Abstract
Background: Worldwide, Hypertension (HTN) has emerged as the most highly prevalent modifiable risk factor for cardiovascular disease related morbidity and mortality, in terms of strongest evidence of causation and high prevalence for exposure. A preventive approach to control blood pressure (BP) may reduce these risks. Oral Magnesium (Mg) intake is inversely related with risk of HTN. Nutritional Magnesium has both direct and indirect impacts on regulation of BP through sodium (Na)-potassium (K) and intracellular Calcium (Ca) mediated Mg-driven Na-K and Ca pumps, impairment of which leads to vasoconstriction and HTN. Additionally, it increases endothelial nitric oxide, improves endothelial dysfunction, apart from inducing direct and indirect vasodilation. The efficacy of Mg, Beta-sitosterol, Pyridoxine, Niacinamide and L-carnitine, as individual ingredients in supporting alleviation of BP or associated conditions has been documented in literatures; however, no study has been done on the effectiveness of combination of these ingredients in HTN, specifically in Indian population. Despite improvement in primary therapeutics for HTN, there are reports of resistant hypertension in patients who are on more than three antihypertensives of different classes, and in many cases, achieving the goal BP becomes difficult in clinical practice. It has been suggested that addition of nutritional management for high blood pressure to the primary regimens can be a safe, sustainable, and cost-effective intervention, but their benefits are yet to be shown through appropriately designed studies. This study is aimed to evaluate the impact of MG-HT® in reduction of blood pressure when administered in conjunction with any antihypertensive therapy, such as, Calcium channel blocker, Angiotensin-converting enzyme (ACE) inhibitors, Thiazide diuretic, or Angiotensin II receptor blockers (ARB), in most cases, a combination of two drugs.
Objective: To evaluate the benefits of supplementation with MG-HT® administered twice daily with ongoing antihypertensive regimen versus MG-HT® administered once daily with ongoing antihypertensive drugs versus standard of care on the reduction of systolic blood pressure (SBP) and diastolic blood pressure (DBP) from baseline to study end at 90 days, in subjects with Stage 1 – Stage 2 Hypertension on any antihypertensive therapy, such as, Calcium channel blocker, ACE inhibitor, Thiazide diuretic, or ARB, in all cases a combination of two drugs.
Design, setting and participants: This is a Prospective, Randomized, Three-Arm, Open-Label, Parallel-Group, Multicentric Study involving 80 patients at 4 sites across India, who have Stage 1 – Stage 2 HTN and are on any existing antihypertensive therapy.
Intervention: Participants were randomized to existing antihypertensive therapy and MG-HT® once daily (Study arm 1), existing antihypertensive therapy and MG-HT® twice daily (Study arm 2), and existing antihypertensive therapy (Study arm 3) alone for 90 days, while all continued on the same dietary and activity advice.
Main outcomes and measures: The primary objective was to evaluate the benefits of additional supplementation with MG-HT® administered once daily with the existing antihypertensive therapy versus MG-HT® administered twice daily with the existing antihypertensive therapy versus standard of care with existing antihypertensive therapy alone in reduction of SBP and DBP from baseline to study end at 90 days from the beginning of the study. The secondary objectives were to evaluate the additional health benefits of MG-HT® in the study groups of subjects in terms of lipid profile from baseline to end of the study period at 90 days while they continued on existing dietary and activity advice and to analyze the relationship between the rise in serum magnesium levels to reduction in BP during the same period. Additionally, safety of MG-HT® in all the two study arms throughout the study period of 90 days was evaluated including the prevalence of subclinical magnesium deficiency at baseline in all enrolled subjects. The relationship between variations of serum magnesium levels (independent variable) and BP (dependent variable) was assessed by calculating the Odds Ratio (OR), using multivariate logistic regression analysis.
Results: The change in the Systolic Blood Pressure (SBP) in MG-HT® once daily arm from baseline to end of study was a reduction of 13.63 mmHg; in MG-HT® twice daily arm, there was a reduction of 7.87 mmHg, and in the comparator arm, and there was a reduction of 10.06 mmHg. The reduction in systolic blood pressure was higher in the arm receiving MG-HT® once daily as compared to twice daily and standard of care, respectively. The change in the Diastolic Blood Pressure (DBP) in MG-HT® once daily arm from baseline to end of study at 90 days was a reduction of 9.81 mmHg; in MG-HT® twice daily arm, there was a reduction of 6.16 mmHg and in the comparator arm, there was a reduction of 7.59 mmHg. The reduction in DBP was higher in the arm receiving MG-HT® once daily as compared to twice daily and the standard of care. However, the differences in SBP and DBP were not statistically significant (p-value >0.05). Subjects receiving MG-HT® once daily reported a greater reduction of BP in terms of returning to pre-hypertensive levels at the end of study when compared to MG-HT® twice daily and to subjects receiving standard of care. This difference was statistically significant (p-value >0.001).
The mean change of Mg levels from baseline to study end at 90 days was not statistically significant between study arms (p-value >0.05). The mean change of laboratory parameters from baseline to study end was not statistically significant between study arms (p-value >0.05).
The subjects who had lower magnesium levels at the baseline achieved normal serum mg levels at study end with an average value of 1.8 mg/dL. Subjects receiving MG-HT® once daily reported a greater reduction in SBP levels at study end with a reduction of 10 mmHg compared to 7 mmHg and 6 mmHg of MG-HT® twice daily arm and standard of care arm respectively. No change in DBP was seen. The SBP and DBP changes were not statistically significant between arms.
Conclusions: In this study, it was found that once-daily oral MG-HT® therapy added to standard antihypertensive regimens for 90 days reduced blood pressure in patients with stage 1 and 2 hypertension, improving clinical outcomes.
Keywords
Calcium channel blocker, Endothelial dysfunction, Hypertension, L-carnitine, Magnesium, MG-HT®, Niacinamide, Pyridoxine, Sterols
Introduction
Hypertension (HTN) has emerged as the most important risk factor for morbidity and mortality, worldwide [1]. It is the single largest contributor to the avoidable deaths and diseases in India [2]. It is also one of the major risk factors for noncommunicable diseases such as cardiovascular diseases, stroke, and renal diseases [3]. As per WHO currently, 35% of the world population is affected by HTN, and it might cross 50% by 2025 [4]. The prevalence of HTN in India is around 29.8%, with a higher prevalence seen in urban areas (33.8%), compared to rural areas (27.6%) [5].
In a latest Indian study, it is reported, that 25% of adults are hypertensive, with a substantial prevalence of 12% observed among the young adults aged between 18 to 25 years [6]. WHO ranks HTN as one of the prime causes of premature deaths globally. In India, 57% of all stroke deaths and 24% of all CHD are directly linked to HTN [7].
Despite availability of several comprehensive medical therapies, HTN remains a challenging clinical problem [8]. Majority of patients fail to achieve an optimal blood pressure control even with combination therapy with two or more drugs including a diuretic [9-13]. In India, a multidisciplinary consensus statement highlights that despite treatment, only about 9-20% of patients achieves goal target BP [10]. Another Indian study has reported that uncontrolled HTN may double the risks of cardiovascular events and stroke [11]. Number of drugs used to control uncontrolled hypertension matters, and any patient with uncontrolled hypertension may develop resistant hypertension, the prevalence of which is 20-30%, which would need addition of further medications for blood pressure control as an adjunct to conventional standard of care therapies [12].
Numerous studies have demonstrated that Mg supplementation may lower BP [14]. Mg may play a crucial role in BP regulation, by directly stimulating nitric oxide and prostacyclin formation, modulating endothelium-dependent and endothelium-independent vasodilation, reducing vascular tone and reactivity, and preventing vascular injury via its anti-inflammatory and antioxidant functions [14,15]. Mg also improves vascular smooth muscle tone and contractility by blocking the calcium channels and by inhibiting norepinephrine release [16,17]. An earlier study published in Japanese explored the relationship between Mg and HTN, demonstrating its relaxant effect on vascular smooth muscle cells through cationic regulation of intracellular Sodium (Na): Potassium (K) ratio and Calcium (Ca). This study also showed the benefits of Mg in hypertensive patients on antihypertensive medications, which required a lower dose in comparison to patients who were not on any antihypertensive medications [13].
Studies have shown that magnesium deficiency states may negatively influence functional and structural vascular changes in HTN. It is involved in the pathogenesis of HTN, endothelial dysfunction, dyslipidemia, and inflammation, contributing to arterial stiffness [14].
Pathophysiologically, several experimental models and cross-sectional and longitudinal population studies have reported an inverse correlation between Mg deficiency states and HTN [11-15]. On the other hand, several trials have reported inconsistent results with Mg supplementation on BP lowering effects with some showing a positive impact, while others showing none [15]. Various meta-analyses of Cohort studies and RCTs have confirmed the protective effects of Mg, establishing the association between dietary and supplemental Mg with HTN [16].
A systematic review by Rosanoff et al. (2021) of 49 clinical trials reported that oral Mg safely lowered BP in uncontrolled hypertensive patients on antihypertensive medications. However, patients with controlled hypertension or with normal blood pressure on oral Mg therapy did not show any BP lowering effect [21]. An interventional study by Banjamin et al. (2018) showed significant reduction in SBP and DBP by 8.9 mmHg and 5.8 mmHg respectively. Mg further significantly improved two hemodynamic parameters, reduced systemic vascular resistance index and left cardiac work index [18]. Several other studies have established that Mg increased the effectiveness of all antihypertensive drug classes due to its calcium channel blocker mimetic effect and circulating Na+K+ATPase suppressor activities that can reduce vascular tone [19-23].
