0) 21 (75.0) Gentamicin 28 (100.0) 0 (0.00) 0 (0.00) Cefoxitin 25 (89.3) 0 (0.00) 3 (10.7) Streptomycin 16 (57.1) 9 (32.1) 3 (10.7) Chloramphenicol 14 (50.0) 9 (32.1) 5 (17.9) Tetracycline 0 (0.00) 1 (3.6) 27 (96.4) Amoxicillin 10 (35.7) 11 (39.3) 7 (25.0) Ampicillin 17 (60.7) 0 (0.00) 11 (39.3) Ciprofloxacin 28 (100) 0 (0.00) 0 (0) Trimethoprim 22 (78.6) 3 (10.7) 3 (10.7) Sulfamethoxazol |
Drug Resistance Development Impact
In Human Antibiotic resistance impact is a global phenomenon resulting in the emergence of pathogens with resistance to clinically important antibiotics, necessitating new treatment strategies. Antibiotic-resistant bacteria cause life-threatening illness in humans and pose a significant threat to health and well-being. It is estimated that antibiotic-resistant pathogens cause ~2 million illnesses and 23,000 deaths annually in the U.S. These illnesses cause an additional health care cost of $20 billion and a productivity loss of $35 billion to the U.S. economy. Also, extensive use of antibiotics predisposes individuals to other serious illnesses [24]. Antimicrobial resistance has led to the failure of treatment in 195,763 cases of pneumococcal disease and 2,925 child deaths annually in Ethiopia. It also resulted in a first-line treatment failure rate of 29.4%. Research has demonstrated that antimicrobial resistance is a significant threat to global public health. The long-term use of antibiotics in food animals creates ideal conditions for the development and spread of resistant strains [27]. Resistant bacteria in animals may directly or indirectly reach humans through food, water, mud, and manure, which are used as fertilizers. In fact, there is irrefutable evidence that foods from many animal sources and all food processing stages contain a large number of resistant bacteria. Homologous relationships between drug-resistant bacteria in humans and animals have been identified in the most common food-borne pathogens, such as E. coli and Salmonella, different types of enterococci, and methicillin-resistant Staphylococcus aureus (MRSA). Horizontal gene transfer (HGT) occurs between different bacterial species via mobile genetic elements such as plasmids, integrases, and transposases. Thus, HGT contributes significantly to the rapid spread of resistance. Farm and slaughterhouse workers, veterinarians, and those in close contact with farm workers are easily infected with resistant bacteria through daily exposure to infected animals [28]. Most of the infections caused by these NTS are self-limiting gastrointestinal disease with symptoms of diarrhoea, fever and abdominal cramps. Bacteremia and other extra intestinal focal manifestations usually do not result from mild forms of the disease. Antimicrobial treatment is reserved only in invasive infections, in immunosuppressed and in extremes of ages as antimicrobials can prolong the illness and excretion in Nontyphoidal Salmonellosis [18]. Commonly used drugs for the treatments are fluoroquinolones and extended spectrum cephalosporins. However there are reports of antimicrobial resistance among these Salmonella strains to different classes of antibiotics and that has left us with only few options for treatment. Multidrug resistant Nontyphoidal Salmonellosis has become a global concern now. Community and healthcare associated outbreaks have been reported all over the world due to these resistant strains. Development of antimicrobial resistance is a naturally occurring phenomenon and it is often enhanced by use of antimicrobial agents for the treatment and prevention of infections in humans and animals as well as addition of these antibiotics as growth promoters or for feed efficiency in the food of animals which has favoured the selection and transference of drug resistant strains of Salmonella [29]. In animal antibiotic resistance impact in foodborne pathogens such as Salmonella is a major concern for public health safety. More focus is required to target them in the animal foods supply. Salmonella is difficult to eliminate from its reservoir hosts, and food animals often serve as reservoirs of the pathogen [30]. Antimicrobials may increase the susceptibility of animals to infection by suppressing normal flora and increasing the probability that pathogens will colonize a site (the “competitive effect”) or, if administered at the time of exposure to a resistant pathogen, by facilitating the infection because of a selective effect (the “selective effect”) (see Barza and Travers, this supplement). Resistant nosocomial salmonellosis attributable to antimicrobial therapy occurs in cattle, horses, cats, and probably other species, although little is published on this subject. Between 3% and 26% of resistant Salmonella infections of humans are acquired through a selective mechanism associated with antimicrobial treatments, according to Barza and Travers (this supplement). Comparable estimates for animals remain to be determined. Antimicrobials may prolong shedding or elevate levels of antimicrobial resistant pathogens in feces [28]. In its Framework document, the FDA states a concern about antimicrobial use in food animals increasing the pathogen load in an animal’s intestinal tract, which could increase infection risks for consumers. When challenged with Salmonella and exposed to antimicrobials in feed. Drug resistance development impact in the environment is one of the most noted consequences of antibiotic misuse and antibiotic pollution is the increased frequency of bacteria harboring ARGs in di_erent environments (here, antibiotic resistance is defined as any reduction in susceptibility in a bacterial strain compared to the susceptible wild type . An increase of antibiotic-resistance genes has also been observed in environmental. For example, ARG abundance for all classes of