Studies by Askarpou et al. (2019) and Craig et al. (2007) demonstrated that L-carnitine supplementation decreased SBP and DBP. It also increased brachial artery diameter by 2.3% [24,25].
Phytosterols (Beta-sitosterol) are the analogues of cholesterol, decrease the circulating cholesterol levels by competing with cholesterol for intestinal absorption. They decrease Low-Density Lipoprotein Cholesterol (LDL-c) by up to 10% and Triglycerides (TG), modulate the expression of lipid regulatory genes and de novo lipogenesis. They increase hepatic β-Hydroxy β-methylglutaryl-CoA (HMG-CoA) Reductase mRNA expression. Studies have shown that Beta-sitosterols lower risk of cardiovascular diseases [26,27]. Experimental studies with Beta-sitosterol showed that it improved nitric oxide levels and, hence, vascular function [28] that may support vasorelaxing effect of Mg.
Study by Zhang et al. (2021) demonstrated niacin-induced primary prevention of hypertension [29]. Bays et al. (2009) in a review reported significant BP lowering effects of Niacin in clinical trials involving hypertensive patients [30]. The role of niacin as an adjuvant therapy for reducing atherogenic lipoprotein levels in dyslipidemic patients has also been reported [31] including its efficacy in controlling high TG, supporting maintenance of High-Density Lipoprotein cholesterol (HDL-c) levels, and thus supporting management of lipid abnormalities associated with metabolic syndrome [32], which, in turn, is commonly associated with HTN.
Pyridoxal 5-phosphate regulates cellular calcium transport and thereby can be useful in controlling HTN (33). Aybak et al. (1995) concluded that pyridoxine administration significantly reduced SBP and DBP in hypertensive patients within 4 weeks [33, 34].
Since the current literature on the effectiveness of Mg, Beta-sitosterol, Pyridoxine, Niacinamide and L-carnitine in reduction of BP is predominantly from the western world and no combination study had been done even there, this study is envisaged to evaluate the benefits of proprietary nutraceutical formulation MG-HT® in reduction of BP when administered concomitantly with antihypertensive as a standard therapy in Indian population. Therefore, this study is the first study of its kind in India, which may contribute to better management of hypertensive patients, as a nutritional adjunct to the standard antihypertensive therapy, optimizing clinical outcomes. This could be more so useful in patients with uncontrolled HTN already on standard antihypertensive therapy, contributing to enhanced risks and resistance to treatment.
Materials and Methods
Study Design
This prospective, randomized, three arm, open label, parallel group, multicentric study investigated the clinical effectiveness of supplementing MG-HT® (Magnesium Bisglycinate 500 mg providing elemental Magnesium 70 mg, L-Carnitine L-Tartrate 500 mg, Niacinamide 8 mg, Pyridoxine Hydrochloride 1 mg, Beta-Sitosterol 20 mg, a proprietary nutraceutical formulation available in Indian market) in supporting reduction of BP in uncontrolled hypertensive patients, on standard antihypertensive therapy.
Site of the Study and Ethics
This is a multi-center, interventional study conducted at the 4 sites geographically distributed across India at Suraksha Polyclinic-Kolkata; Sanjeevani Hospital and Polyclinic-Mumbai; Diabetes Specialty Centre, Dwarka-New Delhi; and SRM Institute of Medical Sciences-Chennai. The study was approved by the Independent Ethics Committee (IEC) of each study center. It was performed in compliance with the ICH guidelines for Good Clinical Practice, ICMR guidelines, and declaration of Helsinki. Written informed consent for participation in the study was obtained from all participants. This study was registered in the Clinical Trials Registry of India (CTRI/2020/01/022864).
Study Population
80 Subjects of both sexes in age bracket 35-65 years with Stage 1 – Stage 2 hypertension as defined by the latest JNC 8 hypertension guidelines (Stage 1 HTN: SBP 140-159 mmHg, DBP 90-99 mmHg and Stage 2 HTN: SBP ≥160 mmHg, DBP ≥100 mmHg) and on any antihypertensive therapy for at least a month who have not shown any improvement in BP control or have mild elevation in BP but continue to fall under the same stage as at the time of diagnosis.
Inclusion Criteria
Subjects
- Able to provide signed informed consent
- Willing to adhere to protocol and study requirements during the entire study duration
Exclusion Criteria
Subjects
- With uncontrolled diabetes mellitus in the opinion of the investigator
- With history of myocardial infarction within the past 3 months of the start of the study, cardiac failure of class III and IV, Atrioventricular block II or III on ECG
- With chronic kidney disease or liver disorder
- With chronic terminal diseases, such as, malignancies, anemias and presence of serum electrolyte disturbances (Na, K, Cl), that might indicate an underlying secondary HTN
- On Mg supplements equal to or above the study dose of 70 mg, in which case a wash-out period of 7 days will be followed prior to enrolment
- With any other condition, which in the opinion of the investigator renders the patient unfit to participate
- Females, who are lactating, pregnant, or planning to conceive during the study period
Randomization
Computer generated random numbers were used to assign participants to the MG-HT® once daily with standard antihypertensive therapy or MG-HT® twice daily with standard antihypertensive therapy or standard of care groups in 1:1:1 allocation ratio.
Outcome Measures
The primary endpoint was the proportion of subjects with a reduction in SBP and DBP from baseline to study end after 90 days, mean change in SBP from baseline to study end after 90 days, and mean change in DBP from baseline to study end after 90 days.
The secondary endpoints were the mean change in lipid profile from baseline to study end after 90 days and mean change in serum Mg levels from baseline to study end after 90 days.
The safety endpoints were the solicited and unsolicited adverse events (AEs) in all the three arms and changes from baseline in the laboratory parameters of renal function tests and liver function tests.
The exploratory end point was the proportion of subjects with subclinical Mg deficiency at baseline.
BP Measurement Procedure: Seated BP of each enrolled subjects was measured using Diamond brand mercurial type BP instrument with standard U-tube manometer at each study visit after a rest period of 10 minutes. Subjects were made to sit comfortably with legs resting on the ground not crossed and with arm supported at the heart level. Each subject was advised to empty the bladder prior to BP recording. Cuff bladder was applied, encircling two-thirds of the subject’s arm circumference. The recordings were made on the right arm. The subject and the person taking the measurements were not allowed to speak during the procedure. The cuff was inflated to at least 30 mmHg above the point at which the radial pulse disappeared. The cuff was then deflated at a rate of 2 to 3 mmHg per second (or per pulse when the heart rate is slow). Deflation rates greater than 2 mmHg per second can cause the systolic pressure to appear lower and the diastolic pressure to appear higher. The first and last audible sounds were recorded as systolic and diastolic pressures, respectively. Measurements were given to the nearest 2 mmHg. An average of two readings was recorded. The BP was recorded by the investigator or a trained delegated study personnel. A calibrated sphygmomanometer was used at all sites. The same delegated personnel for each site recorded the BP at all visits. This maintained consistency and eliminated any interobserver bias.
Procedure to analyze MG-HT® batches allocated to each Intervention Group: The batch number and other details of MG-HT® product were recorded in the form of a list. Each batch allocated in the study had an assay undertaken at the manufacturing unit, and the Certificate of Analysis was available for record and corroboration. It was ascertained that the elemental Mg level in each batch was at least 70 mg.
Study Arms
- Study arm 1: Antihypertensive therapy and MG-HT® once daily (morning).
- Study arm 2: Antihypertensive therapy and MG-HT® twice daily.
- Comparator arm: Standard of care with antihypertensive therapy.
- Study product was supplied by Pharmed Limited, Bangalore, India.
Methodology
80 enrolled subjects ranging 35-65 years of age, diagnosed with Stage 1 – Stage 2 HTN and on any antihypertensive therapy for at least a month that have not shown any improvement in BP control or have mild elevation in BP but continue to fall under the same stage at the time of diagnosis were enrolled into the study. All enrolled subjects were screened according to the pre-defined inclusion and exclusion criteria, and subjects were randomized to either of the three study arms. Subjects randomized to study arm 1 received antihypertensive therapy and MG-HT® once daily (morning), subjects randomized to study arm 2 received antihypertensive therapy and MG-HT® twice daily, and subjects randomized to comparator arm received the investigator prescribed standard of care of antihypertensive therapy. There were three physical visits (V1-Screening, V3 and V6-Study end on Day 1, 30 and 90 respectively) and three telephonic follow-up visits (V2, V4 and V5 on Day 15, 45 and 60 respectively). All the subjects in each group were asked to continue with salt-restricted diet and usual activities, such as, daily walking as a part of standard of care before the screening.
After obtaining written informed consent from the participants in the prescribed format, detailed clinical history, and stage of HTN were recorded along with their relevant medical history and drugs used as antihypertensives or otherwise. Seated BP of all enrolled subjects were measured and recorded as an average of two readings. A fasting blood sample was collected from each subject for baseline laboratory investigations of HbA1c, Lipid Profile, Serum Mg, Renal Function Tests, Liver Function Tests, and an ECG was recorded at baseline on each subject.
Subjects were contacted telephonically at Day 15, Day 45 and Day 60 and enquired about their general well-being and compliance to study product consumption. Subjects were instructed to report to the study sites for the physical follow-up visits on Day 30 and Day 90. At all follow-up visits, a general examination was performed including BP recording, and subjects were enquired about any solicited or unsolicited AEs. Concomitant and rescue medications were reviewed as applicable in each case. Investigational Product (IP) accountability was also performed. At study end visit, a fasting blood sample was collected from each of the subjects for laboratory investigations, and an ECG was also recorded.