antibiotics was found to be significantly increased in soils from the Netherlands since the 1940s [31]. Resistance to antibiotics can be conveyed via a broad range of mechanisms. For example, antibiotics can be inactivated (e.g., beta-lactamases cleaving beta-lactams such as penicillin) or transported outside of the bacterial cell via e_ux pumps (e.g., Tet A proteins pumping tetracyclines outside of cells). The modification of the antibiotic’s target (e.g., point mutations in gyr A prevent binding by ciprofloxacin) is another common mechanism [32]. The prevalence of nosocomial (hospital-acquired) infections with resistant bacteria make hospitals and extended care facilities high interest environments to study the evolution and dissemination of antibiotic resistance. The microbial communities mostly associated with ARGs in hospitals are members of various human microbiomes as well as situated in hospital water and air flow systems [33]. Hospitals employ a broad range of antibiotics over extended time spans, thus enabling de novo resistance evolution, for example during long-term treatment of chronic infections. Environmental contamination and wildlife may also play a role in bovine S. typhimurium infection. Grazing cattle often obtain drinking water from streams and rivers which may receive effluent from sewage and meat processing plants. Streams and rivers can be a source of infection [31].
Transmission Modes
Bovine salmonellosis is spread by direct or indirect means. Infected animals are the source of the organisms; they excrete them and infect other animals, directly or indirectly by contamination of the environment, primarily feed and water supplies [34]. The farm animal may be infected in different ways: by animal-to-animal transmission, especially of host-adapted serovars; by contaminated animal feed; and by a contaminated environment (soil, birds, rodents, insects, water supplies). The excretion of salmonellas is exacerbated by the stress imposed [35]. Transmission of Salmonella to humans traditionally has been attributed to contaminated animal-product foods, but epidemiological studies have demonstrated that cases are sporadic and may more likely involve environmental sources than previously thought. It has been suggested that contaminated soils, sediments and water as well as wildlife may play a significant role in Salmonella transmission. Consumption of raw milk, inadequately pasteurized milk, improperly cooked beef from culled dairy cattle, contaminated water and direct animal contact are the major routes of acquiring dairy associated salmonellosis in humans [25]. Most Salmonella infection in farm animals are likely to acquire from animals of the same species, especially in the case of the host adapted serovars. In adult cattle there are important differences in the behavior of S. Dublin and S. typhimurium. Those animals which recover from S. dublin infection may become persistent excreters, shedding up to 106 organisms per gram of feaces daily. Other herd may harbor infection and excrete the organisms only when stressed particularly at parturition [19]. Aerosol transmission has long been suggested as a means by which Salmonella may be transmitted and experimental infection of calves by aerosol has been reported recently. In addition pasture contamination results when flooding occurs and there are many reports clinical case in adult cattle arising from grazing recently flooded pasture [2]. A wide variety of animal species have been shown to be capable of harboring the organisms and in the developed world turkey, chicken, swine and cattle are found to be infected carriers in the studies conducted in the abattoirs. These carriers may readily shed Salmonella during transportation to the abattoir and contaminate abattoir workers or equipment during slaughter. The progressive trend forwards mass processing and distribution of food products has been an important factor in the increase incidences of Salmonella foodborne diseases. Person to person spread has been demonstrated on many occasions and may take place in young children and group living under poor socioeconomic condition where effective sanitation is lacking. Person to person spread also may occur in hospitals, nursing homes, mental institution in which large number of outbreak has occurred [5]. Amplification of infection in these institutions may occur from contaminated food or asymptomatic carrier’s babies being at special risk [36]. Direct or indirect contact with animals colonized with Salmonella is another source of infection, including contact during visits to petting zoos and farms Fecal oral route and vehicle born infection may result from ingestion of food or water that have been contaminated with human or animal feaces or from direct exposure to animals or their waste. A lower infectious dose of organism is usually required in the elderly, the immunocompromised, antibiotic users and those with a chlorhydria or regular use of antacid and related medication [37]. The commonly recognized vehicle of transmission includes inadequate cooked or raw meat, unpasteurized milk or milk product, contaminated and inadequately treated drinking water [20]. Contamination of milk may occur by a variety of route. Animal may occasionally, excrete the organisms in milk during the febrile stage of the disease or more likely infected feaces, from either a clinically infected cow or healthy carrier may contaminate the milk during the milking process. Milk also may be contaminated from use of polluted water from dirty equipment or from dairy workers. Indirect contamination also has been described when cattle have become contaminated with Salmonella. Contamination of food also may occur directly from Salmonella infected food handlers or indirectly from sewage polluted water (Figure 1) [36].
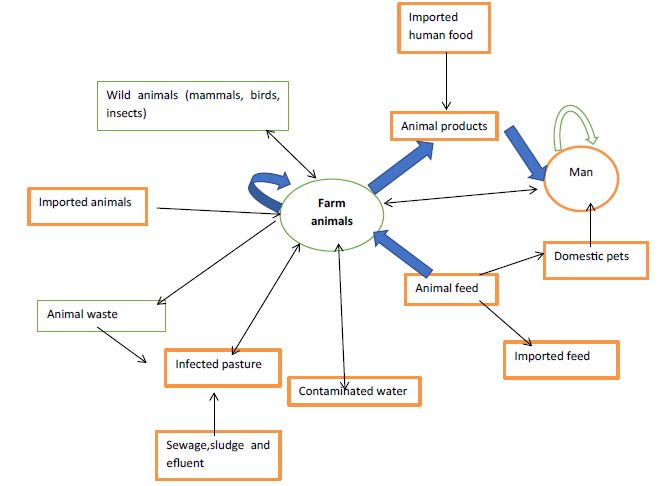
Figure 1: Transmission modes
Potential Risk Factors
Proximity to animals, food consumption behavidor, problems related to contamination of milk and meat, inadequate supply of treatment drugs, harsh environment (hot, dry and dusty zones), and socio economic and cultural practices are the main factors that expose the pastoralists to different zoonotic diseases [38]. Human behavior and level of education are further factors that may influence health status Migration may put nomadic pastoralists at periodical risk of infection, especially around water point. Since the animal and human interface is very intimate and common event in the pastoral areas of Ethiopia, it is very difficult to address the health of animals and humans separately but better if integrated. The pastoral area of Ethiopia is characterized by large size, limited development and inadequate supply of health care materials. The human population tends to be small, highly mobile, and difficult to reach, and derive their food and income from their livestock. The main concerns of the pastoral people are livestock diseases and water supply which contributed to the occurrence of different infectious diseases (Abebe, 2003).
Animal Risk Factors
The clinical characteristics of salmonellosis in large animals vary depending on the various management systems used, the intensity of stocking, whether or not the animals are housed, and the epidemiological characteristics of the different Salmonella species. The response to infection with a Salmonella sp. varies depending on the size of the challenge dose and the immunological status of the animal, itself dependent on colostrum intake in neonates, previous exposure to infection and exposure to stressors, particularly in older animals [39].
Environmental and Management risk Factors
Intensification of husbandry in all species is recognized as a factor contributing significantly to an increase in the new infection rate. Any significant change in management of the herd or a group of animals can precipitate the onset of clinical salmonellosis if the infection preexists in those animals. Temperature and wetness are most important, as salmonellas are susceptible to drying and sunlight [33].
Pathogen Risk Factors
Salmonellas are facultative intracellular organisms that survive in the phagolysosome of macrophages and can therefore evade the bactericidal effect of antibody. Compared to other organisms of the same family, salmonellas are relatively resistant to various environmental factors. They multiply at temperatures between 8°C and 45°C, at water activities above 0.94, and in a pH range of 4-8. They are also able to multiply in an environment with a low level of or no oxygen [40].