Statistical Analysis
To evaluate the overall effects of MG-HT® supplementation on BP, the mean changes of systolic and diastolic BP between treatment groups after treatment was compared by calculating mean differences and 95% confidence intervals (CIs). For comparison of normally distributed variables, One-Way ANOVA Test (or Kruskal-Wallis test for skewed data) to establish the differences between the groups was performed. The relationships between variations of serum Mg levels (independent variable) and BP (dependent variable) were assessed by calculating the Odds Ratio (OR) using multivariate logistic regression analysis. A 95% confidence interval (CI 95%) was considered, and p-value <0.05 defined the level of statistical significance. A sub-group analysis was performed to analyze the correlation between effects of MG-HT® and reduction in BP in various classes of antihypertensives prescribed. Associations between continuous variables were captured using Pearson’s Correlation Coefficient if the data followed normal distribution or Spearman’s Rank Correlation Coefficient if the data did not follow normal distribution. Safety analysis was performed on all enrolled subjects who have received at least one dose of the study product.
Results
A total number of 80 subjects satisfying inclusion and exclusion criteria were enrolled into the study and randomized to study arm 1, 2 and 3 or comparator arm. 28 subjects were enrolled in both the treatment arms of MG-HT® once daily and twice daily and 24 subjects were enrolled in the comparator arm. The lost to followup rate was considerably higher in the study owing to the unprecedented SARS-CoV-2 pandemic; a total of 28 subjects were lost to followup at visit 6, of which 12 were in MG-HT® once daily arm, 9 were in MG-HT® twice daily arm, and 7 were in the comparator arm. However, at each visit subjects were followed up telephonically, and the following is the subject disposition of all subjects who reported physically at site and followed up telephonically. The baseline characteristics of the study patients are shown in Table 1.
Table 1: Baseline characteristics of 80 subjects, the values representing the mean ± standard deviation (SD)
|
Parameters |
Arm-1 (n=28) | Arm-2 (n=28) |
Arm-3 (n=24) |
| Age (Y) |
52.79 ± 8.359 |
52.39 ± 8.5 |
48.08 ± 10.325 |
| Height (cm) |
162 ± 7.779 |
163.87 ± 8.18 |
164.32 ± 8.706 |
| Weight (Kg) |
69.75 ± 10.429 |
71.02 ± 11.262 |
72.63 ± 15.863 |
| BMI (kg/m2) |
26.62 ± 3.812 |
26.43 ± 3.61 |
26.83 ± 5.252 |
| History of duration of hypertension (months) |
49.54 ± 68.692 |
59.83 ± 61.198 |
37.71 ± 60.68 |
| Blood Pressure (mmHg) |
SBP:149.64 ± 7.395 DBP:90.39 ± 4.228 |
SBP:147.55 ± 6.735
DBP:91.61 ± 3.9 |
SBP:148.56 ± 6.589 DBP:91.13 ± 4.785 |
| Hypertension stage wise distribution (n) |
Stage 1: 22 Stage 2: 6 |
Stage 1: 24
Stage 2: 4 |
Stage 1: 20 Stage 2: 4 |
| HbA1c (%) |
6.46 ± 1.181 |
6.83 ± 1.521 |
7.16 ± 1.807 |
| Cholesterol Total (mg/dL) |
168.22 ± 51.147 |
178.96 ± 40.963 |
190.68 ± 42.956 |
| HDL (mg/dL) |
44.94 ± 9.427 |
44.62 ± 10.993 |
44.31 ± 12.119 |
| LDL (mg/dL) |
96.78 ± 41.116 |
106.07 ± 34.422 |
116 ± 35.68 |
| VLDL (mg/dL) |
27.05 ± 12.296 |
27.34 ± 10.869 |
30.85 ± 12.887 |
| Triglyceride (mg/dL) |
145.96 ± 83.259 |
150.94 ± 80.182 |
162.42 ± 90.644 |
| Bilirubin Total (mg/dL) |
0.64 ± 0.375 |
0.61 ± 0.287 |
0.61 ± 0.502 |
| Bilirubin Direct (mg/dL) |
0.23 ± 0.12 |
0.25 ± 0.136 |
0.21 ± 0.097 |
| Bilirubin Indirect (mg/dL) |
0.42 ± 0.3 |
0.37 ± 0.189 |
0.4 ± 0.426 |
| SGPT(ALT) (U/L) |
27.16 ± 11.252 |
25.91 ± 16.611 |
35.63 ± 25.901 |
| SGOT (AST) (U/L) |
28.2 ± 13.319 |
23.94 ± 10.598 |
25.92 ± 11.204 |
| Albumin (g/dL) |
4.63 ± 0.506 |
4.64 ± 0.462 |
4.55 ± 0.384 |
| Alkaline Phosphatase (U/L) |
92.64 ± 46.993 |
85.59 ± 17.885 |
96.25 ± 26.122 |
| Blood Urea Nitrogen (mg/dL) |
11.42 ± 3.38 |
10.43 ± 4.024 |
9.95 ± 2.703 |
| Urea Serum (mg/dL) |
23.93 ± 6.459 |
22.61 ± 8.649 |
21.28 ± 5.785 |
| Creatinine (mg/dL) |
0.86 ± 0.228 |
0.83 ± 0.293 |
0.78 ± 0.173 |
| Magnesium (mg/dL) |
2.02 ± 0.135 |
1.9 ± 0.238 |
2.01 ± 0.226 |
Baseline values represent the mean ± standard deviation. BMI: Body mass index, HbA1c: Hemoglobin A1c, HDL: High-density lipoprotein, LDL: Low-density lipoprotein, VLDL: Very low-density lipoprotein, SGPT: Serum glutamic pyruvic transaminase, ALT: Alanine transaminase, SGOT: Serum glutamic oxaloacetic transaminase, AST: Aspartate transaminase
Blood Pressure Data
Systolic Blood Pressure
Under Intention-to-treat (ITT) analysis, the change in the SBP in study arm-1 from baseline to end of study was a reduction of 13.63 mmHg; in study arm-2, there was a reduction of 7.87 mmHg, and in the comparator arm, there was a reduction of 10.06 mmHg. The reduction in SBP was higher in the arm receiving MG-HT® once daily as compared to twice daily and standard of care. However, this difference was not statistically significant (p-value >0.05). The mean changes in SBP are shown in Table 2. It is also to be recognized at this point that there is high standard deviation noted in the data set, which is commensurate with other studies involving HTN.
Table 2: Mean changes in SBP & DBP at baseline, 1 month & 3 months, the values representing the mean ± standard deviation (SD)
|
Parameter (mmHg) |
Treatment Arms | ||
| Study Arm-1 | Study Arm-2 |
Comparator Arm |
|
| Baseline SBP |
149.64 ± 7.395 |
147.55 ± 6.735 |
148.56 ± 6.589 |
| End of 1-month SBP |
135.5 ± 9.509 |
137.73 ± 8.189 |
140.36 ± 6.801 |
| End of 3 months SBP |
133.75 ± 11.527 |
138.68 ± 8.479 |
137.24 ± 8.864 |
| Difference SBP |
-13.63 ± 9.157 |
-7.87 ± 7.341 |
-10.06 ± 9.666 |
| Baseline DBP |
90.39 ± 4.228 |
91.61 ± 3.9 |
91.13 ± 4.785 |
| End of 1-month DBP |
81.79 ± 5.989 |
84.93 ± 5.637 |
88.91 ± 8.549 |
| End of 3 months DBP |
80.75 ± 7.179 |
84.89 ± 6.402 |
83 ± 6.275 |
| Difference DBP |
-9.81 ± 7.884 |
-6.16 ± 8.14 |
-7.59 ± 6.472 |
Values represent the mean ± standard deviation
Diastolic Blood Pressure
The change in the DBP in study arm-1 from baseline to end of study was a reduction of 9.81 mmHg; in study arm-2, there was a reduction of 6.16 mmHg, and in the comparator arm, there was a reduction of 7.59 mmHg. The reduction in DBP was higher in the arm receiving MG-HT® once daily as compared to twice daily and standard of care. However, this difference was not statistically significant (p-value >0.05). The mean changes in DBP are shown in Table 2; although there was a high standard deviation, this compares well with the comparator arm.
Hypertension Stage Wise Distribution: Change from Baseline between Groups
In MG-HT® once daily arm, there were 22 subjects in stage 1 which reduced to 5 at study end. Similarly, there were 6 subjects in stage 2, and none at study end. Ten subjects became prehypertensive and 1 subject, normotensive; 12 subjects lost to follow-up before the end of the study. In MG-HT® twice daily arm, there were 24 subjects in stage 1 which reduced to 11 at study end. Similarly, there were 4 subjects in stage 2 and none at study end. Eight subjects became prehypertensive and none was normotensive; 9 subjects lost to follow-up before the end of the study. In standard of care arm, there were 20 subjects in stage 1 which reduced to 11 at study end. Similarly, there were 4 subjects in stage 2 and none at study end; 6 subjects became prehypertensive but none were normotensive; 7 subjects lost to follow-up before end of study. Subjects receiving MG-HT® once daily reported a greater reduction of BP in terms of returning to pre hypertensive levels at end of study when compared to MG-HT® twice daily and those receiving standard of care. This difference was statistically significant (p-value >0.001) despite a large standard deviation in the captured data set. The change in hypertension stage wise distribution is shown in Table 3.