Human Source
The environmental and personal hygiene is one of the knowledge and practice restrictions of human from beef/dairy farm and abattoir food processing plants [41]. On the other hand food getting contamination depends largely on the health status of the food handlers. Food borne diseases are a public health problem in developed and developing countries like Ethiopia, the contamination occurs at any point during its journey through production, processing, distribution, and preparation. High standards of hygiene of personnel are required to maintain in food processing industries and dairy farms [8].
The host adapted serovars (some of which are human pathogens and may be contracted from foods): included are S. Gallinarum (poultry), S. Dublin (cattle), S. Abortusovis (sheep) and S. Choleraesuis (swine). Unadapted serovars (no host preference). These are pathogenic for humans and other animals, and they include most foodborne serova [42]. The Host-specific Salmonella serovars and the diseases, disease symptoms and pathological effects see on table 3 below (Table 3).
Table 3: Salmonella serovars, diseases, symptoms and pathological effects
|
Serovars |
Host |
Disease, symptoms, pathological lesions |
| S. Typhi
S. Paratyphi A, B, C S. Dublin S. Choleraesuis S. Pullorum S. Gallinarum S. Abortusequi S. Abortusovis |
Humans
Cattle and calves Pigs Chickens, turkeys Chickens, turkeys Horses Sheep |
Typhoid fever, paratyphoid fever
Cattle: diarrhea, fever necrotic enteritis Calves: diarrhea, fever, enteritis Septicaemia, pneumonia, hepatitis Pullorum disease Fowl typhoid Abortion Abortion |
Status of Bovine Salmonellosis in Ethiopia
Status of Bovine Salmonellosis in Ethiopia from (2003-2017) Food borne diseases are public health problems both in developed and developing countries. Thousands of millions of people fall ill and may die as a result of eating unsafe foods. Biological contaminants largely bacteria, constitute the major cause of food borne diseases. Salmonella infection most commonly occurs in countries with poor standards of hygiene in food preparation and handling and where sanitary disposal of sewage is lacking [43]. Studies indicated the widespread occurrence and distribution of Salmonella in Ethiopia. In Ethiopia, minced beef is usually used for the preparation of a popular traditional Ethiopian dish known as locally “Kitfo” and most of the time it is consumed raw or medium cooked. The habit of raw meat consumption and the presence of Salmonella in minced beef indicate, in addition to the poor hygienic standards in food handling in the country, the presence of great public health hazards of Salmonella. A number of studies conducted by different individuals on various slaughtered beef animals and foods of beef origin are showed the prevalence of Salmonella in the country as indicated in the Table 4 below.
Table 4: Prevalence of Bovine Salmonellosis in different parts of Ethiopia from 2003-2017
|
Area |
Species |
Sample type |
Prevalence |
Year |
| Addis Ababa and Modjo | Sheep and goats | Faeces, mesenteri lymph nodes, liver, spleen, and abdominal and diaphragmatic muscle |
1.80% |
2003/2004 |
| Modjo | Sheep and goats | Skin swabs, mesenteric lymph nodes, hand swabs, caecal contents, knife swabs, carcass and water |
8.90% |
2007/2008 |
| Addis Ababa | Cattle | Faecal and milk |
10.76% |
2010 |
| Addis Ababa Abattoir enterprise | Sheep and goats | Liver, kidney, spleen, muscle, carcass, mesenteric lymph node and feces |
1.04% |
2010-2011 |
| Gondar | Cattle | Raw meat and swab |
17.30% |
2013 |
| Holeta | Cattle | Rectal feces, udder milk, pooled milkers, hand swab, tank milk, tank swabs, and bucket swabs |
5.60% |
2014 |
| Asella | Cattle | Carcass swab, Hanging material swab, Knife swab, Hand swab, lymph node, Faeces, milk |
6.50% |
2014 |
| Gondar | Animal-origin food items | Raw meat, minced meat, burger, raw eggs, and raw milk. |
5.50% |
2014-2015 |
| Eastern Hararghe | Sheep | Faeces |
6.19% |
2014/2015 |
| Addis Ababa | Cattle | Fecal and carcass swab |
3.70% |
2014/2015 |
| Dessie | Cattle | Meat, eviscerating knives and |
4.95% |
2014/2015 |
| Bahir Dar | Cattle | Meat |
70% |
2015 |
| Modjo and Bishoftu | Sheep and goats | Cecum, liver, mesenteric lymph nodes, abdominal muscle |
17.21% |
2015/2016 |
| Eastern Haraghe | Cattle, sheep and goats | Faeces |
5.07% |
2015/2016 |
| Holeta | Dogs | Rectal Swab |
17.10% |
2015/2016 |
| Ambo | Cattle | Mesenteric lymph nodes and feces |
8% |
2015/2016 |
| Wolaita Sodo | Cattle | Abdomen, thorax, crutch, and breast |
12.50% |
2015/2016 |
| Addis Ababa | Cattle | Feces, carcass swabs, milk |
7.50% |
2017 |
Economic Importance
Bovine Salmonellosis is a significant cause of economic loss in farm animals because of the cost of clinical disease, which include death, diagnosis and treatment of clinical cases, diagnostic laboratory cost, the cost of cleaning and disinfection and cost of control and prevention [17]. In addition when the disease is diagnosed in the herd, it can create a considerable apprehension in the producer because of difficulty on identifying infected animals. An estimation of economic impact of an outbreak of S. Dublin infection in calf rearing unit indicate that the cost of disease represented a substantial proportion of gross margin of rearing calves [9]. Estimated annual costs for salmonellosis have ranged from billions of dollars in United States to hundreds to millions of dollars in Canada and millions of pounds in United Kingdom. Analysis of five Salmonella outbreak due to manufactured food in North America gave direct cost with range from $36,400-$62 million, there have been few studies in to the cost and benefit of preventing Salmonella infection, but it has been suggested that for every £1 spent on investigation and curtailment of the outbreak there is a saving of £5 [2]. Both clinical outbreaks and subclinical infections of Salmonella can drain profit from the dairy operation. Salmonella infection in a dairy herd can lead to losses from: milk production decline, death in any age group of livestock, abortions, treatment costs, losses from antibiotic, contaminated milk, increased culling, increased cost due to delayed culling while antibiotic residues clear, increased labor for management of sick animals, reduced feed efficiency, the inability to sell animals originating from an “infected” herd. Salmonella infection in a herd is also a significant public health risk to farm families, employees and visitors. This disease has serious economic, animal health and public health implications. Your veterinarian should become involved as soon as Salmonella is suspected [17]. Costs of animal diseases are normally associated with reductions in animal populations and production. There are also costs related to the mitigation of disease, which include the money and resources expended to monitor, control and, in extreme cases, eliminate the disease agent. Animal diseases that reduce reproductive competence increase the proportion of breeding animals that have to be maintained and thereby reduce the overall efficiency of the population [44]. In a recent edition of the Morbidity and Mortality Weekly reported at the 2018. FoodNet findings citing 25,606 laboratory-confirmed cases of foodborne illness, which led to the hospitalization of 5,893 individuals and 120 deaths. In the 2018 FoodNet data, Salmonella was the second most commonly reported pathogen with 9,084 cases of illness (18.3 cases per 100,000 individuals). The most commonly reported Salmonella serotypes by case were Salmonella serotype Enteritidis (S. Enteritidis: 2.6 per 100,000 individuals), Salmonella serotype Newport (S. Newport: 1.6), and Salmonella serotype Typhimurium (S. Typhimurium) [12].
Conclusion and Recommendation
Bovine Salmonella is a leading cause of foodborne disease in human and consumption of both meat and milk has been implicated in salmonellosis outbreaks of people. Having animals and raw products it is not possible to be free from zoonotic agent; however the occurrences can be minimized by applying high standard of hygiene in all steps of the food production. Infected animals can present with a great variety of clinical symptoms, and risk factors for transmission to humans clearly differ by animal species, age groups, animal purpose and geographic region. In addition, strains of Salmonella resistant to multiple antibiotics have been isolated from dairy cow during salmonellosis outbreak on dairy operation. The same strains have also been isolated from ill people. A high degree of interaction between medical and veterinarian surveillance is needed. Finally, implementing basic and applied research to the agent that cause foodborne salmonellosis will be a crucial point for new approaches to prevent and control the disease. Based on the above facts the following recommendations are forwarded: Strict hygiene of the slaughter house and lairage, People should not drink unpasteurized milk or milk products and should not eat raw meat, Education of food handlers, Vaccination of cattle, Maintenance of cold chains, Setting import standards, Sanitary examination of the product, Collaboration between government agencies, professional organizations and special interest groups.