Table 3: Hypertension stage wise distribution change from baseline between groups
|
Study Arm |
Stage | Baseline |
Visit 6 |
| Antihypertensive therapy and MG-HT® once daily | Stage 1 |
22 |
5 |
| Stage 2 |
6 |
0 |
|
| Pre-Hypertensive |
0 |
10 |
|
| Normal |
0 |
1 |
|
| Lost to follow-up |
0 |
12 |
|
|
Total |
28 |
28 |
|
| Antihypertensive therapy and MG-HT® twice daily | Stage 1 |
24 |
11 |
| Stage 2 |
4 |
0 |
|
| Pre-Hypertensive |
0 |
8 |
|
| Normal |
0 |
0 |
|
| Lost to follow-up |
0 |
9 |
|
| Total |
28 |
28 |
|
| Standard of Care | Stage 1 |
20 |
11 |
| Stage 2 |
4 |
0 |
|
| Pre-Hypertensive |
0 |
6 |
|
| Normal |
0 |
0 |
|
| Lost to follow-up |
0 |
7 |
|
| Total |
24 |
24 |
Change in Magnesium (Mg) Levels from Baseline to Study End
The mean changes of Mg levels from baseline to study end were not statistically significant between study arms (p-value >0.05). The mean change in Mg levels are shown in Table 4.
Table 4: Mean changes in magnesium levels from baseline to study end, values represent the mean ± standard deviation
|
Antihypertensive therapy and MG-HT® once daily |
|||
| Magnesium (mg/dL) | Visit 1
(baseline) |
Visit 6
(study end) |
Difference in Mg levels (Visit 6 – Visit 1) |
| 2.02 ± 0.135 | 2.05 ± 0.172 | 0.04 ± 0.159 | |
|
Antihypertensive therapy and MG-HT® twice daily |
|||
| Magnesium (mg/dL) | Visit 1
(baseline) |
Visit 6
(study end) |
Difference in Mg levels (Visit 6 – Visit 1) |
| 1.9 ± 0.238 | 2.07 ± 0.212 | 0.11 ± 0.125 | |
|
Standard of Care with Antihypertensive therapy |
|||
| Magnesium (mg/dL) | Visit 1
(baseline) |
Visit 6
(study end) |
Difference in Mg levels (Visit 6 – Visit 1) |
| 2.0 ± 0.226 | 2.06 ± 0.166 | 0.03 ± 0.179 | |
Values represent the mean ± standard deviation
Change in Laboratory Parameters from Baseline to End of Study between Groups
The mean changes of laboratory parameters from baseline to study end were not statistically significant between study arms (p-value >0.05). The mean changes in laboratory parameters are shown in Table 5.
Table 5: Mean changes in laboratory parameters from baseline to study end, values represent the mean ± standard deviation
|
Parameter |
HbA1c (%) Visit 1 |
HbA1c (%) Visit 6 |
| Arm-1 |
6.46 ± 1.181 |
6.42 ± 0.848 |
| Arm-2 |
6.83 ± 1.521 |
6.35 ± 0.714 |
| Arm-3 |
7.16 ± 1.807 |
6.61 ± 1.561 |
|
Parameter |
Cholesterol Total (mg/dL) Visit 1 |
Cholesterol Total (mg/dL) Visit 6 |
| Arm-1 |
168.22 ± 51.147 |
168.46 ± 41.559 |
| Arm-2 |
178.96 ± 40.963 |
178.81 ± 37.641 |
| Arm-3 |
190.68 ± 42.956 |
179.45 ± 56.949 |
|
Parameter |
HDL (mg/dL) Visit 1 |
HDL (mg/dL) Visit 6 |
| Arm-1 |
44.94 ± 9.427 |
42.73 ± 8.492 |
| Arm-2 |
44.62 ± 10.993 |
45.97 ± 15.665 |
| Arm-3 |
44.31 ± 12.119 |
43.33 ± 11.732 |
|
Parameter |
LDL (mg/dL) Visit 1 |
LDL (mg/dL) Visit 6 |
| Arm-1 |
96.78 ± 41.116 |
94.69 ± 34.452 |
| Arm-2 |
106.07 ± 34.422 |
100.28 ± 29.648 |
| Arm-3 |
116 ± 35.68 |
99.88 ± 46.098 |
|
Parameter |
VLDL (mg/dL) Visit 1 |
VLDL (mg/dL) Visit 6 |
| Arm-1 |
27.05 ± 12.296 |
31.04 ± 17.201 |
| Arm-2 |
27.34 ± 10.869 |
29.16 ± 10.564 |
| Arm-3 |
30.85 ± 12.887 |
35.74 ± 16.172 |
|
Parameter |
Triglyceride (mg/dL) Visit 1 |
Triglyceride (mg/dL) Visit 6 |
| Arm-1 |
145.96 ± 83.259 |
161.33 ± 105.337 |
| Arm-2 |
150.94 ± 80.182 |
178.27 ± 91.067 |
| Arm-3 |
162.42 ± 90.644 |
196.94 ± 107.201 |
|
Parameter |
Bilirubin Total (mg/dL) Visit 1 |
Bilirubin Total (mg/dL) Visit 6 |
| Arm-1 |
0.64 ± 0.375 |
0.77 ± 0.467 |
| Arm-2 |
0.61 ± 0.287 |
0.65 ± 0.299 |
| Arm-3 |
0.61 ± 0.502 |
0.62 ± 0.468 |
|
Parameter |
Bilirubin Direct (mg/dL) Visit 1 |
Bilirubin Direct (mg/dL) Visit 6 |
| Arm-1 |
0.23 ± 0.12 |
0.29 ± 0.154 |
| Arm-2 |
0.25 ± 0.136 |
0.26 ± 0.157 |
| Arm-3 |
0.21 ± 0.097 |
0.21 ± 0.087 |
|
Parameter |
Bilirubin Indirect (mg/dL) Visit 1 |
Bilirubin Indirect (mg/dL) Visit 6 |
| Arm-1 |
0.42 ± 0.3 |
0.48 ± 0.362 |
| Arm-2 |
0.37 ± 0.189 |
0.38 ± 0.232 |
| Arm-3 |
0.4 ± 0.426 |
0.4 ± 0.397 |
|
Parameter |
SGPT (ALT) (U/L)
Visit 1 |
SGPT (ALT) (U/L) Visit 6 |
| Arm-1 |
27.16 ± 11.252 |
34.04 ± 19.129 |
| Arm-2 |
25.91 ± 16.611 |
24.87 ± 12.862 |
| Arm-3 |
35.63 ± 25.901 |
41.29 ± 32.789 |
|
Parameter |
SGOT (AST) (U/L)
Visit 1 |
SGOT (AST) (U/L) Visit 6 |
| Arm-1 |
28.2 ± 13.319 |
34.89 ± 17.32 |
| Arm-2 |
23.94 ± 10.598 |
25.94 ± 11.662 |
| Arm-3 |
25.92 ± 11.204 |
29.19 ± 11.514 |
|
Parameter |
Albumin (g/dL) Visit 1 |
Albumin (g/dL) Visit 6 |
| Arm-1 |
4.63 ± 0.506 |
4.37 ± 0.253 |
| Arm-2 |
4.64 ± 0.462 |
4.67 ± 0.241 |
| Arm-3 |
4.55 ± 0.384 |
4.47 ± 0.379 |
|
Parameter |
Alkaline Phosphatase (U/L) Visit 1 |
Alkaline Phosphatase (U/L) Visit 6 |
| Arm-1 |
92.64 ± 46.993 |
102.19 ± 53.392 |
| Arm-2 |
85.59 ± 17.885 |
82.74 ± 19.706 |
| Arm-3 |
96.25 ± 26.122 |
88.71 ± 31.612 |
|
Parameter |
Blood Urea Nitrogen (mg/dL) Visit 1 |
Blood Urea Nitrogen (mg/dL) Visit 6 |
| Arm-1 |
11.42 ± 3.38 |
11.44 ± 3.738 |
| Arm-2 |
10.43 ± 4.024 |
10.47 ± 2.552 |
| Arm-3 |
9.95 ± 2.703 |
10.82 ± 2.076 |
|
Parameter |
Serum Urea (mg/dL) Visit 1 |
Serum Urea (mg/dL) Visit 6 |
| Arm-1 |
23.93 ± 6.459 |
25.11 ± 7.854 |
| Arm-2 |
22.61 ± 8.649 |
21.9 ± 4.892 |
| Arm-3 |
21.28 ± 5.785 |
23.15 ± 4.443 |
|
Parameter |
Creatinine (mg/dL) Visit 1 |
Creatinine (mg/dL) Visit 6 |
| Arm-1 |
0.86 ± 0.228 |
0.89 ± 0.342 |
| Arm-2 |
0.83 ± 0.293 |
0.83 ± 0.221 |
| Arm-3 |
0.78 ± 0.173 |
0.82 ± 0.207 |
Values represent the mean ± standard deviation
Changes in Magnesium Levels in Magnesium Deficient Subjects from Baseline to End of 3 Months
There was a total of 9 subjects who had hypomagnesaemia at baseline with serum Mg levels below 1.7 mg/dL. Of these, 1 was in MG-HT® once daily arm, 6 were in MG-HT® twice daily arm out of which 2 subjects completed the visit 6 and remaining 4 were lost to follow-up before visit 6 and 2 were in the standard of care arm out of which, 1 subject completed the visit 6 and remaining 1 was lost to follow-up before visit 6. All subjects achieved normal serum Mg levels at study end with an average of 1.8 mg/dL. The mean change in Mg levels in hypomagnesaemic subjects are shown in Table 6, values representing the mean ± standard deviation.