Acknowledgements
Above all, I would like to praise my Almighty God, for supporting me health wisdom and strength in my work and for his perfect protection and guidance of my life. I would like to express great thanks for my parents for their great consolidation and financial support to educate me and my advisor Dr. Bayan Ahmed for his active gueding and advising me in working this manuscript. Finally, I greatly acknowledge to Haramaya University library workers for supporting me internet service that helps me to get different data sources.
References
- Bhunia AK (2018) Foodborne Microbial Pathogens.
- Kemal J (2014) Journal of Veterinary Science & A Review on the Public Health Importance of Bovine Salmonellosis. 5(2) https://doi.org/10.4172/2157-7579.1000175.
- Kahsay AG (2020) Prevalence and antimicrobial resistance of animal Salmonellosis in Ethiopia Overview of Salmonellosis. 1-17.
- El-Ghany WAA (2006) Salmonellosis : A food borne zoonotic and public health disease in Egypt. 10-14. https://doi.org/10.3855/jidc.12739
- Abebe E, Gugsa G, Ahmed M (2020) Review on Major Food-Borne Zoonotic Bacterial Pathogens. 2020.
- Adem J, Bushra E (2016) Bovine Salmonellosis and Its Public Health Importance : A Review. 62-71.
- Williams BM, Med, BV (2010) Bovine Sal monellosis. 15.
- Cavallo SJ et al. (2015) Human outbreak of Salmonella typhimurium associated with exposure to locally made chicken jerky pet treats, New Hampshire, 2013. Foodborne Pathogens and Disease 12: 441-446. 2015.
- Adem J, Bushra E (2016) Bovine Salmonellosis and Its Public Health Importance : A Review. 62-71.
- Younus M, Idrees, M. A, Akram Q, Ahmad W, Sciences A, Sciences A, Sciences A, Significance, P. H (2021) Pathology and public health significance of salmonella.
- Eduardo S, Monroy A (2000) Salmonella in Domestic Animals.
- Jones P (2000) Living With Bovine Salmonellosis. 2(3), 1-8.
- Williams, B. M, Med, B. V (2010) Bovine Sal monellosis. 15.
- Industry B, Safety F (2020) Salmonella White Paper. January.
- Rohr, J. R, Barrett, C. B, Civitello, D. J, Craft, M. E, Delius B, Deleo, G. A, Hudson, P. J, Jouanard N, Nguyen, K. H, Ostfeld, R. S, Remais, J. V, Riveau G, Sokolow, S. H, Tilman D (2019) to global food production. Nature Sustainability, 2(June), 445-456. https://doi.org/10.1038/s41893-019-0293-3
- Safety F, Practices, F. H (2021) Salmonella , Food Safety and Food Handling Practices. 1-16.
- Majowicz, S. E, Musto J, Scallan E, Angulo, F. J, Kirk M, Brien, S. J. O, Jones, T. F, Fazil A, Hoekstra, R. M, Collaboration I, Burden D (2010) The Global Burden of Nontyphoidal Salmonella Gastroenteritis. 50, 882-889. https://doi.org/10.1086/650733
- Hoelzer K, Isabel A, Switt M, Wiedmann M (2011) Animal contact as a source of human non-typhoidal salmonellosis. 1-28.
- Pal M, Teashal, B. M, Gizaw F, Alemayehu G, Kandi V (2020) Animals and Food of Animal Origin as a Potential Source of Salmonellosis : A Review of the Epidemiology , Laboratory Diagnosis , Economic Impact and Public Health Significance. 8(2), 48-56. https://doi.org/10.12691/ajmr-8-2-2.