Table 6: Mean changes in magnesium levels in subjects with Magnesium Deficiency from baseline to study end, values represent the mean ± standard deviation
|
Antihypertensive therapy and MG-HT® once daily |
||
|
Visit 1 |
Visit 6 |
|
| N |
1 |
1 |
| Magnesium (mg/dl) |
1.7 |
1.8 |
|
Antihypertensive therapy and MG-HT® twice daily |
||
|
Visit 1 |
Visit 6 |
|
| N |
6 |
2 |
| Magnesium (mg/dl) |
1.54 ± 0.15 |
1.8 |
|
Standard of Care with Antihypertensive therapy |
||
|
Visit 1 |
Visit 6 |
|
| N |
2 |
1 |
| Magnesium (mg/dl) |
1.67 ± 0.042 |
1.8 |
Values represent the mean ± standard deviation
Change in SBP in Subjects with Hypomagnesaemia from Baseline to End of 3 Months
The changes in SBP in the 9 (1 subject in MG-HT® once daily arm, 6 subjects in MG-HT® twice daily arm and 2 subjects in Standard of Care arm) subjects who had hypomagnesaemia at baseline were not statistically significant between arms; out of these 9 subjects 4 in MG-HT® twice daily arm and 1 in Standard of Care arm lost to follow-up before study end visit. However, subjects receiving MG-HT® once daily reported a greater reduction in BP levels at study end with a reduction 10 mmHg compared to 7 mmHg and 6 mmHg of MG-HT® twice daily arm and standard of care arm respectively. The mean change in SBP in subjects with hypomagnesaemia is shown in Table 7.
Table 7: Mean changes in SBP in subjects with hypomagnesaemia from baseline to end of visit at 3 months, values represent the mean ± standard deviation
|
Antihypertensive therapy and MG-HT® once daily |
||
|
Visit 1 |
Visit 6 |
|
| N |
1 |
1 |
| SBP (mmHg) |
150 |
140 |
|
Antihypertensive therapy and MG-HT® twice daily |
||
|
Visit 1 |
Visit 6 |
|
| N |
6 |
2 |
| SBP (mmHg) |
144.5 ± 7.791 |
137.5 ± 3.536 |
|
Standard of Care with Antihypertensive therapy |
||
|
Visit 1 |
Visit 6 |
|
| N |
2 |
1 |
| SBP (mmHg) |
146 ± 2.828 |
140 |
Values represent the mean ± standard deviation
Change in DBP in Subjects with Hypomagnesaemia from Baseline to End of 3 Months
The changes in DBP in the 9 subjects who had hypomagnesaemia at baseline were not statistically significant between arms. The mean changes in DBP in subjects with hypomagnesaemia are shown in Table 8.
Table 8: Mean changes in DBP in subjects with hypomagnesaemia from baseline to end of 3 months, values represent the mean ± standard deviation
|
Antihypertensive therapy and MG-HT® once daily |
||
|
Visit 1 |
Visit 6 |
|
| N |
1 |
1 |
| DBP (mmHg) |
90 |
90 |
|
Antihypertensive therapy and MG-HT® twice daily |
||
| DBP (mmHg) |
Visit 1 |
Visit 6 |
| N |
6 |
2 |
| DBP (mmHg) |
88.17 ± 4.491 |
90 |
|
Standard of Care with Antihypertensive therapy |
||
| DBP (mmHg) |
Visit 1 |
Visit 6 |
| N |
2 |
1 |
| DBP (mmHg) |
94 |
90 |
Values represent the mean ± standard deviation
Relationship between Magnesium Levels and SBP and DBP between Arms (N=9)
As depicted in Figure 3, the correlation between changes in serum Mg levels and change in SBP was more marked in subjects receiving MG-HT® once daily compared to the other two arms. No correlation was seen between change in serum Mg levels and change in DBP in the treatment arms (Figure 3).
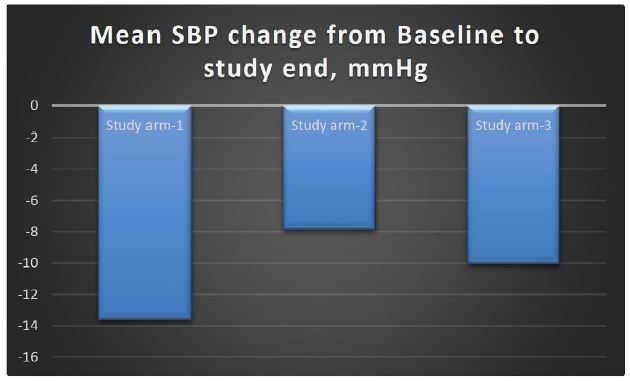
Figure 1: Mean SBP change from baseline to study end
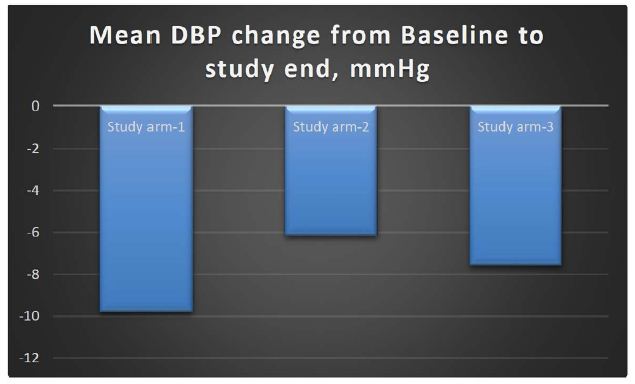
Figure 2: Mean SBP change from baseline to study end
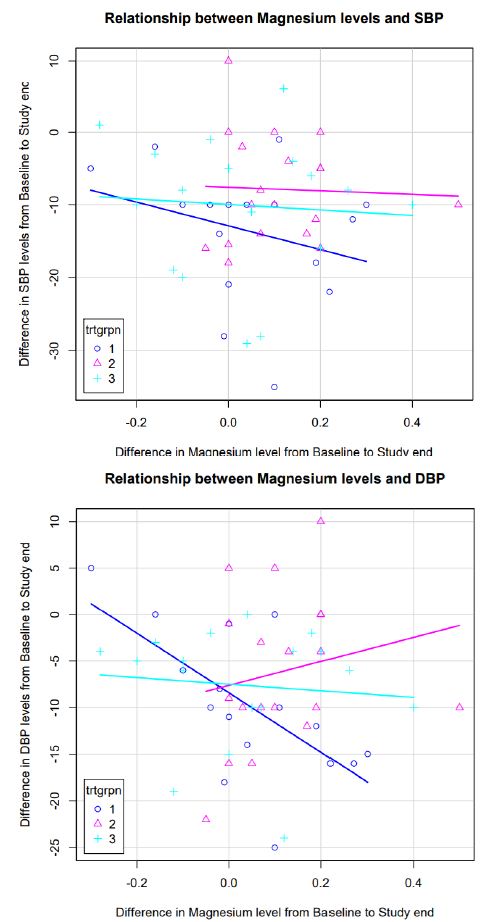
Figure 3: Change in SBP, DBP and levels of magnesium in subjects with combined treatment arms vs. standard of care arm from baseline to end of 3 months
Change in SBP, DBP and Levels of Magnesium in Subjects with Combined Treatment Arms vs. Standard of Care Arm from Baseline to End of 3 Months
The pooled analysis of changes in SBP and DBP between control vs treatment (MGHT® once daily and twice daily combined) was not statistically significant. Mean changes are shown in Table 9.
Table 9: Mean change in SBP, DBP and levels of magnesium in subjects with combined treatment arms vs. standard of care arm from baseline to end of 3 months
|
SBP (mmHg) Visit 1 |
SBP (mmHg) Visit 6 |
||||
|
N |
Mean ± SD | N | Mean ± SD |
p-value |
|
| Treatment |
56 |
148.6 ± 7.087 | 35 |
136.43 ± 10.144 |
0.83 |
| Standard of Care |
24 |
148.56 ± 6.589 | 17 |
137.24 ± 8.864 |
|
|
DBP (mmHg) Visit 1 |
DBP (mmHg) Visit 6 |
||||
|
N |
Mean ± SD | N | Mean(SD) |
p-value |
|
| Treatment |
56 |
91 ± 4.077 | 35 |
83 ± 6.987 |
0.982 |
| Standard of Care |
24 |
91.13 ± 4.785 | 17 |
83 ± 6.275 |
|
|
Magnesium (mg/dL) Visit 1 |
Magnesium (mg/dL) Visit 6 |
||||
|
N |
Mean ± SD | N | Mean ± SD |
p-value |
|
| Treatment |
55 |
1.96 ± 0.199 | 35 |
2.06 ± 0.192 |
0.419 |
| Standard of Care |
24 |
2.01 ± 0.226 | 17 |
2.06 ± 0.166 |
|
Change in Lab Parameters in Combined Treatment Arms vs. Standard of Care Arm from Baseline to End of 3 Months
The pooled analysis of change in laboratory parameters between control vs. treatment arms (MG-HT® once daily and twice daily combined) was not statistically significant. Mean changes are shown in Table 10.
Table 10: Mean change in lab parameters in combined treatment arms vs. standard of care arm from baseline to end of 3 months
|
|
HbA1c (%) Visit 1 |
HbA1c (%) Visit 6 |
p-value |
|
| Treatment | N |
55 |
35 |
0.986 |
| Mean ± SD |
6.64 ± 1.359 |
6.38 ± 0.767 |
||
| Standard of Care | N |
24 |
17 |
|
| Mean ± SD |
7.16 ± 1.807 |
6.61 ± 1.561 |
||
|
Cholesterol Total (mg/dl) Visit 1 |
Cholesterol Total (mg/dl) Visit 6 |
p-value |
||
| Treatment | N |
55 |
35 |
0.391 |
| Mean ± SD |
173.49 ± 46.318 |
174.08 ± 39.236 |
||
| Standard of Care | N |
24 |
17 |
|
| Mean ± SD |
190.68 ± 42.956 |
179.45 ± 56.949 |
||
|
HDL (mg/dl) Visit 1 |
HDL (mg/dl) Visit 6 |
p-value |
||
| Treatment | N |
55 |
35 |
0.297 |
| Mean ± SD |
44.78 ± 10.132 |
44.49 ± 12.822 |
||
| Standard of Care | N |
24 |
17 |
|
| Mean ± SD |
44.31 ± 12.119 |
43.33 ± 11.732 |
||
|
LDL (mg/dl) Visit 1 |
LDL (mg/dl) Visit 6 |
p-value |
||
| Treatment | N |
55 |
35 |
0.606 |
| Mean ± SD |
101.34 ± 37.918 |
97.72 ± 31.575 |
||
| Standard of Care | N |
24 |
17 |
|
| Mean ± SD |
116 ± 35.68 |
99.88 ± 46.098 |
||
|
VLDL (mg/dl) Visit 1 |
VLDL (mg/dl) Visit 6 |
p-value |
||
| Treatment | N |
54 |
32 |
0.551 |
| Mean ± SD |
27.19 ± 11.523 |
30.1 ± 14.074 |
||
| Standard of Care | N |
24 |
16 |
|
| Mean ± SD |
30.85 ± 12.887 |
35.74 ± 16.172 |
||
|
Triglyceride (mg/dl) Visit 1 |
Triglyceride (mg/dl) Visit 6 |
p-value |
||
| Treatment | N |
55 |
35 |
0.888 |
| Mean ± SD |
148.41 ± 81.042 |
170.53 ± 96.742 |
||
| Standard of Care | N |
24 |
17 |
|
| Mean ± SD |
162.42 ± 90.644 |
196.94 ± 107.201 |
||
|
Bilirubin Total (mg/dL) Visit 1 |
Bilirubin Total (mg/dL) Visit 6 |
p-value |
||
| Treatment | N |
55 |
35 |
0.294 |
| Mean ± SD |
0.63 ± 0.332 |
0.7 ± 0.384 |
||
| Standard of Care | N |
24 |
17 |
|
| Mean ± SD |
0.61 ± 0.502 |
0.62 ± 0.468 |
||
|
Bilirubin Direct (mg/dL) Visit 1 |
Bilirubin Direct (mg/dL) Visit 6 |
p-value |
||
| Treatment | N |
55 |
35 |
0.256 |
| Mean ± SD |
0.24 ± 0.127 |
0.27 ± 0.154 |
||
| Standard of Care | N |
24 |
17 |
|
| Mean ± SD |
0.21 ± 0.097 |
0.21 ± 0.087 |
||
|
Bilirubin Indirect (mg/dL) Visit 1 |
Bilirubin Indirect (mg/dL) Visit 6 |
p-value |
||
| Treatment | N |
55 |
35 |
0.593 |
| Mean ± SD |
0.39 ± 0.251 |
0.43 ± 0.298 |
||
| Standard of Care | N |
24 |
17 |
|
| Mean ± SD |
0.4 ± 0.426 |
0.4 ± 0.397 |
||
|
SGPT(ALT) (U/L) Visit 1 |
SGPT (ALT) (U/L) Visit 6 |
p-value |
||
| Treatment | N |
55 |
35 |
0.661 |
| Mean ± SD |
26.55 ± 14.02 |
29.07 ± 16.447 |
||
| Standard of Care | N |
24 |
17 |
|
| Mean ± SD |
35.63 ± 25.901 |
41.29 ± 32.789 |
||
|
SGOT (AST) (U/L) Visit 1 |
SGOT (AST) (U/L) Visit 6 |
p-value |
||
| Treatment | N |
55 |
35 |
0.552 |
| Mean ± SD |
26.11 ± 12.14 |
30.03 ± 14.994 |
||
| Standard of Care | N |
24 |
17 |
|
| Mean ± SD |
25.92 ± 11.204 |
29.19 ± 11.514 |
||
|
Albumin (g/dL) Visit 1 |
Albumin (g/dL) Visit 6 |
p-value |
||
| Treatment | N |
55 |
35 |
0.509 |
| Mean ± SD |
4.63 ± 0.481 |
4.54 ± 0.286 |
||
| Standard of Care | N |
24 |
17 |
|
| Mean ± SD |
4.55 ± 0.384 |
4.47 ± 0.379 |
||
|
Alkaline Phosphatase (U/L) Visit 1 |
Alkaline Phosphatase (U/L) Visit 6 |
p-value |
||
| Treatment | N |
55 |
35 |
0.143 |
| Mean ± SD |
89.18 ± 35.649 |
91.63 ± 39.495 |
||
| Standard of Care | N |
24 |
17 |
|
| Mean ± SD |
96.25 ± 26.122 |
88.71 ± 31.612 |
||
|
Blood Urea Nitrogen (mg/dL) Visit 1 |
Blood Urea Nitrogen (mg/dL) Visit 6 |
p-value |
||
| Treatment | N |
55 |
35 |
0.795 |
| Mean ± SD |
10.93 ± 3.709 |
10.91 ± 3.138 |
||
| Standard of Care | N |
24 |
17 |
|
| Mean ± SD |
9.95 ± 2.703 |
10.82 ± 2.076 |
||
|
Urea Serum (mg/dL) Visit 1 |
Urea Serum (mg/dL) Visit 6 |
p-value |
||
| Treatment | N |
52 |
32 |
0.706 |
| Mean ± SD |
23.27 ± 7.587 |
23.41 ± 6.546 |
||
| Standard of Care | N |
24 |
17 |
|
| Mean ± SD |
21.28 ± 5.785 |
23.15 ± 4.443 |
||
|
Creatinine (mg/dL) Visit 1 |
Creatinine (mg/dL) Visit 6 |
p-value |
||
| Treatment | N |
55 |
35 |
0.931 |
| Mean ± SD |
0.84 ± 0.26 |
0.86 ± 0.28 |
||
| Standard of Care | N |
24 |
17 |
|
| Mean ± SD |
0.78 ± 0.173 |
0.82 ± 0.207 |
||
Adverse Events
There were 8 adverse events in the study. The list of adverse events with the study arm distribution has been presented below. The causality assessment of all AEs reported were assessed to be unrelated to the study products. This means although sinus bradycardia and first-degree heart block had been reported to be associated with Mg administration, the incidence of such events are very low. None of the patients in study groups once or twice daily MG-HT® ever demonstrated any event of hypermagnesemia or critical hypomagnesemia, and thus it can be safely concluded that these adverse events did not result from any episode of hyper or hypomagnesemia, related to MG-HT® intervention. Additionally, the subjects had these problems even before the study. Therefore, these events were considered negligible, and all the adverse events were considered unrelated to the intervention.
The following AEs were reported, sinus bradycardia, first-degree heart block, insomnia, COVID-19, herpes zoster, weakness of leg and arm suspected due to Vitamin D deficiency, dyspepsia and constipation.
Discussion
The results of the present study show that MG-HT® once daily with continued antihypertensive regimen, MG-HT® twice daily with continued antihypertensive therapy and standard of care reduced BP in patients with stage 1 and 2 HTN. The mean change in SBP from baseline to end of study at 90 days was a reduction of 13.63 mmHg, 7.87 mmHg and 10.06 mmHg in MG-HT® once daily, MG-HT® twice daily and standard of care arm respectively. MG-HT® once daily arm showed higher SBP reduction as compared to MG-HT® twice daily arm and standard of care arm. However, this difference was not statistically significant (p-value > 0.05).
The mean change in DBP from baseline to end of study at 90 days was a reduction of 9.81 mmHg, 6.16 mmHg and 7.59 mmHg in MG-HT® once daily, MG-HT® twice daily and standard of care arm respectively. The reduction in DBP was higher in the arm receiving MG-HT® once daily as compared to MG-HT® twice daily and standard of care. However, this difference was not statistically significant (p-value > 0.05).
The pooled analysis of changes in SBP and DBP from baseline to end of 90 days between control vs. treatment (MG-HT® once daily and twice daily combined) was not statistically significant (p-value > 0.05).
These data showed wide variations in recorded BP, which could be due to two reasons, (1) wide variations in BP recordings in various other studies involving BP as a variable (2) a larger percentage of patients who were lost to follow-up due to prevalent COVID-19 situation in India. This wide variation led to a large standard deviation which might have contributed to lack of statistical significance of the findings in different data sets. The result also showed statistically non-significant improvement of lipid profile and serum Mg from baseline to study end between study arms (p-value > 0.05). Nine subjects who had hypomagnesaemia at baseline, achieved normal serum Mg levels at study end with an average value of 1.8 mg/dL. The mean change in SBP and DBP from baseline to end of study at 90 days in these 9 subjects with hypomagnesaemia was not statistically significant between study arms; however, subjects receiving MG-HT® once daily reported a greater reduction in SBP levels at the end of study with a reduction 10 mmHg compared to 7 mmHg and 6 mmHg for MG-HT® twice daily arm and standard of care arm respectively.
The change from baseline to study end in the stage wise distribution of number of subjects was statistically significant (p-value <0.001). There were 66 subjects (82.5%) in stage 1 which reduced to 27 subjects (33.8%) at study end. Similarly, there were 14 subjects (17.5%) in stage 2 and none at study end. A statistically significant high proportion of subjects, i.e., 24 subjects (30%) moved to prehypertensive levels with 15 subjects (18.8%) reporting prehypertensive levels of BP as early as, a month after treatment. One subject reported normal levels of BP at one month of treatment and maintained the same levels at the end of study.