- Zekarias T, Mandado T (2020) Public Health Importance of Bovine Salmonellosis in Ethiopia : A Review. 22(1), 1-8. https://doi.org/10.5829/idosi.gv.2020.01.08
- Johnson, T. J, Wannemuehler, Y. M, Johnson, S. J, Logue, C. M, White, D. G, Doetkott C, Nolan, L. K (2007) Plasmid replicon typing of commensal and pathogenic Escherichia coli isolates. Applied and Environmental Microbiology, 73(6), 1976-1983. https://doi.org/10.1128/AEM.02171-06
- Heredia N, García S (2018) Animals as sources of food-borne pathogens : A review. Animal Nutrition, 4(3), 250-255. https://doi.org/10.1016/j.aninu.2018.04.006
- Muktar Y, Abera G, Kemal J, Medicine V, Box, P. O, Dawa D (2015) Antibiotic Resistance in Salmonella Species , a Serious Public Health Problem : A Review. 15(5), 469-479. https://doi.org/10.5829/idosi.gv.2015.15.05.96225
- Sahoo, K. C, Tamhankar, A. J, Johansson E, Lundborg, C. S (2010) Antibiotic use , resistance development and environmental factors : a qualitative study among healthcare professionals in Orissa , India.
- Molossi, F. A, Lorenzett, M. P, , Sonne L, Driemeier D (2021) Epidemiological and pathological aspects of salmonellosis in cattle in southern Brazil.
- AC F (2005) Towards more virulent and antibiotic-resistant Salmonella ? FEMS Immunol Med Microbiol 43:1-11.
- Nair, D. V. T, Venkitanarayanan K, Johny, A. K (2018) Antibiotic-Resistant Salmonella in the Food Supply and the Potential Role of Antibiotic Alternatives for Control. https://doi.org/10.3390/foods7100167
- Casarin, R. A (2018) Antimicrobial Resistance : Its Surveillance , Impact , and Alternative Management Strategies in Dairy Animals. 4(January), 1-27. https://doi.org/10.3389/fvets.2017.00237
- Article R (2017) Drug Resistance in Nontyphoidal Salmonella- Challenges for the Future. 6-11.
- Mcewen, S. A, Fedorka-cray, P. J (2002) Antimicrobial Use and Resistance in Animals. 34(Suppl 3), 93-106.
- Kraemer, S. A, Ramachandran A, Perron, G. G (2019) Antibiotic Pollution in the Environment : From Microbial Ecology to Public Policy. 1-24.
- Review S, Ramatla T, Tawana M, Onyiche, T. E, Lekota, K. E, Thekisoe O (2021) Prevalence of Antibiotic Resistance in Salmonella Serotypes Concurrently Isolated from the Environment , Animals , and Humans in South Africa : A Systematic Review and.
- Agency, U. S, Development I (2019) Livestock growth, public health and the environment.
- States U, States U (2013) Signs and Symptoms Diagnosis Treatment Transmission. 2011-2013.
- Kachni, J. Č, Sasáková N, Papajová I, Ová, K. V. L. Č, Hromada R (2013) The risk to human health related to disposal of animal wastes to soil – microbiological and parasitical aspects. 147-154. https://doi.org/10.2478/s11687-013-0124-4.
- Barrow P.A, Jones MA, T. N (2010) Pathogenesis of bacterial infection in animals 4th edition. Edited by Gyles, C.L, Songer, G. Theon, C.O: Blackwell publishing 233. 2010.
- Orriss, G. D (1997) Animal Diseases of Public Health Importance. 3(4), 497-502.
- Takele S, Woldemichael K, Gashaw, M., Tassew H, Yohannes M, Abdissa A (2018) Prevalence and drug susceptibility pattern of Salmonella isolates from apparently healthy slaughter cattle and personnel working at the Jimma municipal abattoir ,. 1-7.
- Mcdaniel, C. J, Cardwell, D. M, Moeller, R. B, Gray, G. C (2014) Humans and Cattle : A Review of Bovine Zoonoses. 14(1) https://doi.org/10.1089/vbz.2012.1164
- Jajere, S. M (2019) A review of Salmonella enterica with particular focus on the pathogenicity and virulence factors, host specificity and antimicrobial resistance including multidrug resistance. Vet World. 12, 2019.
- CDC (2014) Control and Prevention, Eight multistate outbreaks of human Salmonella infections linked to small turtles (final update)
- DSM (2009) Diagnostic Services of Manitoba (DSM) (2009): Salmonella Nomenclature and the Reporting of Results on Salmonella Species: Memorandum. 2009.
- Husbandry A (2020) Salmonella and Its Status in Ethiopia. 7(1)
- Federation I, Health A (2012) The Costs of Animal Disease. October.