In our study the causality assessment of all AEs reported were assessed to be unrelated to the study products. There were no serious adverse events reported in the present study.
Several studies have shown that inadequate intake of Mg may cause essential hypertension. Mg supplementation has been documented to decrease BP acting as Calcium antagonist on smooth muscle tone, leading to vasorelaxation, which appears to be the desired end result of all antihypertensive treatments and could be the final common physiological pathway for blood pressure regulation. This suggests an inverse correlation between dietary Mg and BP. Some studies have shown that Mg supplementation has been shown to decrease BP in normotensives; despite that, even now the clinical practice does not routinely recommend Mg as an active treatment for HTN. However, reduction of BP, albeit low, has been shown to be clinically significant in hypertensive patients, in that this can reduce the incidence of coronary heart disease, heart failure, and stroke or other complications, particularly in high-risk individuals. Thus, the reduction of BP as a result of Mg over and above that effected by standard of care can be of great and significant importance in management of HTN, more so when the clinical outcomes with standard of care are suboptimal or hypo-responsive, in addition to reduction of complications [35-38].
Some studies have established that Mg deficiency caused by lack of dietary or supplemental Mg intake leads to HTN. Due to the correlation between Mg and HTN, it has been suggested that supranutritional Mg intake may act as a mild antihypertensive agent. Although this antihypertensive effect of Mg is existent, it is also to be noted that these studies failed to demonstrate any significant association between serum Mg concentration and the risk of HTN. On the other hand, although most studies on Mg supplementation showed a reduction of 3-4 mmHg of SBP and 2-3 mmHg of DBP, one study reported significantly higher reduction of BP to the tune of 18.7 mmHg in SBP and 10.9 mmHg in DBP when the SBP was higher than 155 mmHg. This finding is significant and comparable to our study findings [21].
A landmark categorized systematic review of 49 clinical trials was published in 2021. In this, Rosanoff et al. categorized studies involving 4 categories; our study may belong to the second category which involved uncontrolled hypertensives, who were subjects using antihypertensive medications during and prior to the study but were still hypertensive at baseline. This study has demonstrated conclusively that uncontrolled hypertensive subjects respond to oral Mg therapy consistently and significantly lowered both SBP and DBP. This is in consonance with the findings in our study where once daily dose of Mg containing nutraceutical formulation added to the standard of care antihypertensive therapy leads to reduction of both SBP and DBP, which was resistant to treatment with standard of care therapy [21].
In our study, once daily MG-HT® added to standard antihypertensive regimen led to clinically significant BP reduction, in comparison to twice daily MG-HT®, contrary to general expectation that twice daily regimen will offer more Mg and thus will be more potent in terms of BP reduction. This can be explained from the findings of the categorized systematic review by Rosanoff et al. (2022) which showed that although all Mg doses ranging from 240-607 mg/day showed decreased BP in all uncontrolled hypertensives, it failed to reduce BP in controlled hypertensives or normotensive subjects. The Mg-replete patients showed no change in BP even at high Mg dose [21]. Another meta-analysis by Zhang et al. (2016) has demonstrated that the relationship between rise in Mg level and drop in BP is nonlinear and although there is a tendency of reduction of DBP by 2.26 mmHg for every 0.1 mmol/L increase in serum Mg level, depending on baseline Mg status. This relationship ceased to exist when the subjects were Mg replete, following which the BP response to Mg was invisible [15]. In another review by Houston, it has been stated that with Mg, patients with highest BP levels at entry had the largest reduction in BP. Additionally, in this review, the author quotes the findings of a randomized controlled study which shows that although Mg and potassium combination reduced BP to a significant extent, further addition of Mg failed to reduce BP further [23]. From these data, it is clear that just numerical enhancement of Mg dosage would not linearly reduce the BP, and there are several physiological factors that control the impact of Mg on BP, and Mg repletion annuls the impact of additional dose of Mg for further reduction of BP. This may explain why in our study a dose of 70 mg of Mg was effective in reducing BP, but a twice daily dose of Mg could not further enhance the BP reduction.
In our study intake of MG-HT® could reduce the nominal values of different laboratory parameters, but these lacked significances due mainly to the contracted sample size issue, and this is an important finding in that many of these parameters can reduce the CV risks, particularly in patients with HTN. Several studies have shown that oral Mg therapy could improve several cardiovascular health parameters, such as, serum and plasma Mg, endothelial function, fasting glucose and insulin resistance, triglycerides, and total cholesterol as well as high-density lipoproteins. Although adequately structured studies are needed to establish these parameters, it can be clearly stated that in uncontrolled hypertensives, addition of Mg in the management regimen would not only reduce the BP, would also reduce the hypertensive risks.
It has been shown that all forms of Mg, inorganic or organic are effective in reducing BP in uncontrolled hypertensive subjects. Additionally, some authors also have demonstrated that patients who are Mg replete respond poorly to Mg therapy in comparison to Mg deplete individuals. Several studies have also shown that many patients with baseline HTN, be it untreated or uncontrolled, respond to oral Mg therapy to demonstrate a BP lowering effect. Obviously, in relation to this, another prudent question may arise, particularly in case of hypertensive patients who are on standard of care therapy, like those who were randomized as subjects in this study, whether routine use of Mg should be considered in addition to antihypertensive therapy. Mg has vasorelaxant effect, leading to lowering down of both SBP and DBP. Therefore, patients who are already on antihypertensive therapy as standard of care at baseline may have additive effect on reduction of BP due to individual and independent action of both. This may lead to a clinical situation of hypotension with administration of Mg just in case the patient is normotensive at baseline with the standard of care therapy. One study by Hattori et al. (1998) has conclusively demonstrated that oral Mg does not demonstrate BP lowering effect on normotensive subjects, which is only demonstrable in hypertensive subjects [38]. Another systematic review in 2021 had also shown that a range of doses of Mg could not effect any change in normotensive subjects consistently [23].
Several studies including reviews and meta-analyses demonstrated minor adverse effects only among the participants, which were transient. Additionally, these adverse effects were reported in both experimental and control groups. This finding is identical to that in our study and therefore, it can be commented that the treatment offered by MG-HT® is also devoid of any considerable adverse effects. The effective dose in our study was 70 mg of elemental Mg from 500 mg of organic Mg salt. It has been stated in literature that the tolerable upper limit of Mg intake from non-food sources is 350 mg/day, and ICMR states, in Indian population, the RDA ranges from 370 to 440 mg (440 mg for males and pregnant women; 370 mg for nonpregnant females). Many studies reported mild gastrointestinal symptoms in this dosage range. It is also important to note that many studies which have supplemented substantially more than this range did not demonstrate any such adverse effects. It is well known from other literature that very high Mg intake can be dangerous to people despite not having renal or intestinal disease, but such concentrations are in the range of more than 5000 mg, about 70 times more than the strength used in our study product. This makes this product safe beyond any doubts or concerns, which has been demonstrated in the findings of this study, and this is presumably due to combination with other ingredients which have innate capability to reduce BP, in conjunction with oral Mg.
The evidence for a positive effect of Mg on high BP risk accentuates the importance of largely encouraging the intake of foods, such as, vegetables, nuts, whole cereals and legumes, restricting processed foods, which are deprived in Mg and lack other fundamental nutrients as well, in order to prevent high BP. In some cases when diet is not adequate to sustain a sufficient Mg status, Mg supplementation may be of advantage and has been shown to be well tolerated [35], particularly when the standard of care therapy fails to produce desired or optimum results in terms of BP in a hypertensive patient. This bears further importance in terms of risk reduction in vulnerable patient groups or in hypertensive patients who pose risk of complications related to uncontrolled HTN.
A pooled analysis of 7 RCTs showed that Mg supplementation significantly reduced SBP and DBP in type 2 diabetes mellitus patients [36]. A meta-analysis, based on evidence from 34 randomized, double-blind, placebo-controlled trials, showed a significant antihypertensive effect of Mg supplementation on both systolic and diastolic BP among normotensive or hypertensive adults. Findings from this meta-analysis suggested that oral Mg supplements can be recommended for the prevention of high BP or as adjunct to antihypertensive therapy [37] in patients where there are considerable risks of HTN or hypertensive complications. Our study also shows similar findings, and this is the first study of this kind in Indian population. Therefore, in the Indian population, the findings of this study can be translated into management practices in that all patients with Stage 1 and 2 uncontrolled HTN on any antihypertensive regimen can have clinically beneficial outcomes when a Mg containing supplement, MG-HT® is added to the regimen.
However, this study has limitations hindering the generalization of the findings. Due to COVID-19, the number of patients lost to followup in each arm was large, totalling 28, which has contributed to large variation in data and has compromised the statistical significance. However even then, it could yield a very important finding of clinically significant numerical reduction of BP numbers with the study product. A large multicentric study in the same design may improve the different outcome parameters to a level of statistical significance which may impact the clinical management of HTN in future.
Conclusion
The findings of the present prospective, randomized, three-arm, open label, parallel group, multicentric study showed that adding oral MG-HT® to the existing antihypertensive regimens reduced BP in patients with stage 1 and 2 HTN. MG-HT® therapy holds potential as a way of safely achieving lower BP without increasing antihypertensive medications, specifically in persons where standard of care therapy fails to provide optimum BP control increasing risks of complications. Our findings suggested that oral MG-HT® supplement can be recommended for the prevention of HTN or as an adjuvant to antihypertensive therapy in patients with inadequate control, specifically in India. However, future large-scale, well-designed studies are warranted to provide more consistent evidence of MG-HT® supplementation benefits on BP among these patients.
Acknowledgments
The authors thank Dr. DB Anantha Narayana, Research Scholar, Bangalore, and Prof Roop Krishen Khar, Professor and Director, B.S. Anangpuria Institute of Pharmacy- Faridabad for their support in review and comments prior to publication.
Source of Funding
This study was funded by Pharmed Limited, Bangalore.
Availability of Data and Material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Rajeev Gupta, Kiran Gaur, C Venkata S Ram (2019) Emerging trends in hypertension epidemiology in India. J Hum Hypertens 33: 575-587.
- Soumitra Ghosh, Manish Kumar (2019) Prevalence and associated risk factors of hypertension among persons aged 15-49 in India: a cross-sectional study. BMJ Open 9: e029714.
- Majid Jalalyazdi, Javad Ramezani, Azadeh Izadi-Moud, Fereshteh Madani-Sani, Shokufeh Shahlaei, et al. (2019) Effect of hibiscus sabdariffa on blood pressure in patients with stage 1 hypertension. J Adv Pharm Technol Res 10: 107-111.
- Hui-Fang Chiu, Kamesh Venkatakrishnan, Oksana Golovinskaia, Chin-Kun Wang (2021) Impact of Micronutrients on Hypertension: Evidence from Clinical Trials with a Special Focus on Meta-Analysis. Nutrients 13: 588.
- Senkadhirdasan Dakshinamurthy, Vartika Saxena, Ranjeeta Kumari, Anissa Atif Mirza, Minakshi Dhar (2020) Prevalence of hypertension and associated cardiometabolic risk factors in urban Rishikesh, Uttarakhand. J Family Med Prim Care 9: 2931-2939.
- Jonas Prenissl, Jennifer Manne-Goehler, Lindsay M Jaacks, Dorairaj Prabhakaran, Ashish Awasthi, et al. (2019) Hypertension screening, awareness, treatment, and control in India: A nationally representative cross-sectional study among individuals aged 15 to 49 years. PLoS Med 16: e1002801.
- Raghupathy Anchala, Nanda K Kannuri, Hira Pant, Hassan Khan, Oscar H Franco, et al. (2014) Hypertension in India: a systematic review and meta-analysis of prevalence, awareness, and control of hypertension. J Hypertens 32: 1170-1177.
- Carolina Guerrero-García, Alberto Francisco Rubio-Guerra (2018) Combination therapy in the treatment of hypertension. Drugs Context 7: 212531.
- Julian P Yaxley, Sam V Thambar (2015) Resistant hypertension: an approach to management in primary care. J Family Med Prim Care 4: 193-199.
- R Padmanabhan, Rana Gopal Singh, Govindan Unni, Bhupen Desai, Sanjeev Kumar Hiremath, et al. (2020) Multidisciplinary Consensus Document on the Management of Uncontrolled Hypertension in India. High Blood Pressure & Cardiovascular Prevention.
- Mayank Kapoor, Minakshi Dhar, Anissa Mirza, Vartika Saxena, Monika Pathania (2021) Factors responsible for Uncontrolled Hypertension in the Adults over 50 years of age: A pilot study from Northern India. Indian Heart Journal 73: 644-646.
- Rajkumar Bharatia, Manoj Chitale, Ganesh Narain Saxena, Raman Ganesh Kumar, Chikkalingaiah, et al. (2016) Management Practices in Indian Patients with Uncontrolled Hypertension 64: 14-21.
- Andrea Rosanoff (2005) Magnesium and hypertension. Clin Calcium 15: 255-260.
- Omid Asbaghi, Reza Hosseini, Behnoosh Boozari, Ehsan Ghaedi, Sara Kashkooli, et al. (2021) The Effects of Magnesium Supplementation on Blood Pressure and Obesity Measure among Type 2 Diabetes Patient: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Biol Trace Elem Res 199: 413-424.
- Xi Zhang, Yufeng Li, Liana C Del Gobbo, Andrea Rosanoff, Jiawei Wang, et al. (2016) Effects of Magnesium Supplementation on Blood Pressure: A Meta-Analysis of Randomized Double-Blind Placebo-Controlled Trials. Hypertension 68: 324-333.
- Nikolina Banjanin, Goran Belojevic (2018) Changes of Blood Pressure and Hemodynamic Parameters after Oral Magnesium Supplementation in Patients with Essential Hypertension-An Intervention Study. Nutrients 10: 581.
- Ana Rosa Cunha, Jenifer D’El-Rei, Fernanda Medeiros, Bianca Umbelino, Wille Oigman, et al. (2017) Oral magnesium supplementation improves endothelial function and attenuates subclinical atherosclerosis in thiazide-treated hypertensive women. J Hypertens 35: 89-97.
- F Guerrero-Romero, M Rodríguez-Morán (2009) The effect of lowering blood pressure by magnesium supplementation in diabetic hypertensive adults with low serum magnesium levels: a randomized, double-blind, placebo-controlled clinical trial. J Hum Hypertens 23: 245-251.
- Ligia J Dominguez, Nicola Veronese, Mario Barbagallo (2021) Magnesium and Hypertension in Old Age. Nutrients 13: 139.
- Andrea Rosanoff, Rebecca B Costello, Guy H Johnson (2021) Effectively Prescribing Oral Magnesium Therapy for Hypertension: A Categorized Systematic Review of 49 Clinical Trials. Nutrients 13: 195.
- Nikolina Banjanin, Goran Belojevic (2018) Changes of Blood Pressure and Hemodynamic Parameters after Oral Magnesium Supplementation in Patients with Essential Hypertension-An Intervention Study. Nutrients 10: 581.
- Mark Houston (2011) The role of magnesium in hypertension and cardiovascular disease. J Clin Hypertens (Greenwich) 13: 843-847.
- Moein Askarpour, Amir Hadi, Azadeh Dehghani Kari Bozorg, Omid Sadeghi, Ali Sheikhi, et al. (2019) Effects of L-carnitine supplementation on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Hum Hypertens 33: 725-734.
- Craig J McMackin, Michael E Widlansky, Naomi M Hamburg, Alex L Huang, Susan Weller, et al. (2007) Effect of combined treatment with alpha-Lipoic acid and acetyl-L-carnitine on vascular function and blood pressure in patients with coronary artery disease. J Clin Hypertens (Greenwich) 9: 249-255.
- Chuanxun Yuan, Xueru Zhang, Xue Long, Jing Jin, Risheng Jin (2019) Effect of β-sitosterol self-microemulsion and β-sitosterol ester with linoleic acid on lipid-lowering in hyperlipidemic mice. Lipids Health Dis18: 157.
- Simin Feng, Zhuqing Dai, Anna B Liu, Jinbao Huang, Nihal Narsipur, et al. (2018) Intake of stigmasterol and β-sitosterol alters lipid metabolism and alleviates NAFLD in mice fed a high-fat western-style diet. Biochim Biophys Acta Mol Cell Biol Lipids 1863: 1274-1284.
- M K Radika, P Viswanathan, C V Anuradha (2013) Nitric oxide mediates the insulin sensitizing effects of β-sitosterol in high fat diet-fed rats. Nitric Oxide 32: 43-53.
- Zhuxian Zhang, Mengyi Liu, Chun Zhou, Panpan He, Yuanyuan Zhang, et al. (2021) Evaluation of Dietary Niacin and New-Onset Hypertension Among Chinese Adults. JAMA Netw Open 4: e2031669.
- H E Bays, D J Rader (2009) Does nicotinic acid (niacin) lower blood pressure? Int J Clin Pract 63: 151-159.
- William E Boden, Mandeep S Sidhu, Peter P Toth (2014) The therapeutic role of niacin in dyslipidemia management. J Cardiovasc Pharmacol Ther 19: 141-158.
- James McKenney (2003) Niacin for dyslipidemia: considerations in product selection. Am J Health Syst Pharm 60: 995-1005.
- Shyamala Dakshinamurti, Krishnamurti Dakshinamurti (2015) Antihypertensive and neuroprotective actions of pyridoxine and its derivatives. Can J Physiol Pharmacol 93: 1083-1090.
- M Aybak, A Sermet, M O Ayyildiz, A Z Karakilçik (1995) Effect of oral pyridoxine hydrochloride supplementation on arterial blood pressure in patients with essential hypertension. Arzneimittelforschung 45: 1271-1273.
- Ligia Dominguez, Nicola Veronese, Mario Barbagallo (2020) Magnesium and Hypertension in Old Age. Nutrients 13: 139.
- Omid Asbaghi, Reza Hosseini, Behnoosh Boozari, Ehsan Ghaedi, Sara Kashkooli, et al. (2021) The Effects of Magnesium Supplementation on Blood Pressure and Obesity Measure Among Type 2 Diabetes Patient: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Biol Trace Elem Res 199: 413-424.
- Xi Zhang, Yufeng Li, Liana C Del Gobbo, Andrea Rosanoff, Jiawei Wang, et al. (2016) Effects of Magnesium Supplementation on Blood Pressure: A Meta-Analysis of Randomized Double-Blind Placebo-Controlled Trials. Hypertension 68: 324-333.
- L Kass, J Weekes, L Carpenter (2012) Effect of magnesium supplementation on blood pressure: a meta-analysis. Eur J Clin Nutr 66: 411-418.
- K Hattori 1, K Saito, H Sano, H Fukuzaki (1988) Intracellular magnesium deficiency and effect of oral magnesium on blood pressure and red cell sodium transport in diuretic-treated hypertensive patients. Jpn Circ J 52: 1249-1256.